The Role of Brain-Derived Neurotrophic Factor (BDNF) in the Relation between Physical Activity and Executive Functioning in Children
Abstract: Background
1. Background
2. Methods
2.1. Recruitment
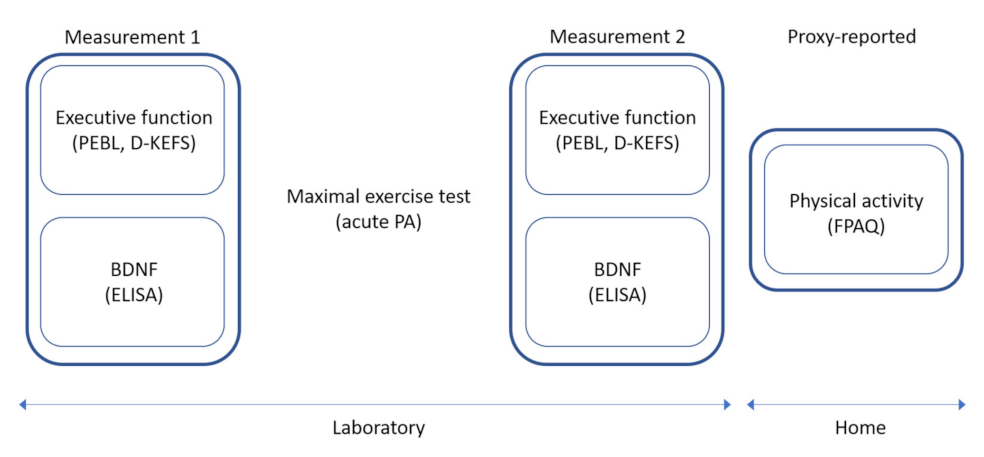
2.2. Procedure
2.3. Measures
- The Flanker Test: this test measures inhibition by measuring the effect of conflicting information within a stimulus set. The child receives an image of five arrows and should only look at the arrow in the middle. If the middle arrow is pointing right, then the right shift key should be pressed. If the middle arrow is pointing left, then the left shift key should be pressed. The other arrows are causing conflicting information. The outcomes of the Flanker test are mean accuracy and mean reaction time.
- The Go/No-Go Test: The child sees four different squares on the screen. In one of the four squares, either the letter ‘P’ appears or the letter ‘R’. When the letter P appears, the child should press the right shift button. When the letter ‘R’ appears, the child should not press a key or click on the mouse. The response accuracy of each No-Go trial is used as a measurement for inhibitory control. The outcomes of the Go/No-Go test are expressed as mean accuracy and conflict–cost reaction time.
- The Corsi Block-Tapping Test: The Corsi Block-Tapping Task is a widely used test to assess visuospatial working memory. The child sees nine blocks on the screen. The blocks light up one by one in a random order. The child needs to replicate the order of the blocks. The first trial lights up two blocks, the next trial lights up three blocks, and each trial gradually increases until there are nine blocks in length. The outcome of the Corsi Test was the total score which reflects the total number of correct blocks replicated.
- Trail-Making Test. The D–KEFS Trail Making subtest (Delis et al., 2001) contains five conditions. The first and fourth conditions were a focus of this study. During the first condition, “Visual Scanning”, the participant is asked to cross-out circles that contain a particular number. On the fourth condition, “Number–Letter Switching”, the participant is asked to draw a line connecting dots, alternating between dots containing numbers and dots containing letters. This condition is a commonly used measure of set-switching, or cognitive flexibility, and has high reliability (Delis et al., 2001). For this study, the scaled scores of completion time in each condition were used as a measure of visual scanning and memory (condition 1) and switching/cognitive flexibility (condition 4), with lower scores indicating better performance.
- Color–Word Interference Test. This D–KEFS subtest consists of four parts: color naming (condition 1), word reading (condition 2), inhibition (condition 3), and inhibition/switching (condition 4). The inhibition trial is the third condition, which was the focus of this study. In this condition, the participant is presented with a page containing the words “red,” “green,” and “blue” printed incongruently in red, green, or blue ink. The participant is asked to say the color of the ink in which each word is printed as quickly as he/she can without making mistakes. Performance is measured by accuracy and reaction time on this condition.
- Design Fluency Test. The D–KEFS Design Fluency (DF) Test consists of three trials in which participants create novel designs by connecting dots in a series of five dot matrices. The three conditions are referred to as Filled Dots (connecting filled dots), Empty Dots (connecting empty dots while filled dots function as distractors), and Switch (switching between connecting filled and empty dots). The third condition includes switching, which was examined in the current study, resulting in a score representing the total number of correct unique designs/patterns generated during the “switch” condition.
3. Data Analysis
4. Results
4.1. Descriptive Statistics
4.2. Research Question 1. Effect of Acute PA on BDNF and EFs
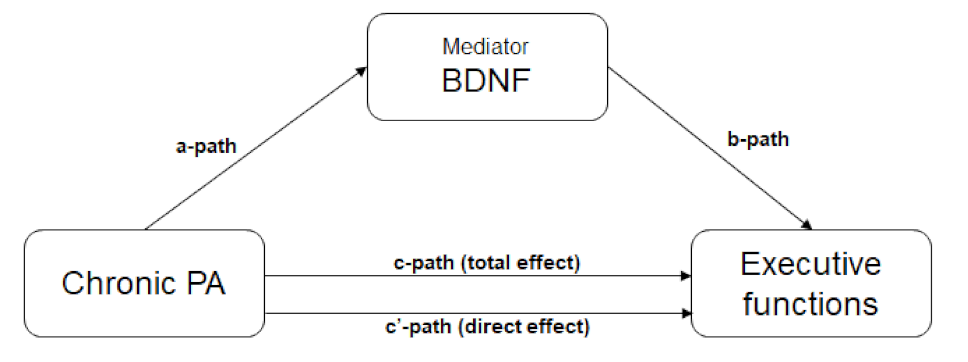
4.3. Research Question 2. Chronic PA and (Mediation) Effect on BDNF and EFs
Correlation Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomporowski, P.D.; McCullick, B.; Pendleton, D.M.; Pesce, C. Exercise and children’s cognition: The role of exercise characteristics and a place for metacognition. J. Sport Health Sci. 2015, 4, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, J.E.; Hillman, C.H.; Castelli, D.; Etnier, J.L.; Lee, S.; Tomporowski, P.; Lambourne, K.; Szabo-Reed, A.N. Physical activity, fitness, cognitive function, and academic achievement in children: A systematic review. Med. Sci. Sports Exerc. 2016, 48, 1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkey, C.S.; Rockett, H.R.; Gillman, M.W.; Colditz, G.A. One-year changes in activity and in inactivity among 10-to 15-year-old boys and girls: Relationship to change in body mass index. Pediatrics 2003, 111, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Stamatakis, E.; Mishra, G. Psychological distress, television viewing, and physical activity in children aged 4 to 12 years. Pediatrics 2009, 123, 1263–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibley, B.A.; Etnier, J.L. The relationship between physical activity and cognition in children: A meta-analysis. Pediatric. Exerc. Sci. 2003, 15, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Burton, L.J.; VanHeest, J.L. The importance of physical activity in closing the achievement gap. Quest 2007, 59, 212–218. [Google Scholar] [CrossRef]
- Alvarez-Bueno, C.; Pesce, C.; Cavero-Redondo, I.; Sanchez-Lopez, M.; Martínez-Hortelano, J.A.; Martinez-Vizcaino, V. The effect of physical activity interventions on children’s cognition and metacognition: A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 729–738. [Google Scholar] [CrossRef]
- De Greeff, J.W.; Bosker, R.J.; Oosterlaan, J.; Visscher, C.; Hartman, E. Effects of physical activity on executive functions, attention and academic performance in preadolescent children: A meta-analysis. J. Sci. Med. Sport 2018, 21, 501–507. [Google Scholar] [CrossRef]
- Hillman, C.H.; Biggan, J.R. A review of childhood physical activity, brain, and cognition: Perspectives on the future. Pediatric. Exerc. Sci. 2017, 29, 170–176. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [Green Version]
- Diamond, A. Executive functions: Insights into ways to help more children thrive. Zero Three 2014, 35, 9–17. [Google Scholar]
- Van Waelvelde, H.; Vanden Wyngaert, K.; Mariën, T.; Baeyens, D.; Calders, P. The relation between children’s aerobic fitness and executive functions: A systematic review. Infant Child Dev. 2020, 29, e2163. [Google Scholar] [CrossRef]
- Diamond, A.; Lee, K. Interventions shown to aid executive function development in children 4 to 12 years old. Science 2011, 333, 959–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, R. Physical education and sport in schools: A review of benefits and outcomes. J. Sch. Health 2006, 76, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Etnier, J.L.; Salazar, W.; Landers, D.M.; Petruzzello, S.J.; Han, M.; Nowell, P. The influence of physical fitness and exercise upon cognitive functioning: A meta-analysis. J. Sport Exerc. Psychol. 1997, 19, 249–277. [Google Scholar] [CrossRef] [Green Version]
- Best, J.R. Effects of physical activity on children’s executive function: Contributions of experimental research on aerobic exercise. Dev. Rev. 2010, 30, 331–351. [Google Scholar] [CrossRef]
- Kashihara, K.; Maruyama, T.; Murota, M.; Nakahara, Y. Positive effects of acute and moderate physical exercise on cognitive function. J. Physiol. Anthropol. 2009, 28, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Tomporowski, P.D. Effects of acute bouts of exercise on cognition. Acta Psychol. 2003, 112, 297–324. [Google Scholar] [CrossRef]
- Pesce, C. Shifting the focus from quantitative to qualitative exercise characteristics in exercise and cognition research. J. Sport Exerc. Psychol. 2012, 34, 766–786. [Google Scholar] [CrossRef] [Green Version]
- Gray, C.; Gibbons, R.; Larouche, R.; Sandseter, E.B.H.; Bienenstock, A.; Brussoni, M.; Chabot, G.; Herrington, S.; Janssen, I.; Pickett, W. What is the relationship between outdoor time and physical activity, sedentary behaviour, and physical fitness in children? A systematic review. Int. J. Environ. Res. Public Health 2015, 12, 6455–6474. [Google Scholar] [CrossRef] [Green Version]
- Rowland, T.W. Developmental Exercise Physiology; Human Kinetics Publishers: Champaign, IL, USA, 1996. [Google Scholar]
- Fedewa, A.L.; Ahn, S. The effects of physical activity and physical fitness on children’s achievement and cognitive outcomes: A meta-analysis. Res. Q. Exerc. Sport 2011, 82, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.H.; Buck, S.M.; Themanson, J.R.; Pontifex, M.B.; Castelli, D.M. Aerobic fitness and cognitive development: Event-related brain potential and task performance indices of executive control in preadolescent children. Dev. Psychol. 2009, 45, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaynman, S.; Gomez-Pinilla, F. License to run: Exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabilit. Neural Repair 2005, 19, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, J.S.; Prakash, R.; McAuley, E.; Elavsky, S.; Marquez, D.X.; Hu, L.; Kramer, A.F. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1166–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colcombe, S.J.; Kramer, A.F.; Erickson, K.I.; Scalf, P.; McAuley, E.; Cohen, N.J.; Webb, A.; Jerome, G.J.; Marquez, D.X.; Elavsky, S. Cardiovascular fitness, cortical plasticity, and aging. Proc. Natl. Acad. Sci. USA 2004, 101, 3316–3321. [Google Scholar] [CrossRef] [Green Version]
- Kramer, A.F.; Hahn, S.; Cohen, N.J.; Banich, M.T.; McAuley, E.; Harrison, C.R.; Chason, J.; Vakil, E.; Bardell, L.; Boileau, R.A. Ageing, fitness and neurocognitive function. Nature 1999, 400, 418–419. [Google Scholar] [CrossRef]
- Philippaerts, R.; Matton, L.; Wijndaele, K.; Balduck, A.-L.; De Bourdeaudhuij, I.; Lefevre, J. Validity of a physical activity computer questionnaire in 12-to 18-year-old boys and girls. Int. J. Sports Med. 2006, 27, 131–136. [Google Scholar] [CrossRef]
- Delis, D.C.; Kramer, J.H.; Kaplan, E.; Holdnack, J. Reliability and validity of the Delis-Kaplan executive function system: An update. J Int Neuropsych Soc 2004, 10, 301–303. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 26.0; Released 2013; IBM Corp: Armonk, NY, USA, 2020. [Google Scholar]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [Green Version]
- Mandelman, S.D.; Grigorenko, E.L. BDNF Val66Met and cognition: All, none, or some? A meta-analysis of the genetic association. Genes Brain Behav. 2012, 11, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef] [Green Version]
- Warnault, V.; Darcq, E.; Morisot, N.; Phamluong, K.; Wilbrecht, L.; Massa, S.M.; Longo, F.M.; Ron, D. The BDNF valine 68 to methionine polymorphism increases compulsive alcohol drinking in mice that is reversed by tropomyosin receptor kinase B activation. Biol. Psychiatry 2016, 79, 463–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St Clair-Thompson, H.L.; Gathercole, S.E. Executive functions and achievements in school: Shifting, updating, inhibition, and working memory. Q. J. Exp. Psychol. 2006, 59, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Toplak, M.E.; West, R.F.; Stanovich, K.E. Practitioner review: Do performance-based measures and ratings of executive function assess the same construct? J. Child Psychol. Psychiatry 2013, 54, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Barkley, R.A. Response inhibition in attention-deficit hyperactivity disorder. Ment. Retard. Dev. Disabil. Res. Rev. 1999, 5, 177–184. [Google Scholar] [CrossRef]
- Bexkens, A.; van den Wildenberg, W.P.; Tijms, J. Rapid automatized naming in children with dyslexia: Is inhibitory control involved? Dyslexia 2015, 21, 212–234. [Google Scholar] [CrossRef]
- Hogervorst, E.; Riedel, W.; Jeukendrup, A.; Jolles, J. Cognitive performance after strenuous physical exercise. Percept. Mot. Ski. 1996, 83, 479–488. [Google Scholar] [CrossRef]
- Lichtman, S.; Poser, E.G. The effects of exercise on mood and cognitive functioning. J. Psychosom. Res. 1983, 27, 43–52. [Google Scholar] [CrossRef]
- Diamond, A.; Barnett, W.S.; Thomas, J.; Munro, S. Preschool program improves cognitive control. Science 2007, 318, 1387. [Google Scholar] [CrossRef] [Green Version]
- Merkt, J.; Gawrilow, C. Measures of Inhibition Support the Prediction of Early Academic Skills. In Proceedings of the 3rd International Congress on ADHD, Berlin, Germany, 26–29 May 2011. [Google Scholar]
- Bledsoe, J.C.; Semrud-Clikeman, M.; Pliszka, S.R. Response inhibition and academic abilities in typically developing children with attention-deficit-hyperactivity disorder-combined subtype. Arch. Clin. Neuropsychol. 2010, 25, 671–679. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Mavilidi, M.F.; Singh, A.; Englert, C. Combining physical and cognitive training to improve kindergarten children’s executive functions: A cluster randomized controlled trial. Contemp. Educ. Psychol. 2020, 63, 101908. [Google Scholar] [CrossRef]
- Van der Niet, A.G.; Smith, J.; Scherder, E.J.; Oosterlaan, J.; Hartman, E.; Visscher, C. Associations between daily physical activity and executive functioning in primary school-aged children. J. Sci. Med. Sport 2015, 18, 673–677. [Google Scholar] [CrossRef] [PubMed]

| Executive Functions | Pre-Test (Acute PA) Mean (SD) | Post-Test (Acute PA) Mean (SD) | p-Value | ||
|---|---|---|---|---|---|
| Executive Functioning Test | Executive Functioning Component | Measurement | |||
| PEBL | |||||
| Flanker Test | Inhibition | Reaction time, in msec | 557.00 (106.94) | 535.11 (97.70) | p = 0.509 |
| Accuracy, in % | 70.99 (15.69) | 76.66 (12.96) | p = 0.034 * | ||
| Go/No-Go Test | Inhibition | Accuracy, in % | 84.10 (5.77) | 84.25 (7.12) | p = 0.022 * |
| Reaction time (conflict–cost), in msec | 55.16 (61.66) | 52.31 (54.27) | p = 0.484 | ||
| Corsi Test | Visuospatial working memory | Total score, n | 29.96 (10.56) | 34.24 (12.78) | p = 0.747 |
| D–KEFS | |||||
| Color–Word Inference Test | Condition 3: inhibition | Reaction time, in msec | 82.67 (21.07) | 71.61 (24.61) | p = 0.624 |
| Accuracy, in % | 93.11 (4.49) | 95.50 (4.18) | p = 0.795 | ||
| Design Fluency Test | Condition 3: switching | Correct patterns, n | 11.23 (2.60) | 13.70 (15.39) | p = 0.508 |
| Trail-Making Test | Condition 1: visual scanning and memory Condition 4: switching & cognitive flexibility | Completion time, in sec | 11.82 (2.46) 11.18 (3.29) | 13.16 (2.03) 12.45 (2.93) | p = 0.323 p = 0.479 |
| BDNF (in pq/mL) | 28.58 (63.06) | 30.59 (88.20) | p = 0.075 | ||
| Pre-Exercise Executive Functions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PEBL | D-KEFS | |||||||||
| Flanker Test (Reaction Time) | Flanker Test (Accuracy) | Corsi Test (Total Score) | Go/No-Go Test (Reaction time) | Go/No-Go Test (Accuracy) | Color–Word Interference Test (Reaction Time) | Color–Word Interference Test (Accuracy) | Design Fluency Test | Trail-Making Test (C1) | Trail-Making Test (C4) | |
| Chronic PA | −0.157 | 0.171 | 0.046 | 0.102 | 0.006 | −0.285 | −0.141 | −0.136 | 0.091 | 0.290 |
| BDNF | −0.094 | 0.095 | 0.064 | 0.083 | −0.039 | 0.005 | 0.053 | 0.191 | 0.018 | 0.211 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latomme, J.; Calders, P.; Van Waelvelde, H.; Mariën, T.; De Craemer, M. The Role of Brain-Derived Neurotrophic Factor (BDNF) in the Relation between Physical Activity and Executive Functioning in Children. Children 2022, 9, 596. https://doi.org/10.3390/children9050596
Latomme J, Calders P, Van Waelvelde H, Mariën T, De Craemer M. The Role of Brain-Derived Neurotrophic Factor (BDNF) in the Relation between Physical Activity and Executive Functioning in Children. Children. 2022; 9(5):596. https://doi.org/10.3390/children9050596
Chicago/Turabian StyleLatomme, Julie, Patrick Calders, Hilde Van Waelvelde, Tineke Mariën, and Marieke De Craemer. 2022. "The Role of Brain-Derived Neurotrophic Factor (BDNF) in the Relation between Physical Activity and Executive Functioning in Children" Children 9, no. 5: 596. https://doi.org/10.3390/children9050596







