Gait Adaptation Is Different between the Affected and Unaffected Legs in Children with Spastic Hemiplegic Cerebral Palsy While Walking on a Changing Slope
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Gait Protocol
2.4. Gait Analysis
2.5. Statistical Analysis
3. Results
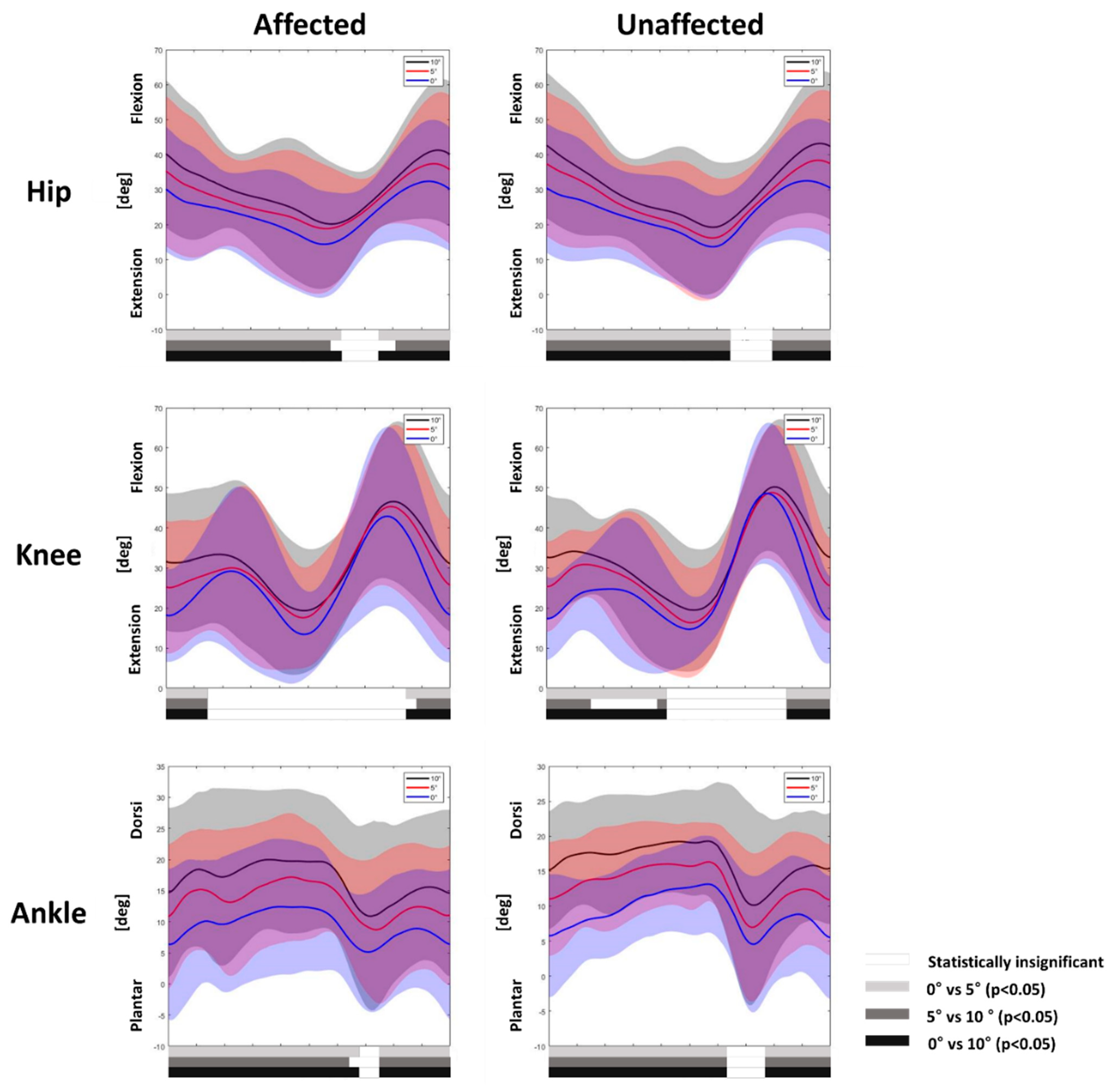
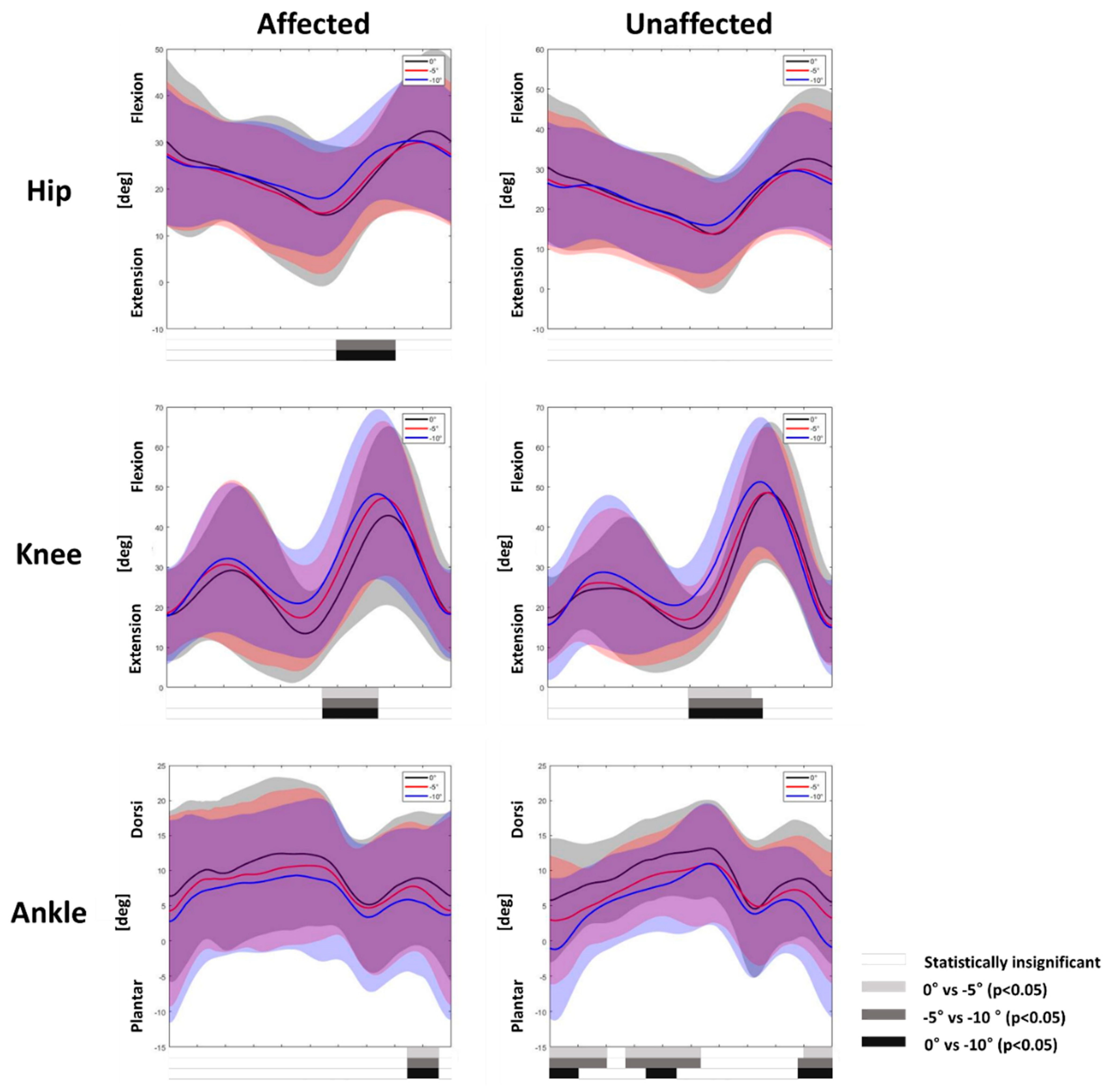
3.1. Gait Adaptation during Uphill Gait
3.2. Gait Adaptation during Downhill Gait
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerr Graham, H.; Selber, P. Musculoskeletal aspects of cerebral palsy. J. Bone Jt. Surg. Br. 2003, 85, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Redfern, M.S.; Dipasquale, J. Biomechanics of descending ramps. Gait Posture 1997, 6, 119–125. [Google Scholar] [CrossRef]
- Lay, A.N.; Hass, C.J.; Gregor, R.J. The effects of sloped surfaces on locomotion: A kinematic and kinetic analysis. J. Biomech. 2006, 39, 1621–1628. [Google Scholar] [CrossRef]
- Kuster, M.; Sakurai, S.; Wood, G.A. Kinematic and kinetic comparison of downhill and level walking. Clin. Biomech. 1995, 10, 79–84. [Google Scholar] [CrossRef]
- Prentice, S.D.; Hasler, E.N.; Groves, J.J.; Frank, J.S. Locomotor adaptations for changes in the slope of the walking surface. Gait Posture 2004, 20, 255–265. [Google Scholar] [CrossRef]
- Leroux, A.; Fung, J.; Barbeau, H. Postural adaptation to walking on inclined surfaces: I. Normal strategies. Gait Posture 2002, 15, 64–74. [Google Scholar] [CrossRef]
- Full, R.J.; Kubow, T.; Schmitt, J.; Holmes, P.; Koditschek, D. Quantifying dynamic stability and maneuverability in legged locomotion. Integr. Comp. Biol. 2002, 42, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Liang, Y.; Kang, X.; Shao, M.; Siemelink, L.; Zhang, Y. Gait Characteristics of Children with Spastic Cerebral Palsy during Inclined Treadmill Walking under a Virtual Reality Environment. Appl. Bionics Biomech. 2019, 2019, 8049156. [Google Scholar] [CrossRef] [Green Version]
- Stott, N.S.; Reynolds, N.; McNair, P. Level versus inclined walking: Ambulatory compensations in children with cerebral palsy under outdoor conditions. Pediatr. Phys. Ther. 2014, 26, 428–435. [Google Scholar] [CrossRef]
- Yilmaz Topcuoglu, M.S.; Krautwurst, B.K.; Klotz, M.; Dreher, T.; Wolf, S.I. How do children with bilateral spastic cerebral palsy manage walking on inclines? Gait Posture 2018, 66, 172–180. [Google Scholar] [CrossRef]
- Mélo, T.R.; Guimarães, A.T.B.; Israel, V.L. Spastic diparetic does not directly affect the capacity to ascend and descend access ramps: Three-dimensional analysis. Fisioter. Mov. 2017, 30, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Hosl, M.; Bohm, H.; Arampatzis, A.; Keymer, A.; Doderlein, L. Contractile behavior of the medial gastrocnemius in children with bilateral spastic cerebral palsy during forward, uphill and backward-downhill gait. Clin. Biomech. 2016, 36, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Cimolin, V.; Galli, M.; Tenore, N.; Albertini, G.; Crivellini, M. Gait strategy of uninvolved limb in children with spastic hemiplegia. Eur. Med. 2007, 43, 303–310. [Google Scholar]
- Bulea, T.C.; Stanley, C.J.; Damiano, D.L. Part 2: Adaptation of Gait Kinematics in Unilateral Cerebral Palsy Demonstrates Preserved Independent Neural Control of Each Limb. Front. Hum. Neurosci. 2017, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Zonta, M.B.; Ramalho Júnior, A.; Camargo, R.M.; Dias, F.H.; Santos, L.H. Two-dimensional analysis of gait asymmetry in spastic hemiplegia. Einstein 2010, 8, 343–349. [Google Scholar] [CrossRef] [Green Version]
- van den Bogert, A.J.; Geijtenbeek, T.; Even-Zohar, O.; Steenbrink, F.; Hardin, E.C. A real-time system for biomechanical analysis of human movement and muscle function. Med. Biol. Eng. Comput. 2013, 51, 1069–1077. [Google Scholar] [CrossRef] [Green Version]
- Pataky, T.C. Generalized n-dimensional biomechanical field analysis using statistical parametric mapping. J. Biomech. 2010, 43, 1976–1982. [Google Scholar] [CrossRef]
- Leroux, A.; Fung, J.; Barbeau, H. Adaptation of the walking pattern to uphill walking in normal and spinal-cord injured subjects. Exp. Brain Res. 1999, 126, 359–368. [Google Scholar] [CrossRef]
- Balasubramanian, C.K.; Neptune, R.R.; Kautz, S.A. Foot placement in a body reference frame during walking and its relationship to hemiparetic walking performance. Clin. Biomech. 2010, 25, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Roerdink, M.; Beek, P.J. Understanding inconsistent step-length asymmetries across hemiplegic stroke patients: Impairments and compensatory gait. Neurorehabilit. Neural Repair 2011, 25, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, C.K.; Bowden, M.G.; Neptune, R.R.; Kautz, S.A. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch. Phys. Med. Rehabil. 2007, 88, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Kautz, S.A.; Neptune, R.R. Step length asymmetry is representative of compensatory mechanisms used in post-stroke hemiparetic walking. Gait Posture 2011, 33, 538–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Pierce, R.; Do, K.P.; Aiona, M. Motion of the center of mass in children with spastic hemiplegia: Balance, energy transfer, and work performed by the affected leg vs. the unaffected leg. Gait Posture 2014, 39, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Madehkhaksar, F.; Klenk, J.; Sczuka, K.; Gordt, K.; Melzer, I.; Schwenk, M. The effects of unexpected mechanical perturbations during treadmill walking on spatiotemporal gait parameters, and the dynamic stability measures by which to quantify postural response. PLoS ONE 2018, 13, e0195902. [Google Scholar] [CrossRef] [PubMed]
- Moyer, B.E.; Chambers, A.J.; Redfern, M.S.; Cham, R. Gait parameters as predictors of slip severity in younger and older adults. Ergonomics 2006, 49, 329–343. [Google Scholar] [CrossRef]
- Espy, D.D.; Yang, F.; Bhatt, T.; Pai, Y.C. Independent influence of gait speed and step length on stability and fall risk. Gait Posture 2010, 32, 378–382. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, T.; Wening, J.D.; Pai, Y.C. Influence of gait speed on stability: Recovery from anterior slips and compensatory stepping. Gait Posture 2005, 21, 146–156. [Google Scholar] [CrossRef]
- Hak, L.; Houdijk, H.; Beek, P.J.; van Dieen, J.H. Steps to take to enhance gait stability: The effect of stride frequency, stride length, and walking speed on local dynamic stability and margins of stability. PLoS ONE 2013, 8, e82842. [Google Scholar] [CrossRef] [Green Version]
- Hak, L.; van Dieen, J.H.; van der Wurff, P.; Prins, M.R.; Mert, A.; Beek, P.J.; Houdijk, H. Walking in an unstable environment: Strategies used by transtibial amputees to prevent falling during gait. Arch. Phys. Med. Rehabil. 2013, 94, 2186–2193. [Google Scholar] [CrossRef] [Green Version]
- Nadeau, S.; Gravel, D.; Arsenault, A.B.; Bourbonnais, D. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clin. Biomech. 1999, 14, 125–135. [Google Scholar] [CrossRef]
- Milot, M.H.; Nadeau, S.; Gravel, D. Muscular utilization of the plantarflexors, hip flexors and extensors in persons with hemiparesis walking at self-selected and maximal speeds. J. Electromyogr. Kinesiol. 2007, 17, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Rodda, J.; Graham, H.K. Classification of gait patterns in spastic hemiplegia and spastic diplegia: A basis for a management algorithm. Eur. J. Neurol. 2001, 8 (Suppl. 5), 98–108. [Google Scholar] [CrossRef] [PubMed]
- Winters, T.F., Jr.; Gage, J.R.; Hicks, R. Gait patterns in spastic hemiplegia in children and young adults. J. Bone Jt. Surg. Am. 1987, 69, 437–441. [Google Scholar]

| Characteristics | Number/Value * |
|---|---|
| Number of participants | 17 |
| Sex, male:female | 12:5 |
| Age at assessment (years) | 10.0 ± 1.8 (7–13) |
| Involved side, right:left | 7:10 |
| Affected plantarflexor tone (MAS, G1:G1+:G2) | 11:3:3 |
| Affected dorsiflexor range of motion | 15.9 ± 6.9 (0–20) |
| GMFCS, I:II | 15:2 |
| Slope Angle | 0° | 5° | 10° | p-Value | ||
|---|---|---|---|---|---|---|
| Slope Angle | Slope Angle × Limb | |||||
| Step length (m) | Affected leg | 0.280 (0.024) | 0.306 a (0.022) | 0.314 a (0.028) | <0.01 b | 0.01 b |
| Unaffected leg | 0.294 (0.025) | 0.289 (0.027) | 0.292 (0.029) | 0.74 | ||
| Swing time (s) | Affected leg | 0.363 (0.012) | 0.376 (0.013) | 0.387 (0.020) | 0.16 | 0.69 |
| Unaffected leg | 0.352 (0.013) | 0.355 (0.014) | 0.370 (0.015) | 0.19 | ||
| Stance time (s) | Affected leg | 0.821 (0.039) | 0.845 (0.043) | 0.884 (0.041) | 0.11 | 0.96 |
| Unaffected leg | 0.839 (0.039) | 0.861 (0.039) | 0.898 (0.041) | 0.08 | ||
| Stance phase (%) | Affected leg | 69.048 (0.689) | 68.805 (0.938) | 69.349 (1.057) | 0.82 | 0.81 |
| Unaffected leg | 70.176 (0.692) | 70.553 (0.679) | 70.633 (0.538) | 0.57 | ||
| Swing phase (%) | Affected leg | 30.952 (0.689) | 31.195 (0.938) | 30.651 (1.057) | 0.82 | 0.81 |
| Unaffected leg | 29.824 (0.692) | 29.447 (0.679) | 29.367 (0.538) | 0.57 |
| Slope Angle | 0° | −5° | −10° | p-Value | ||
|---|---|---|---|---|---|---|
| Slope Angle | Slope Angle × Limb | |||||
| Step length (m) | Affected leg | 0.280 (0.024) | 0.257 (0.023) | 0.245 a (0.023) | <0.01 b | 0.46 |
| Unaffected leg | 0.294 (0.025) | 0.280 (0.029) | 0.278 (0.028) | 0.13 | ||
| Swing time (s) | Affected leg | 0.363 (0.012) | 0.355 (0.013) | 0.349 (0.013) | 0.29 | 0.52 |
| Unaffected leg | 0.352 (0.015) | 0.331 (0.015) | 0.333 (0.015) | 0.10 | ||
| Stance time (s) | Affected leg | 0.821 (0.039) | 0.757 a (0.037) | 0.744 a (0.033) | <0.01 b | 0.13 |
| Unaffected leg | 0.839 (0.039) | 0.796 (0.035) | 0.741 a,c (0.036) | <0.01 b | ||
| Stance phase (%) | Affected leg | 69.048 (0.689) | 67.758 (0.763) | 67.827 (0.719) | 0.10 | 0.17 |
| Unaffected leg | 70.176 (0.692) | 70.582 (0.925) | 68.801 (0.812) | 0.04 b | ||
| Swing phase (%) | Affected leg | 30.952 (0.689) | 32.242 (0.763) | 32.173 (0.719) | 0.10 | 0.17 |
| Unaffected leg | 29.824 (0.692) | 29.418 (0.925) | 31.199 (0.812) | 0.04 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, T.Y.; Park, D.; Shim, D.; Choi, J.-o.; Hong, J.; Ahn, Y.; Park, E.S.; Rha, D.-w. Gait Adaptation Is Different between the Affected and Unaffected Legs in Children with Spastic Hemiplegic Cerebral Palsy While Walking on a Changing Slope. Children 2022, 9, 593. https://doi.org/10.3390/children9050593
Choi TY, Park D, Shim D, Choi J-o, Hong J, Ahn Y, Park ES, Rha D-w. Gait Adaptation Is Different between the Affected and Unaffected Legs in Children with Spastic Hemiplegic Cerebral Palsy While Walking on a Changing Slope. Children. 2022; 9(5):593. https://doi.org/10.3390/children9050593
Chicago/Turabian StyleChoi, Tae Young, Dongho Park, Dain Shim, Joong-on Choi, Juntaek Hong, Yongjin Ahn, Eun Sook Park, and Dong-wook Rha. 2022. "Gait Adaptation Is Different between the Affected and Unaffected Legs in Children with Spastic Hemiplegic Cerebral Palsy While Walking on a Changing Slope" Children 9, no. 5: 593. https://doi.org/10.3390/children9050593
APA StyleChoi, T. Y., Park, D., Shim, D., Choi, J.-o., Hong, J., Ahn, Y., Park, E. S., & Rha, D.-w. (2022). Gait Adaptation Is Different between the Affected and Unaffected Legs in Children with Spastic Hemiplegic Cerebral Palsy While Walking on a Changing Slope. Children, 9(5), 593. https://doi.org/10.3390/children9050593







