Long Term Neurodevelopmental Outcomes after Sevoflurane Neonatal Exposure of Extremely Preterm Children: A Cross-Sectional Observationnal Study
Abstract
:1. Introduction
2. Method
2.1. Subjects and Methods
2.1.1. General Framework
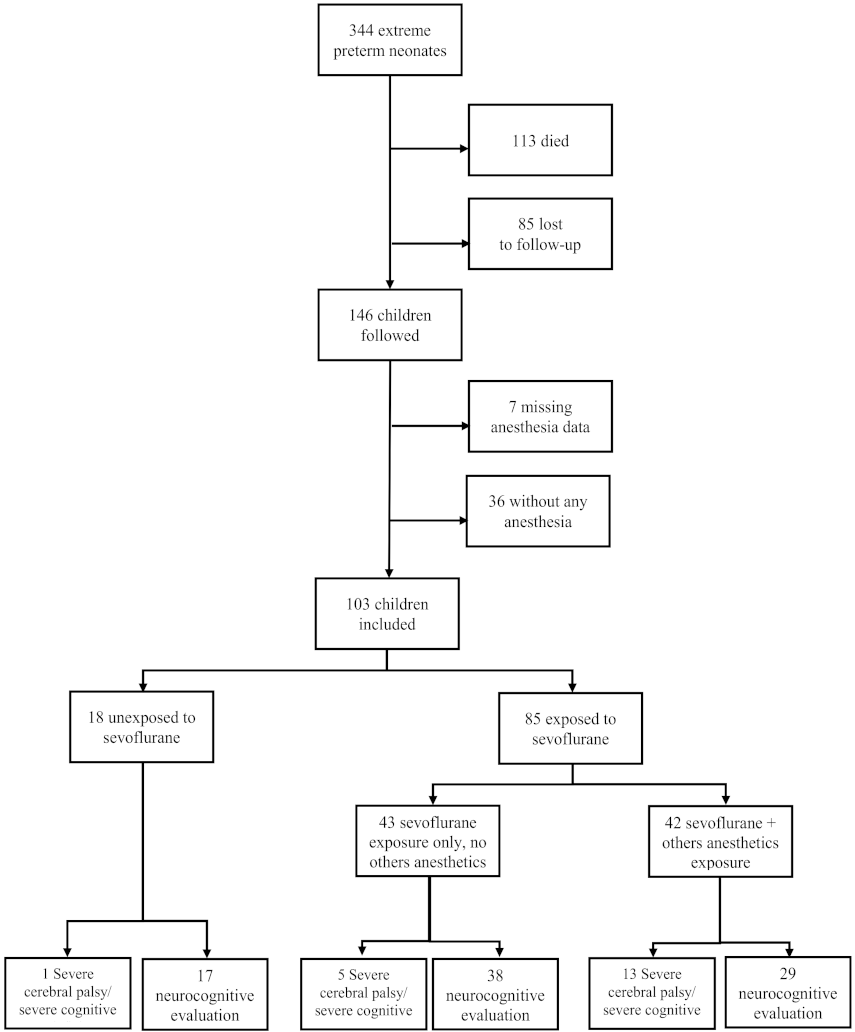
2.1.2. Participants
2.2. Ethics
2.3. Data Collection
2.3.1. Neonatal and Anesthesia Data
2.3.2. Perinatal Data
2.3.3. Evaluation between Seven and Nine Years Old
2.3.4. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Population
3.2. Propensity Score (PS) Matched Analysis
3.3. Comparison Exposed/Unexposed in Matched Group
3.4. Effect of Exposure to Sevoflurane in the Neonatal Period for the Long Term Outcome in Multivariate Models and with the Propensity Score
3.5. Effect of Exposure to Sevoflurane in the Neonatal Period Alone or in Association for the Long-Term Outcome in Multivariate Models and with the Propensity Score
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ancel, P.-Y.; Goffinet, F.; EPIPAGE-2 Writing Group. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: Results of the EPIPAGE-2 cohort study. JAMA Pediatr. 2015, 169, 230–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lago, P.; Garetti, E.; Merazzi, D.; Pieragostini, L.; Ancora, G.; Pirelli, A.; Bellieni, C.V. Guidelines for procedural pain in the newborn. Acta Paediatr. 2009, 98, 932–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbajal, R.; Rousset, A.; Danan, C.; Coquery, S.; Nolent, P.; Ducrocq, S.; Saizou, C.; Lapillonne, A.; Granier, M.; Durand, P.; et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA J. Am. Med. Assoc. 2008, 300, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Grunau, R.E. Neonatal pain in very preterm infants: Long-term effects on brain, neurodevelopment and pain reactivity. Rambam Maimonides Med. J. 2013, 4, e0025. [Google Scholar]
- Ranger, M.; Grunau, R.E. Early repetitive pain in preterm infants in relation to the developing brain. Pain. Manag. 2014, 4, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valeri, B.O.; Holsti, L.; Linhares, M.B.M. Neonatal pain and developmental outcomes in children born preterm: A systematic review. Clin. J. Pain. 2015, 31, 355–362. [Google Scholar] [CrossRef]
- Carbajal, R.; Eriksson, M.; Courtois, E.; Boyle, E.; Avila-Alvarez, A.; Andersen, R.D.; Sarafidis, K.; Polkki, T.; Matos, C.; Lago, P.; et al. EUROPAIN Survey Working Group: Sedation and analgesia practices in neonatal intensive care units (EUROPAIN): Results from a prospective cohort study. Lancet Respir. Med. 2015, 3, 796–812. [Google Scholar] [CrossRef]
- Loepke, A.W. Developmental neurotoxicity of sedatives and anesthetics: A concern for neonatal and pediatric critical care medicine? Pediatr. Crit. Care Med. 2010, 11, 217–226. [Google Scholar] [CrossRef]
- Sanders, R.D.; Davidson, A. Anesthetic-induced neurotoxicity of the neonate: Time for clinical guidelines? Paediatr. Anaesth. 2009, 19, 1141–1146. [Google Scholar] [CrossRef]
- Jevtovic-Todorovic, V.; Hartman, R.E.; Izumi, Y.; Benshoff, N.D.; Dikranian, K.; Zorumski, C.F.; Olney, J.W.; Wozniak, D.F. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J. Neurosci. 2003, 23, 876–882. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Zhang, J.; Wei, L.; Yu, S.P. Neurodevelopmental implications of the general anesthesia in neonate and infants. Exp. Neurol. 2015, 272, 50–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goa, K.L.; Noble, S.; Spencer, C.M. Sevoflurane in paediatric anaesthesia: A review. Paediatr. Drugs 1999, 1, 127–153. [Google Scholar] [CrossRef] [PubMed]
- De Hert, S.; Moerman, A. Sevoflurane. F1000Research 2015, 4, 626. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.J.; Disma, N.; De Graaff, J.C.; Withington, D.E.; Dorris, L.; Bell, G.; Stargatt, R.; Bellinger, D.C.; Schuster, T.; Arnup, S.J.; et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): An international multicentre, randomised controlled trial. Lancet 2016, 387, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.; Flick, R.P.; Zaccariello, M.J.; Colligan, R.C.; Katusic, S.K.; Schroeder, D.R.; Hanson, A.C.; Buenvenida, S.L.; Gleich, S.J.; Wilder, R.T.; et al. Association between Exposure of Young Children to Procedures Requiring General Anesthesia and Learning and Behavioral Outcomes in a Population-based Birth Cohort. Anesthesiology 2017, 127, 227–240. [Google Scholar] [CrossRef]
- Sun, L.S.; Li, G.; Miller, T.L.K.; Salorio, C.; Byrne, M.W.; Bellinger, D.C.; Ing, C.; Park, R.; Radcliffe, J.; Hays, S.R.; et al. Association Between a Single General Anesthesia Exposure Before Age 36 Months and Neurocognitive Outcomes in Later Childhood. JAMA 2016, 315, 2312–2320. [Google Scholar] [CrossRef]
- Hadders-Algra, M.; Heineman, K.R.; Bos, A.F.; Middelburg, K.J. The assessment of minor neurological dysfunction in infancy using the Touwen Infant Neurological Examination: Strengths and limitations. Dev. Med. Child. Neurol. 2010, 52, 87–92. [Google Scholar] [CrossRef]
- Canivez, G.L. Construct validity of the WISC-IV with a referred sample: Direct versus indirect hierarchical structures. Sch. Psychol. Q. 2014, 29, 38–51. [Google Scholar] [CrossRef]
- Senese, V.P.; De Lucia, N.; Conson, M. Cognitive predictors of copying and drawing from memory of the Rey-Osterrieth complex figure in 7- to 10-year-old children. Clin. Neuropsychol. 2015, 29, 118–132. [Google Scholar] [CrossRef]
- Korkman, M.; Mikkola, K.; Ritari, N.; Tommiska, V.; Salokorpi, T.; Haataja, L.; Tammela, O.; Pääkkönen, L.; Olsén, P.; Fellman, V. Neurocognitive test profiles of extremely low birth weight five-year-old children differ according to neuromotor status. Dev. Neuropsychol. 2008, 33, 637–655. [Google Scholar] [CrossRef]
- Gire, C.; Resseguier, N.; Brévaut-Malaty, V.; Marret, S.; Cambonie, G.; Souksi-Medioni, I.; Müller, J.-B.; Garcia, P.; Berbis, J.; Tosello, B.; et al. GPQoL study Group: Quality of life of extremely preterm school-age children without major handicap: A cross-sectional observational study. Arch. Dis. Child. 2019, 104, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Michel, F.; Vialet, R.; Hassid, S.; Nicaise, C.; Garbi, A.; Thomachot, L.; DI Marco, J.N.; Lagier, P.; Martin, C. Sevoflurane for central catheter placement in neonatal intensive care: A randomized trial. Paediatr. Anaesth. 2010, 20, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Torsheim, T.; Cavallo, F.; Levin, K.A.; Schnohr, C.; Mazur, J.; Niclasen, B.; Currie, C. FAS Development Study Group: Psychometric Validation of the Revised Family Affluence Scale: A Latent Variable Approach. Child. Indic. Res. 2016, 9, 771–784. [Google Scholar] [CrossRef] [Green Version]
- Bax, M.C.O.; Flodmark, O.; Tydeman, C. Definition and classification of cerebral palsy. From syndrome toward disease. Dev. Med. Child. Neurol. Suppl. 2007, 109, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.J.; Cameron, D.; Rosenbaum, P.L.; Walter, S.D.; Russell, D. Stability of the gross motor function classification system. Dev. Med. Child. Neurol. 2006, 48, 424–428. [Google Scholar] [CrossRef]
- Elvrum, A.-K.G.; Andersen, G.L.; Himmelmann, K.; Beckung, E.; Öhrvall, A.-M.; Lydersen, S.; Vik, T. Bimanual Fine Motor Function (BFMF) Classification in Children with Cerebral Palsy: Aspects of Construct and Content Validity. Phys. Occup. Ther. Pediatr. 2016, 36, 1–16. [Google Scholar] [CrossRef]
- Pull, C.B. [DSM-IV]. Encephale 1995, 15–20. [Google Scholar]
- Gire, C.; Tosello, B.; Marret, S.; Cambonie, G.; Souksi-Medioni, I.; Müller, J.-B.; Garcia, P.; Berbis, J.; Auquier, P.; Brévaut-Malaty, V.; et al. GPQoL Study Group: Specific cognitive correlates of the quality of life of extremely preterm school-aged children without major neurodevelopmental disability. Pediatr. Res. 2020, 88, 642–652. [Google Scholar] [CrossRef]
- Heinze, G.; Schemper, M. A solution to the problem of separation in logistic regression. Stat. Med. 2002, 21, 2409–2419. [Google Scholar] [CrossRef]
- Heinze, G. A comparative investigation of methods for logistic regression with separated or nearly separated data. Stat. Med. 2006, 25, 4216–4226. [Google Scholar] [CrossRef]
- Vialet, R.; Michel, F.; Hassid, S.; Di Marco, J.-N.; Martin, C. Sevoflurane for central venous catheterization in non-intubated neonates. Indian J. Pediatr. 2009, 76, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Sury, M.R.J.; Harker, H.; Thomas, M.L. Sevoflurane sedation in infants undergoing MRI: A preliminary report. Paediatr. Anaesth. 2005, 15, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Sun, H.; Yao, L.; Feng, Y.; Yang, B. Comparison of effective inspired concentration of sevoflurane in preterm infants with different postconceptual ages. Paediatr. Anaesth. 2011, 21, 148–152. [Google Scholar] [CrossRef]
- Sale, S.M.; Read, J.A.; Stoddart, P.A.; Wolf, A.R. Prospective comparison of sevoflurane and desflurane in formerly premature infants undergoing inguinal herniotomy. Br. J. Anaesth. 2006, 96, 774–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Liu, C.-M.; Sun, J.; Jin, W.-J.; Wu, Y.-Q.; Chen, J. Repeated 2% sevoflurane administration in 7- and 60-day-old rats: Neurotoxicity and neurocognitive dysfunction. Anaesthesist 2017, 66, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Amrock, L.G.; Starner, M.L.; Murphy, K.L.; Baxter, M.G. Long-term effects of single or multiple neonatal sevoflurane exposures on rat hippocampal ultrastructure. Anesthesiology 2015, 122, 87–95. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, Y.; Jiang, J.; Hu, R.; Yang, Y.; Jiang, H.; Yan, J. Neuronal apoptosis may not contribute to the long-term cognitive dysfunction induced by a brief exposure to 2% sevoflurane in developing rats. Biomed. Pharm. 2016, 78, 322–328. [Google Scholar] [CrossRef]
- Yon, J.-H.; Daniel-Johnson, J.; Carter, L.B.; Jevtovic-Todorovic, V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience 2005, 135, 815–827. [Google Scholar] [CrossRef]
- Aksenov, D.P.; Miller, M.J.; Dixon, C.J.; Drobyshevsky, A. Impact of anesthesia exposure in early development on learning and sensory functions. Dev. Psychobiol. 2020, 62, 559–572. [Google Scholar] [CrossRef]
- Serenius, F.; Ewald, U.; Farooqi, A.; Fellman, V.; Hafström, M.; Hellgren, K.; Maršál, K.; Ohlin, A.; Olhager, E.; Stjernqvist, K.; et al. Extremely Preterm Infants in Sweden Study Group: Neurodevelopmental Outcomes Among Extremely Preterm Infants 6.5 Years After Active Perinatal Care in Sweden. JAMA Pediatr. 2016, 170, 954–963. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Li, W.; Chen, X.; Yang, X.; Zhou, Z.; Lu, D.; Feng, X. Dose-dependent effects of sevoflurane exposure during early lifetime on apoptosis in hippocampus and neurocognitive outcomes in Sprague-Dawley rats. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 111–119. [Google Scholar] [PubMed]
- Loepke, A.W.; Soriano, S.G. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth. Analg. 2008, 106, 1681–1707. [Google Scholar] [CrossRef] [PubMed]
- Baudesson de Chanville, A.; Brevaut-Malaty, V.; Garbi, A.; Tosello, B.; Baumstarck, K.; Gire, C. Analgesic Effect of Maternal Human Milk Odor on Premature Neonates: A Randomized Controlled Trial. J. Hum. Lact. 2017, 33, 300–308. [Google Scholar] [CrossRef] [PubMed]
| Number (n) | % or Mean (±SD) | |
|---|---|---|
| Neurocognitive evaluation | ||
| Mean age at testing in years (±SD) | 91/103 | 8.68 (±0.49) |
| WISC-IV | ||
| Mean FSIQ (±SD) | 82/84 | 84.80 (±18.41) |
| FSIQ ≥ 115 | 5/82 | 6.10% |
| FSIQ ≤ 85 | 43/82 | 52.44% |
| Mean VCI (Verbal Comprehension Index) (±SD) | 83/84 | 95.04 (±19.75) |
| Mean PRI (Perceptual Reasoning Index) (±SD) | 84/84 | 86.18 (±15.99) |
| Mean WMI (Working Memory index) (±SD) | 83/84 | 87.30 (±15.27) |
| WMI ≤ 85 | 32/83 | 38.55% |
| Mean PSI (Processing Speed Index) (±SD) | 84/84 | 84.89 (±14.97) |
| PSI ≤ 85 | 45/84 | 53.57% |
| NEPSY | ||
| Mean executive function/planning score (Tower) (±SD) | 84/84 | 10.60 (±2.72) |
| Mean auditory attention score (±SD) | 81/84 | 9.19 (±1.42) |
| Mean visual attention score (±SD) | 83/84 | 9.02 (±2.95) |
| Inhibition score (Statue) ≤ 10th percentile | 2/82 | 2.44% |
| Mean design fluency score | 84/84 | 7.04 (±2.67) |
| Neurocognitive outcome | ||
| 1 Cerebral palsy (CP) (10 Severe and 17 Minor CPs) | 27/101 | 26.73% |
| Severe CP: GMFCS and/or BMF 3 or 4 | 10/101 | 10% |
| Minor CP: GMFCS and/or BMF type < or = 2 | 17/101 | 16.8% |
| 2 Severe disability | 37/101 | 36.63% |
| 3 Specific neurocognitive impairment | 59/84 | 70.24% |
| Dysexecutive disorders | 28/83 | 33.73% |
| Attention deficit | 20/82 | 24.39% |
| Visuo-spatial integration delay | 22/84 | 26.19% |
| Motor ideomotor dyspraxia | 25/83 | 30.12% |
| Language delay | 42/88 | 47.73% |
| Overall (n = 103) | PS ** Matched Cohort (n = 54) | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-Exposed (n = 18) (%) | Exposed to Sevoflurane (n = 85) (%) | *** Standardized Difference | p-Value | Non-Exposed (n = 18) | Exposed to Sevoflurane (n = 36) | *** Standardized Difference | p-Value | |
| Antenatal data | ||||||||
| Antenatal steroids | 16 (88.89) | 82 (96.47) | 0.2 | 0.20 | 16 (88.89) | 33 (91.67) | 0.094 | >0.99 |
| Perinatal data | ||||||||
| Male gender | 9 (50.00%) | 45 (52.94) | 0.29 | 0.82 | 9 (50.00%) | 17 (47.22) | 0.056 | 0.84 |
| Mean GA at birth in Weeks GA (±SD) | 25.89 (±0.96) | 26.27 (±0.79) | 0.43 | 0.10 | 25.89 (±0.96) | 26.31 (±0.71) | 0.49 | 0.11 |
| Mean BW in grams (±SD) | 739.72 (±143.78) | 861.64 (±186.54) | 0.020 * | 739.72 (±143.78) | 877.34 (±175.50) | 0.0094 * | ||
| Neonatal morbidities | ||||||||
| CLD | 11 (61.11) | 43 (52.44) | 0.17 | 0.50 | 11 (61.11) | 20 (66.67) | 0.69 | 0.697 |
| Nosocomial infections | 12 (66.67) | 51 (60.00) | 0.98 | 0.19 | 12 (66.67) | 24 (66.67) | .0.00 | >0.99 |
| PDA | 11 (61.11) | 55 (64.71) | 0.77 | 0.074 | 11(61.11) | 25(69.44) | 0.17 | 0.54 |
| Retinopathy of prematurity (all stages) | 6 (33.33) | 10 (11.76) | 0.53 | 0.033 * | 6(33.33) | 7(19.44) | 0.31 | 0.31 |
| Surgery required (all indications) | 2 (11.11) | 36 (42.35) | 0.75 | 0.013 * | 2(11.1) | 5(13.89) | 0.084 | >0.99 |
| Overall Cohort (n = 103) | PS-Matched Cohort (n = 54) | |||||
|---|---|---|---|---|---|---|
| Non-Exposed (n = 18) | Exposed to Sevoflurane (n = 85) | p-Value | Non-Exposed (n = 18) | Exposed to Sevoflurane (n = 36) | p-Value | |
| Treatment with Sevoflurane | ||||||
| Corrected age (Week GA) exposure | 28.61 (5.07) | 27.28 (3.36) | ||||
| Age exposure (Days) | 16.82 (37.29) | 6.89 (23.7) | ||||
| Mean Exposure time (minutes) | 151 (138) | 149 (109) | ||||
| Treatment with anesthesia * | ||||||
| Other anesthesia | 18 (100) | 42 (49.4) | 0.0001 | 18 (100) | 16 (44.4) | 0.0001 |
| Corrected Weeks GA exposure anesthesia | 27.61 (2.3) | 27.10 (3.37) | 0.161 | 27.61 (2.3) | 26.5 (0.7) | 0.062 |
| Age exposure anesthesia | 12.44 (14.75) | 6.23 (22.59) | 0.0003 | 12.44(14.75) | 1.83 (2.32) | 0.003 |
| Number of general anaesthesia | 0.22 (0.55) | 3.02 (2.24) | 0.0000 | 0.22 (0.55) | 2.81 (1.89) | 0.000 |
| Total dose of sufenta received (microgram by kilogram by hour) | 0.61 (0.36) | 0.72 (0.46) | 0.156 | |||
| Total dose of midazolam received (microgram by kilogram by hour) | 29.4 (15.1) | 31.7 (19.2) | 0.377 | |||
| Treatment with analgesia ** | ||||||
| Number of sedations | 1.44 (0.7) | 0.87 (1.11) | 0.0027 | 1.44 (0.7) | 0.69 (1.01) | 0.0005 |
| Total dose of morphine received (microgram by kilogram by hour) | 7.31 (3.95) | 9.74 (4.92) | 0.0003 | |||
| Duration of sedation (days) | 10.3 (7.6) | 9.5 (17.5) | 0.0026 | 10.3 (7.6) | ||
| Indication for anesthesia | ||||||
| Central line placement | 2 (11.1) | 41 (48.2) | 0.004 | |||
| Nasotracheal intubation | 0 (0) | 75 (88.2) | 0.000 | |||
| Surgery (all indication) | 2 (11.1) | 36 (42.3) | 0.013 | |||
| Patent ductus arteriosus | 2 (11.1) | 16 (18.8) | 0.732 | |||
| Necrotizing enterocolitis | 0 (0) | 25 (29.4) | 0.005 | |||
| Others indications | 0 (0) | 5 (5.8) | 0.584 | |||
| Multivariate Model a (18 vs. 85) | Propensity Score b (18 vs. 36) | |
|---|---|---|
| 1 Cerebral palsy | 5.09 (1.18–32.99) * | 3.96 (1.01–22.26) * |
| 2 Severe disability | 3.75 (1.10–15.26) * | 3.17 (0.96–12.17) * |
| Visuo-spatial integration delay | 6.97 (1.21–90.67) * | 5.05 (1.24–29.18) * |
| Ideomotor dyspraxia | 7.50 (1.38–87.66) * | 7.86 (1.60–77.92) * |
| Attention deficit | 1.65 (0.46–6.90) | 1.72 (0.51–6.31) |
| FSIQ ≤ 85 | 2.16 (0.62–8.13) | 2.41 (0.70–8.61) |
| WMI ≤ 85 | 1.01 (0.31–3.33) | 1.35 (0.41–4.59) |
| PSI ≤ 85 | 2.70 (0.82–9.70) | 4.20 (1.24–15.41) * |
| Overall Cohort (n: 103) | PS-Matched Cohort (n: 54) | |||||
|---|---|---|---|---|---|---|
| Non-Exposed (n = 18) | Exposed to Sevoflurane (n = 85) | p-Value | Non-Exposed (n = 18) | Exposed to Sevoflurane (n = 36) | p-Value | |
| WISC IV results | ||||||
| Verbal comprehension index | 93.44 (21.64) | 95.42 (19.43) | 0.7 | 93.44 (21.64) | 89.24(19.21) | 0.34 |
| Visual -perceptual reasoning index | 87.24 (17.75) | 85.95 (15.65) | 0.8 | 87.24 (17.75) | 77.07(13.13) | 0.037 |
| Working memory index | 87.06 (17.14) | 87.36 (14.93) | 0.7 | 87.06 (17.14) | 82.66(14.84) | 0.46 |
| Processing speed index | 87.76 (16.98) | 84.16 (14.46) | 0.28 | 87.76 (16.98) | 78.62(12.92) | 0.05 |
| Full scale intelligent quotient (FSIQ) | 86.06 (22.51) | 84.5 (17.47) | 0.74 | 86.06 (22.51) | 75.93(15.86) | 0.05 |
| Subtest NePSY results | ||||||
| Tower | 10.47 (3.26) | 10.63 (2.59) | 0.45 | 10.47 (3.26) | 10.03(2.96) | 0.5 |
| Fluence of design | 7.35 (3.46) | 6.96 (2.46) | 0.87 | 7.35 (3.46) | 6.48(2.03) | 0.49 |
| Visual attention | 9.06 (2.88) | 9.02 (2.99) | 0.45 | 9.06 (2.88) | 8.54(2.88) | 0.47 |
| Auditive attention | 8.93 (1.22) | 9.24 (1.46) | 0.82 | 8.93 (1.22) | 9(1.54) | 0.94 |
| Touwen results (developmental coordination disorder) | 1 (5.88) | 24 (35.82) | 0.015 | 1 (5.88) | 13 (44.83) | 0.05 |
| Cerebral palsy (10 Severe CP 3 or 4 and 17 Minor CP <or =2) | 3 (11) | 25 (30) | 0.14 | 3 (11) | 13(33.34) | 0.04 |
| Severe disabilities | 4 (23.53) | 33 (39.2) | 0.21 | 4 (23.53) | 18(51.48) | 0.05 |
| Major sequelae | 5 (27.78) | 42 (50.00) | 0.087 | 5(27.78) | 21 (60) | 0.026 |
| Specific cognitive impairment | ||||||
| Dysexecutive disorders | 12 (70.56) | 47 (70.15) | 0.9 | 12 (70.56) | 23(79.31) | 0.72 |
| Attention deficit | 5 (29.41) | 23 (34.85) | 0.67 | 5 29.41 | 12(42.86) | 0.36 |
| Visuo-spatial integration delay | 2 (12.5) | 18(27.27) | 0.33 | 2 12.5 | 13(46.43) | 0.022 |
| Motor ideomotor dyspraxia | 1 (5.88) | 21 (31.34) | 0.034 | 1 (5.88) | 12(41.38) | 0.015 |
| Language delay | 4 (25) | 21 (31.34) | 0.76 | 4 (25) | 14(48.28) | 0.12 |
| A. Multivariate Model a | |||
|---|---|---|---|
| Non-Exposed Reference | Sevoflurane Exposure Only Odd-Ratio (CI 95%) | Sevoflurane and Others Anesthetics Odd-Ratio (CI 95%) | |
| FSIQ ≤ 85 | 1 | 1.94 (0.51–7.83) | 2.52 (0.54–13.80) |
| 1 Cerebral palsy | 1 | 3.16 (0.65–21.85) | 8.88 (1.70–67.91) * |
| 2 Severe disability | 1 | 2.86 (0.77–12.37) | 5.96 (1.35–32.21) * |
| Visuo-spatial integration delay | 1 | 3.48 (0.52–46.27) | 18.65 (2.43–293.12) * |
| Ideomotor dyspraxia | 1 | 9.27 (1.44–129.41) * | 6.24 (0.97–77.34) |
| Attention deficit | 1 | 1.63 (0.40–7.47) | 1.67 (0.36–8.74) |
| WMI ≤ 85 | 1 | 0.82 (0.23–2.94) | 1.44 (0.35–6.42) |
| PSI ≤ 85 | 1 | 2.27 (0.63–8.75) | 3.62 (0.83–18.43) |
| B. Propensity Score Model b | |||
| Non-Exposed Reference | Sevoflurane Exposure Only Odd-Ratio (CI 95%) | Sevoflurane and Others Anesthetics Odd-Ratio (CI 95%) | |
| FSIQ ≤ 85 | 1 | 1.63 (0.47–5.67) | 3.30 (0.78–13.61) |
| 1 Cerebral palsy | 1 | 2.47 (0.45–13.62) | 5.45 (0.96–31.13) |
| 2 Severe disability | 1 | 2.64 (0.69–10.14) | 5.38 (1.31–22.06) * |
| Visuo-spatial integration delay | 1 | 1.26 (0.21–7.58) | 9.14 (1.49–56.04) * |
| Ideomotor dyspraxia | 1 | 10.11 (1.14–90.07) * | 8.26 (0.81–84.19) |
| Attention deficit | 1 | 1.26 (0.34–4.66) | 1.50 (0.32–6.95) |
| WMI ≤ 85 | 1 | 0.70 (0.19–2.54) | 2.35 (0.58–9.46) |
| PSI ≤ 85 | 1 | 1.70 (0.49–5.93) | 6.10 (1.55–23.97) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brévaut-Malaty, V.; Resseguier, N.; Garbi, A.; Tosello, B.; Thomachot, L.; Vialet, R.; Gire, C. Long Term Neurodevelopmental Outcomes after Sevoflurane Neonatal Exposure of Extremely Preterm Children: A Cross-Sectional Observationnal Study. Children 2022, 9, 548. https://doi.org/10.3390/children9040548
Brévaut-Malaty V, Resseguier N, Garbi A, Tosello B, Thomachot L, Vialet R, Gire C. Long Term Neurodevelopmental Outcomes after Sevoflurane Neonatal Exposure of Extremely Preterm Children: A Cross-Sectional Observationnal Study. Children. 2022; 9(4):548. https://doi.org/10.3390/children9040548
Chicago/Turabian StyleBrévaut-Malaty, Véronique, Noémie Resseguier, Aurélie Garbi, Barthélémy Tosello, Laurent Thomachot, Renaud Vialet, and Catherine Gire. 2022. "Long Term Neurodevelopmental Outcomes after Sevoflurane Neonatal Exposure of Extremely Preterm Children: A Cross-Sectional Observationnal Study" Children 9, no. 4: 548. https://doi.org/10.3390/children9040548
APA StyleBrévaut-Malaty, V., Resseguier, N., Garbi, A., Tosello, B., Thomachot, L., Vialet, R., & Gire, C. (2022). Long Term Neurodevelopmental Outcomes after Sevoflurane Neonatal Exposure of Extremely Preterm Children: A Cross-Sectional Observationnal Study. Children, 9(4), 548. https://doi.org/10.3390/children9040548






