Surgical Treatment of Short Bowel Syndrome—The Past, the Present and the Future, a Descriptive Review of the Literature
Abstract
:1. Introduction
1.1. Definitions
1.2. Clinical Implications of SBS
1.2.1. Proximal Versus Distal Small Bowel Loss
1.2.2. Choleretic Diarrhea
1.2.3. Small Intestinal Bacterial Overgrowth
2. Conservative Treatment
2.1. Parenteral Nutrition
2.2. Medical Management
2.2.1. Antimotility Agents
2.2.2. Choleretic Diarrhea Treatment
2.2.3. Inhibition of Gastric Hypersecretion
2.2.4. Treatment of Small Intestinal Bacterial Overgrowth
2.2.5. Glucagon-Like Peptide-2
2.2.6. Pancreatic Enzyme Replacement and Bile Acid Supplementation
2.2.7. Chyme Reinfusion
3. Surgical Treatment
3.1. Indications
3.2. Procedures Increasing Small Intestinal Length
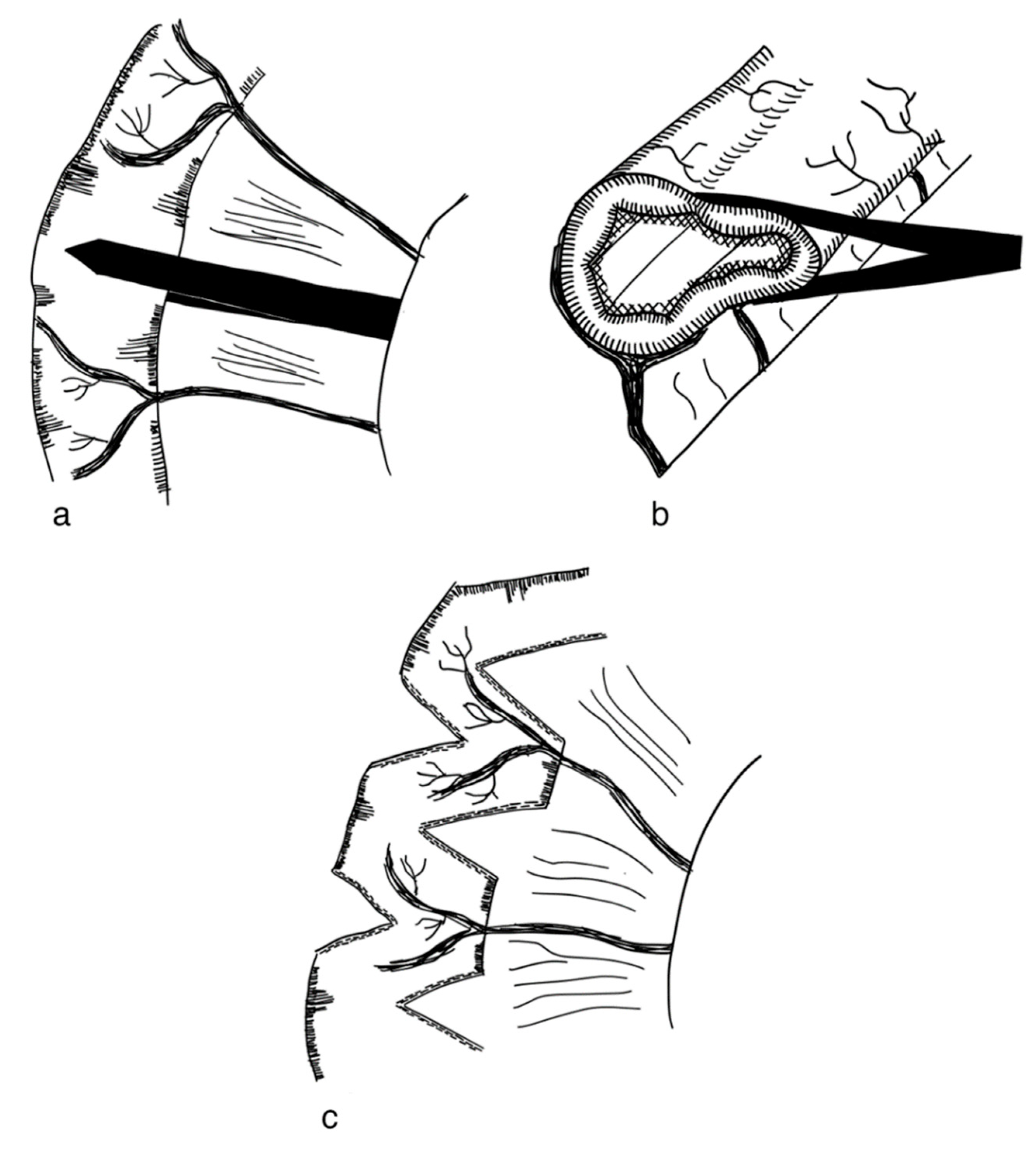
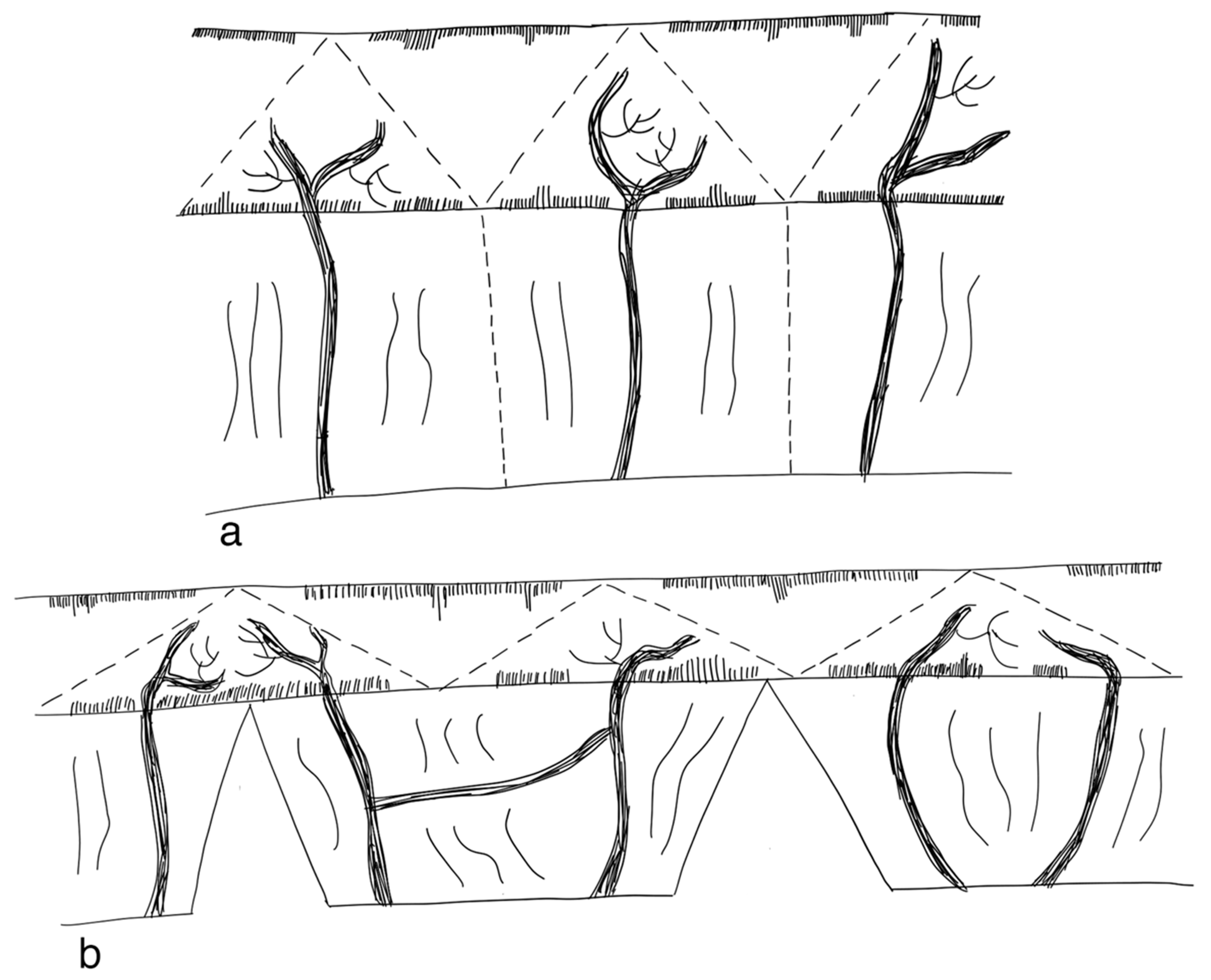
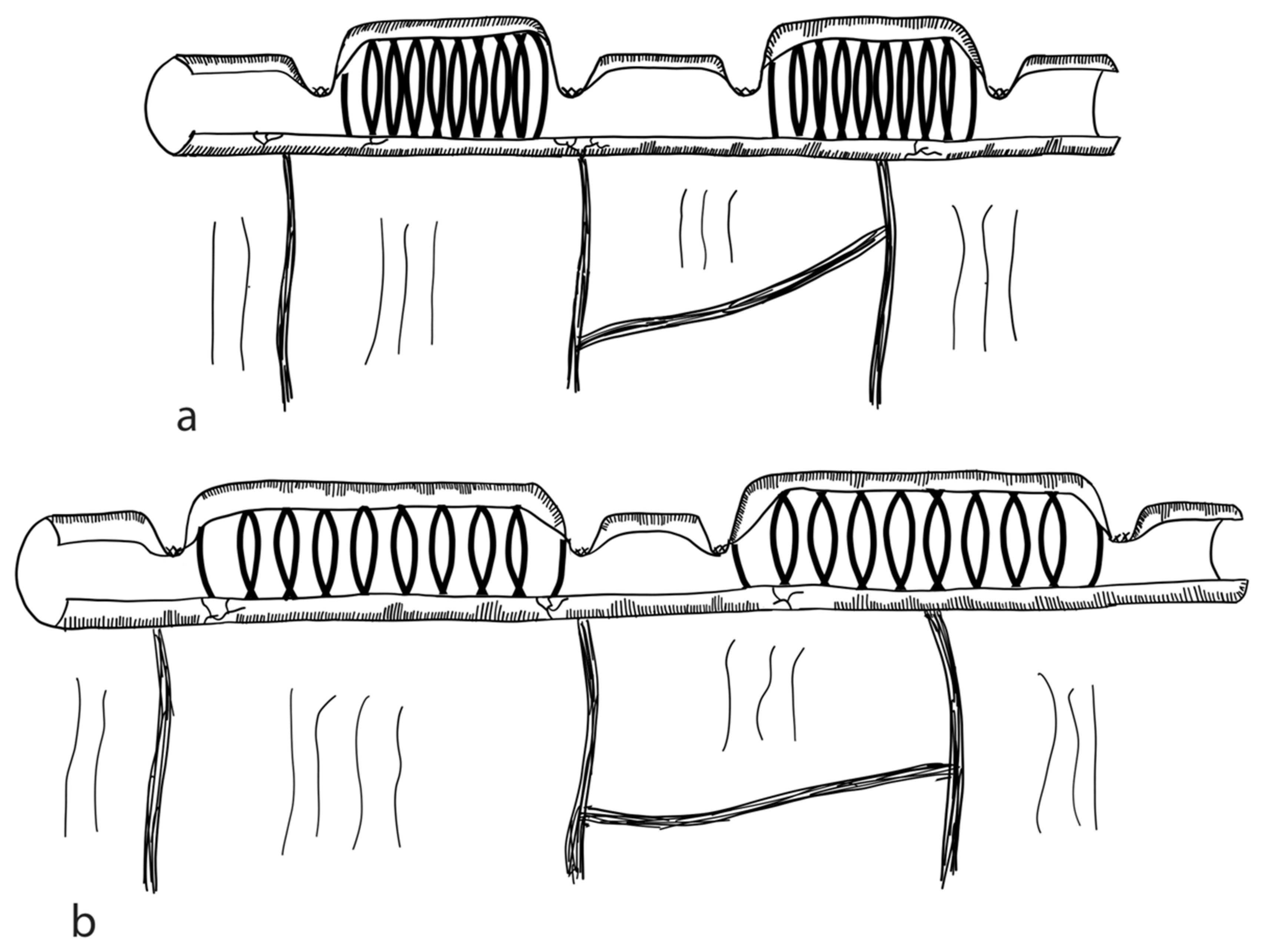
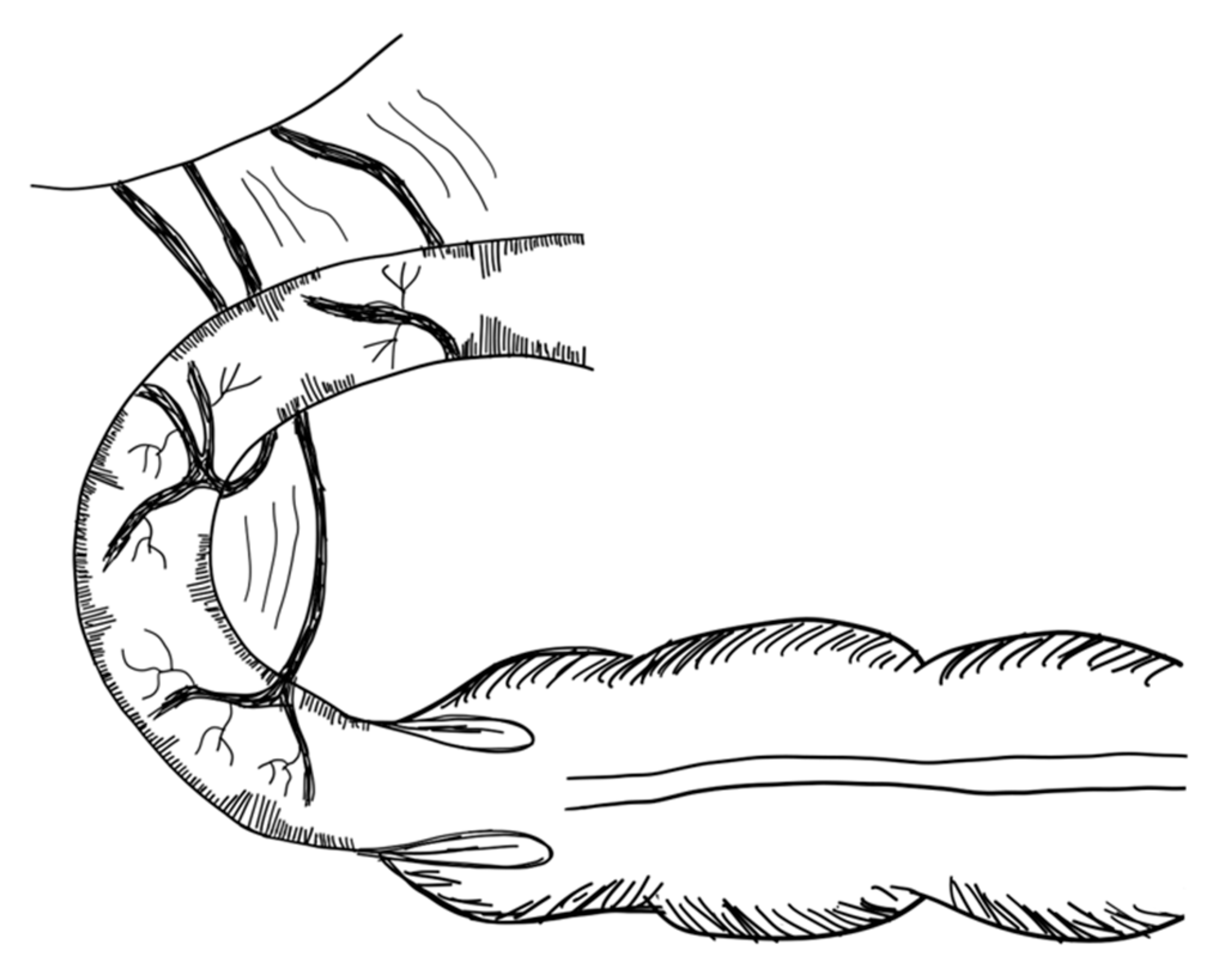
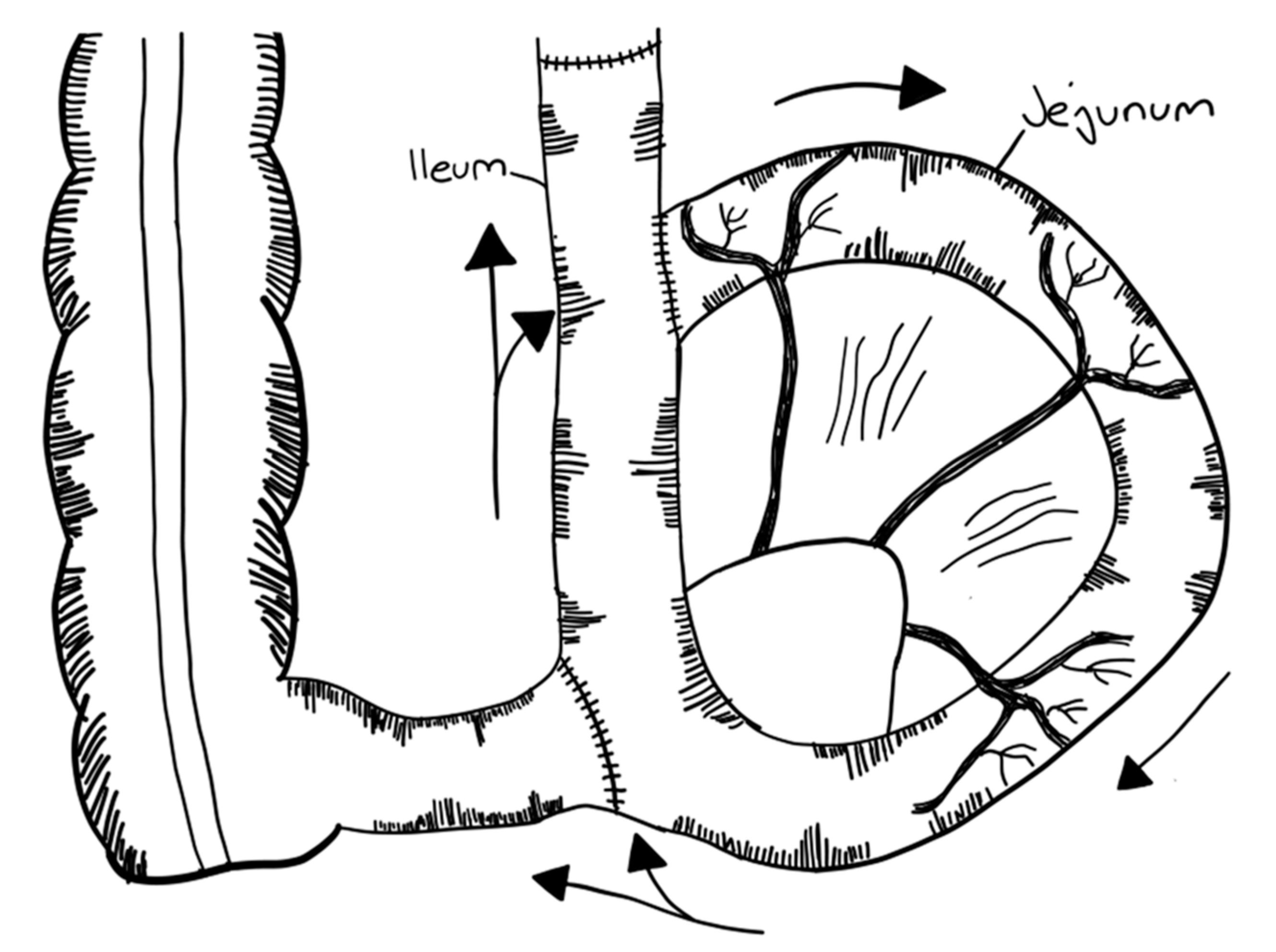
3.2.1. Serial Transverse Enteroplasty Procedure (STEP)
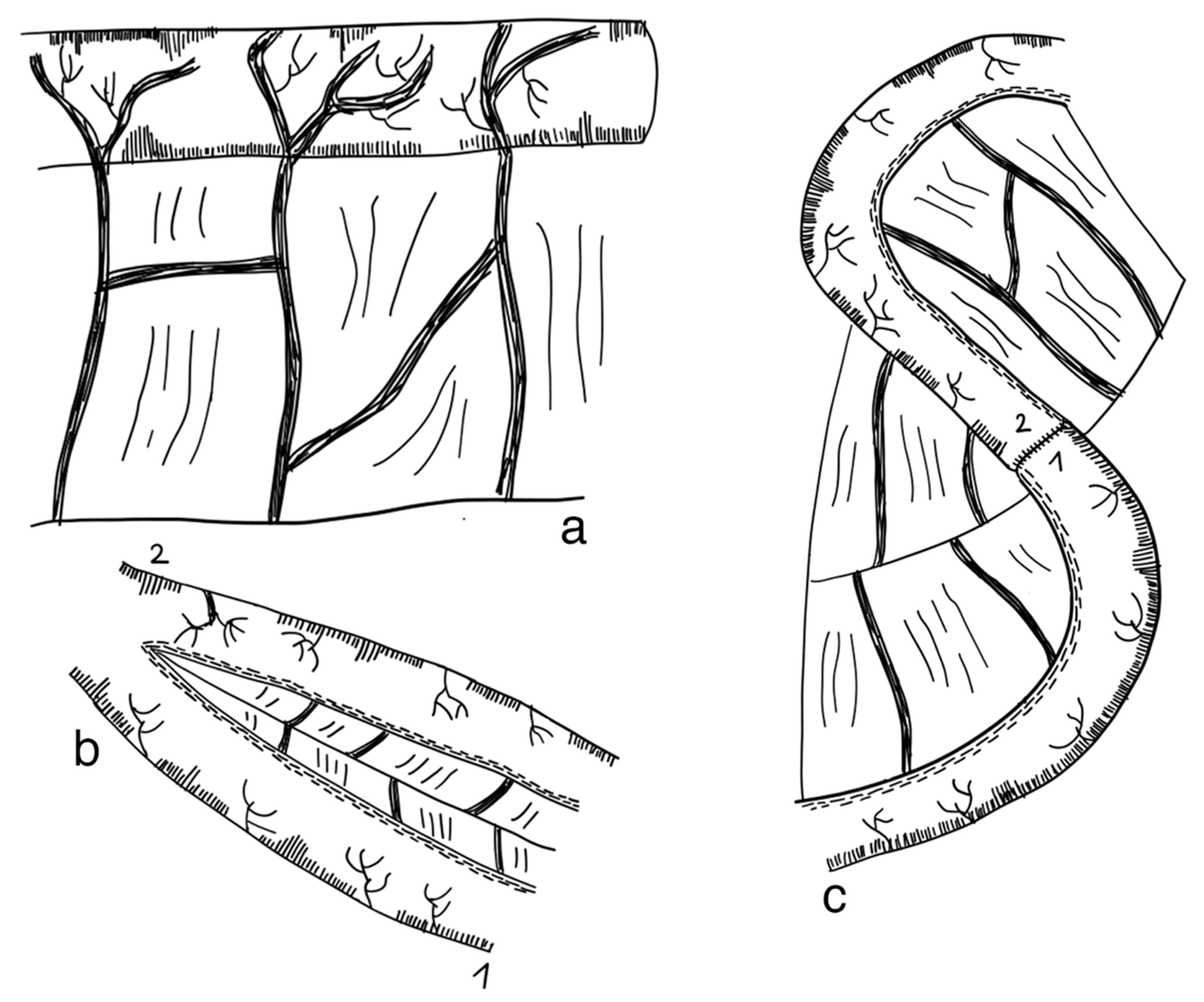
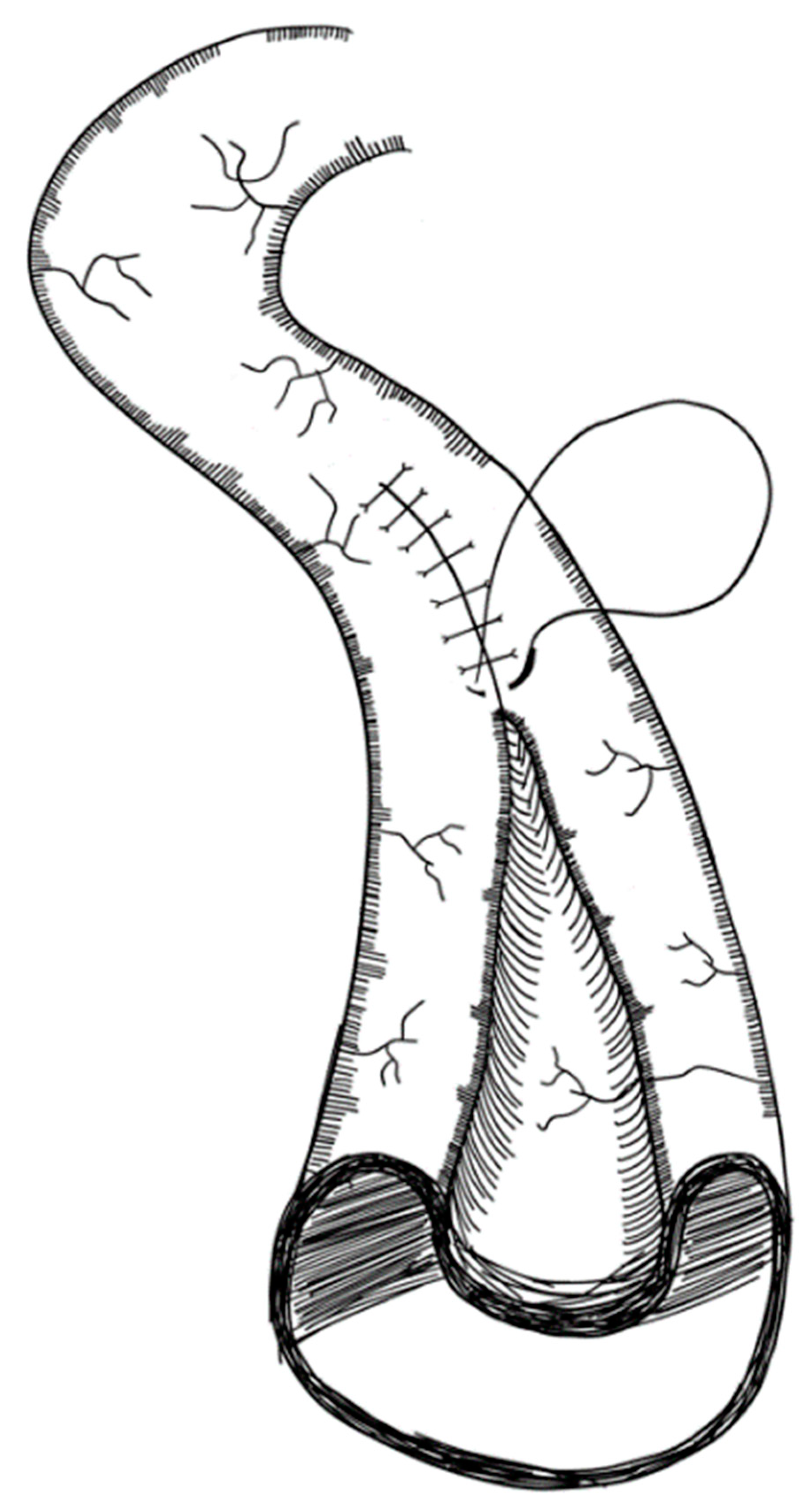
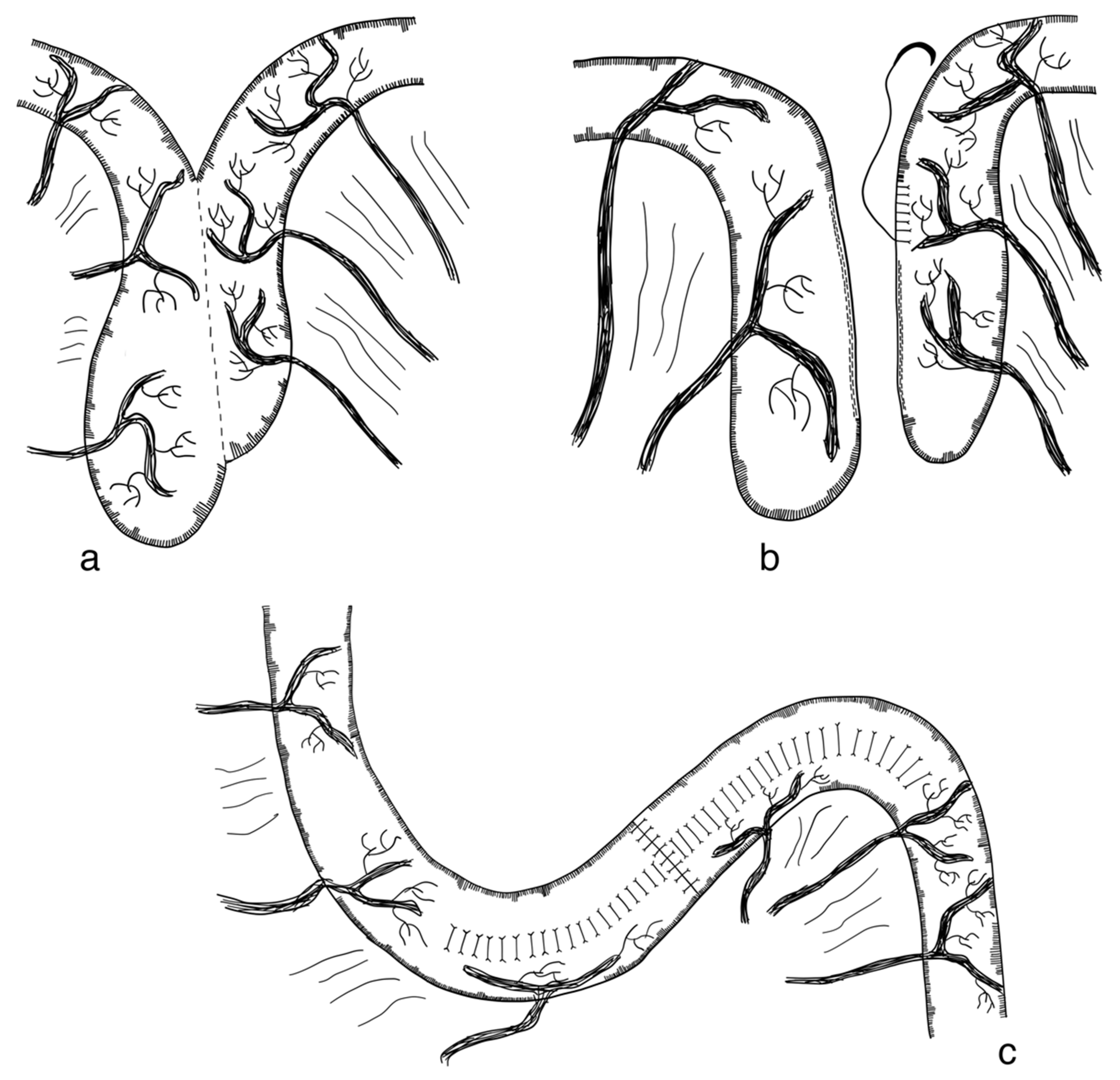
3.2.2. Longitudinal Intestinal Lengthening and Tailoring Procedure (Bianchi Procedure)
3.2.3. Modification of LILT with One Anastomosis
3.2.4. Double Barrel Enteroplasty (DBE)
3.2.5. Kimura Iowa Procedure
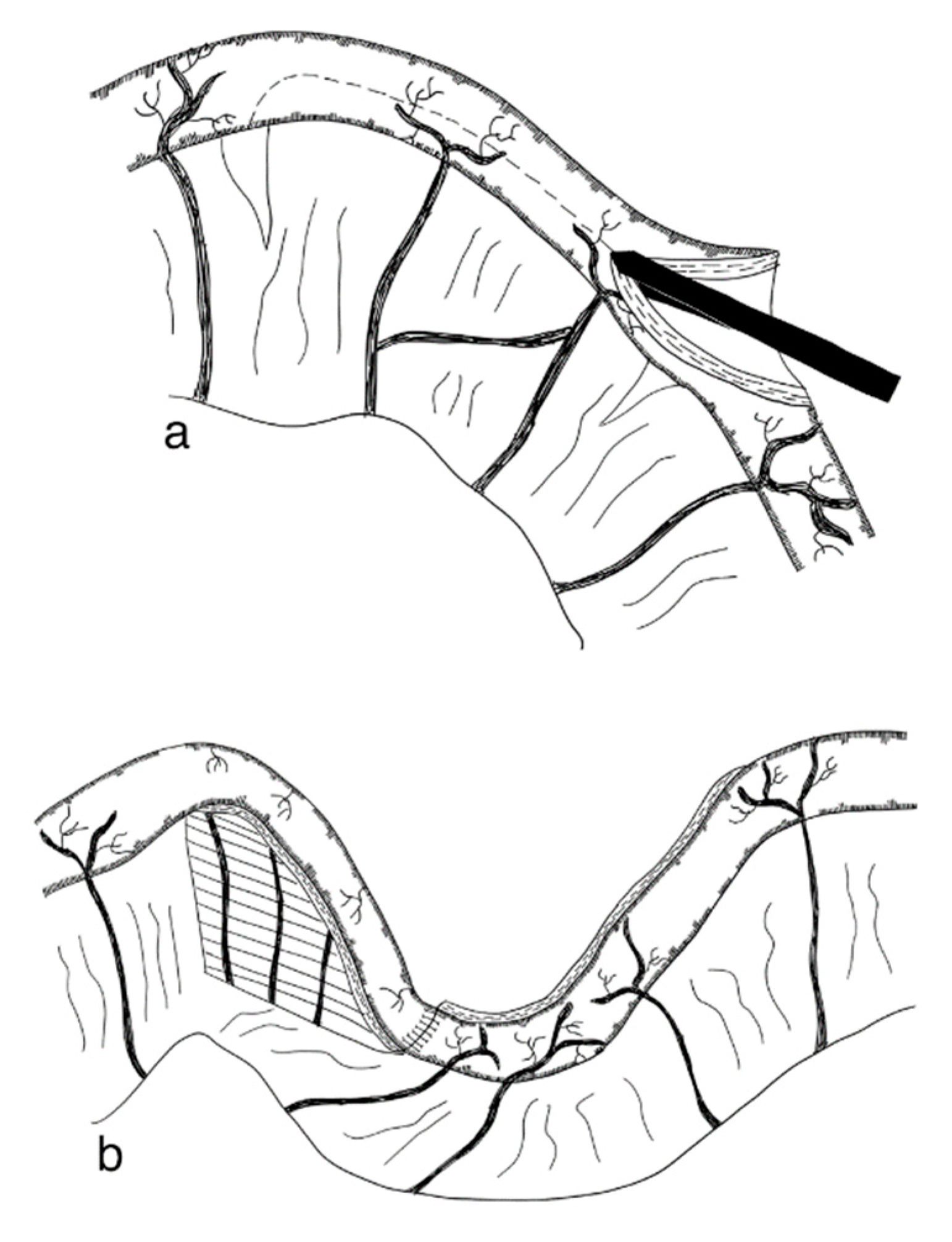
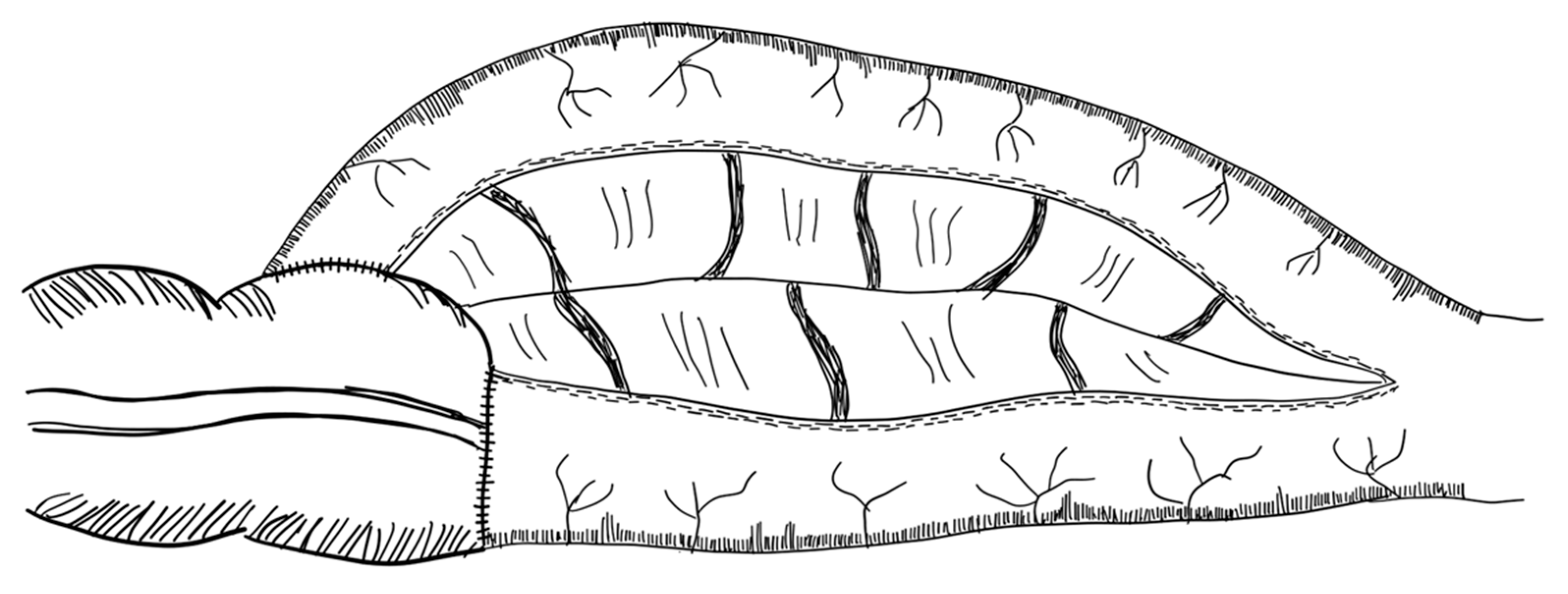
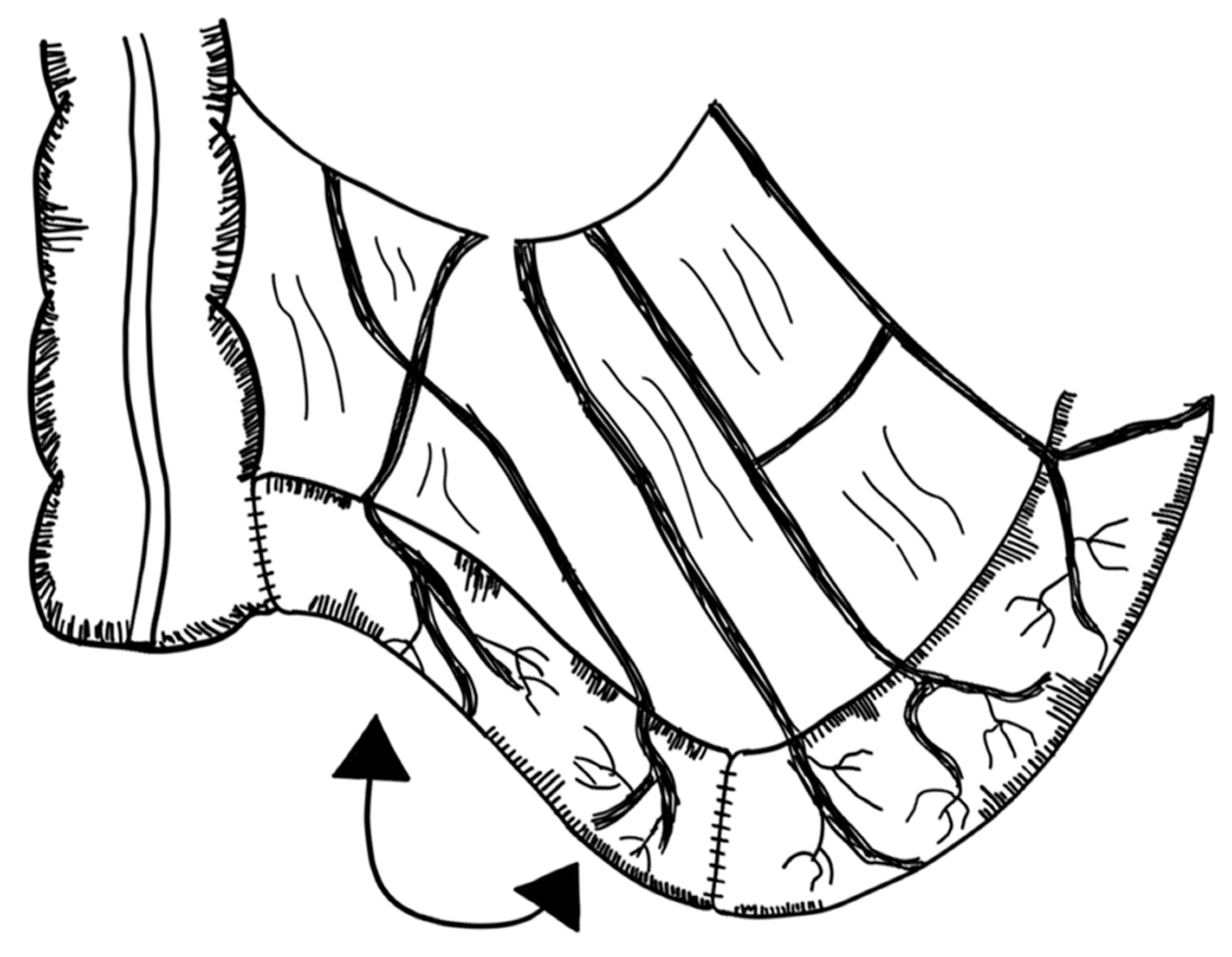
3.2.6. Spiral Intestinal Lengthening and Tailoring (SILT)
3.2.7. Transverse Flap Duodenoplasty (TFD)
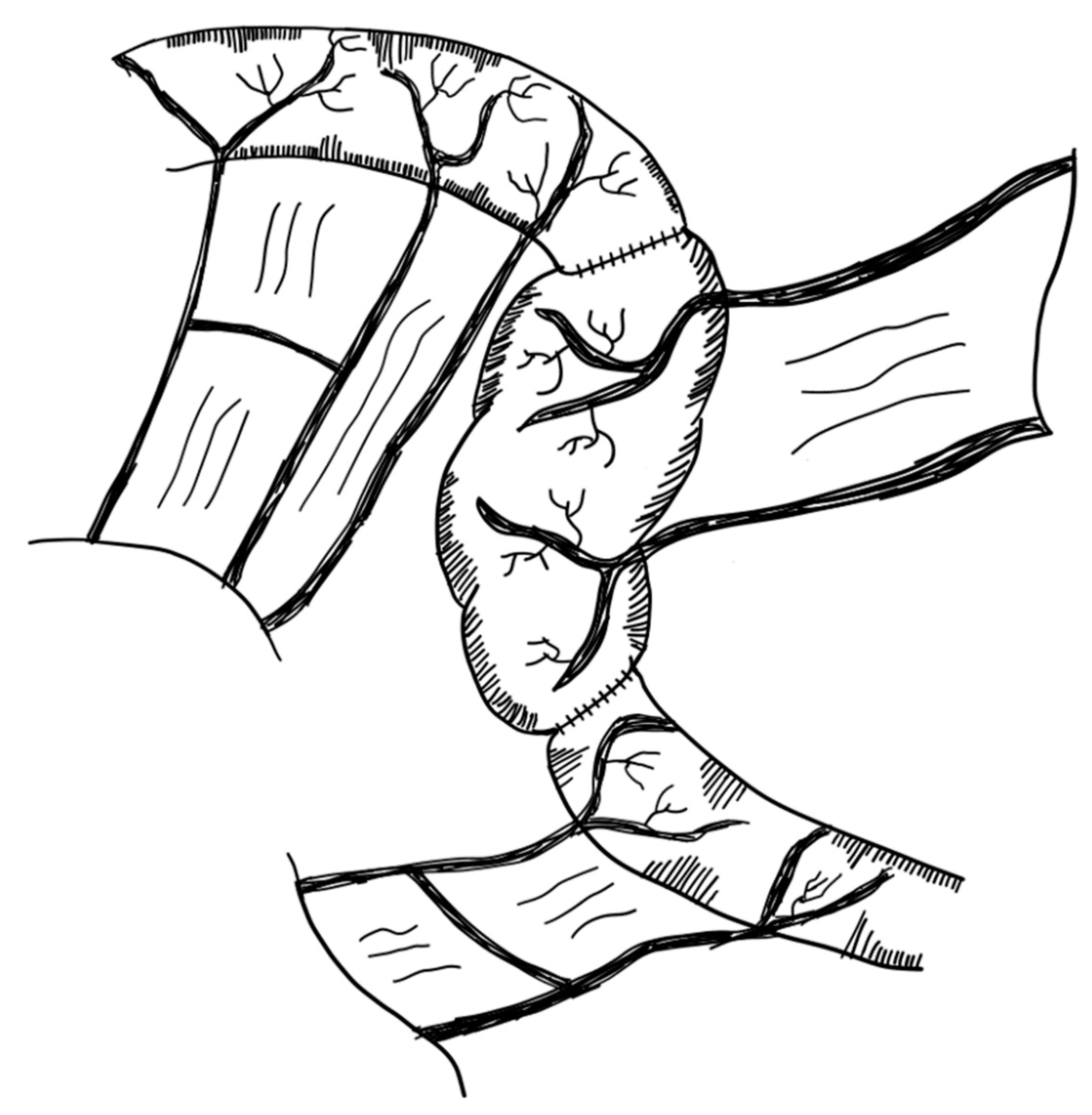
3.2.8. Composite Ileo-Colic Loop (CBT)
3.2.9. Mechanical Distraction
3.3. Procedures Slowing down Intestinal Transit without Bowel Lengthening
3.3.1. Antiperistaltic Small Intestinal Segment
3.3.2. Colon Interposition
3.3.3. Intestinal Valves and Sphincters
3.3.4. Recirculating Bowel Loops
3.4. Procedures Improving Small Intestinal Motility without Bowel Lengthening
3.4.1. Tailoring and Plication
3.4.2. Modified Antimesenteric Tapering Enteroplasty
3.5. Small Bowel Transplantation
3.6. Tissue Engineered Small Intestines (TESI)
3.6.1. Small Intestinal Submucosa (SIS) Grafts
3.6.2. Stem Cells
3.6.3. Organoid Units
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merritt, R.J.; Cohran, V.; Raphael, B.P.; Sentongo, T.; Volpert, D.; Warner, B.W.; Goday, P.S. Intestinal Rehabilitation Programs in the Management of Pediatric Intestinal Failure and Short Bowel Syndrome. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L. Definitions of Intestinal Failure and the Short Bowel Syndrome. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 173–185. [Google Scholar] [CrossRef]
- Nightingale, J.; Woodward, J.M. Guidelines for Management of Patients with a Short Bowel. Gut 2006, 55, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rickham, P.P. Massive Small Intestinal Resection in Newborn Infants. Hunterian Lecture Delivered at the Royal College of Surgeons of England on 13th April 1967. Ann. R. Coll. Surg. Engl. 1967, 41, 480–492. [Google Scholar] [PubMed]
- Lauro, A.; Lacaille, F. Short Bowel Syndrome in Children and Adults: From Rehabilitation to Transplantation. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Batra, A.; Keys, S.C.; Johnson, M.J.; Wheeler, R.A.; Beattie, R.M. Epidemiology, Management and Outcome of Ultrashort Bowel Syndrome in Infancy. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F551–F556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gondolesi, G.; Ramisch, D.; Padin, J.; Almau, H.; Sandi, M.; Schelotto, P.B.; Fernandez, A.; Rumbo, C.; Solar, H. What Is the Normal Small Bowel Length in Humans? First Donor-Based Cohort Analysis. Am. J. Transplant. 2012, 12, 49–54. [Google Scholar] [CrossRef]
- Hounnou, G.; Destrieux, C.; Desmé, J.; Bertrand, P.; Velut, S. Anatomical Study of the Length of the Human Intestine. Surg. Radiol. Anat. 2002, 24, 290–294. [Google Scholar] [CrossRef]
- Touloukian, R.J.; Smith, G.J.W. Normal Intestinal Length in Preterm Infants. J. Pediatr. Surg. 1983, 18, 720–723. [Google Scholar] [CrossRef]
- Struijs, M.C.; Diamond, I.R.; de Silva, N.; Wales, P.W. Establishing Norms for Intestinal Length in Children. J. Pediatr. Surg. 2009, 44, 933–938. [Google Scholar] [CrossRef]
- Buchman, A.L. Etiology and Initial Management of Short Bowel Syndrome. Gastroenterology 2006, 130, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, J.; Gates, A.; Parish, A. Medical Management of Short Gut Syndrome. J. Perinatol. 2010, 30, S2–S5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiBaise, J.K.; Young, R.J.; Vanderhoof, J.A. Intestinal Rehabilitation and the Short Bowel Syndrome: Part 1. Am. J. Gastroenterol. 2004, 99, 1386–1395. [Google Scholar] [CrossRef]
- Wales, P.W.; De Silva, N.; Kim, J.; Lecce, L.; To, T.; Moore, A. Neonatal Short Bowel Syndrome: Population-Based Estimates of Incidence and Mortality Rates. J. Pediatr. Surg. 2004, 39, 690–695. [Google Scholar] [CrossRef]
- Van Gossum, A.; Bakker, H.; Bozzetti, F.; Staun, M.; Leon-Sanz, M.; Hebuterne, X.; Pertkiewicz, M.; Shaffer, J.; Thul, P. Home Parenteral Nutrition in Adults: A European Multicentre Survey in 1997: ESPEN-Home Artificial Nutrition Working Group. Clin. Nutr. 1999, 18, 135–140. [Google Scholar] [CrossRef]
- Howard, L.; Ament, M.; Richard Fleming, C.; Shike, M.; Steiger, E. Current Use and Clinical Outcome of Home Parenteral and Enteral Nutrition Therapies in the United States. Gastroenterology 1995, 109, 355–365. [Google Scholar] [CrossRef]
- Chandra, R.; Kesavan, A. Current Treatment Paradigms in Pediatric Short Bowel Syndrome. Clin. J. Gastroenterol. 2018, 11, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Modi, B.P.; Galloway, D.P.; Gura, K.; Nucci, A.; Plogsted, S.; Tucker, A.; Wales, P.W. ASPEN Definitions in Pediatric Intestinal Failure. J. Parenter. Enter. Nutr. 2022, 46, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Tappenden, K.A. Pathophysiology of Short Bowel Syndrome: Considerations of Resected and Residual Anatomy. J. Parenter. Enter. Nutr. 2014, 38, S14–S22. [Google Scholar] [CrossRef]
- Kumpf, V.J. Pharmacologic Management of Diarrhea in Patients with Short Bowel Syndrome. J. Parenter. Enter. Nutr. 2014, 38, S38–S44. [Google Scholar] [CrossRef]
- Mayer, O.; Kerner, J.A. Management of Short Bowel Syndrome in Postoperative Very Low Birth Weight Infants. Semin. Fetal Neonatal Med. 2017, 22, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Camargo, S.M.R.; Vuille-dit-Bille, R.N.; Meier, C.F.; Verrey, F. ACE2 and Gut Amino Acid Transport. Clin. Sci. 2020, 134, 2823–2833. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.; Camargo, S.M.; Hunziker, S.; Moehrlen, U.; Gros, S.J.; Bode, P.; Leu, S.; Meuli, M.; Holland-Cunz, S.; Verrey, F.; et al. Intestinal IMINO Transporter SIT1 Is Not Expressed in Human Newborns. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 315, G887–G895. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.F.; Camargo, S.M.R.; Hunziker, S.; Leu, S.; Holland-Cunz, S.G.; Verrey, F.; Vuille-Dit-Bille, R.N. Mucosal Monosaccharide Transporter Expression in Newborns With Jejunoileal Atresia and Along the Adult Intestine. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Vuille-dit-Bille, R.N.; Camargo, S.M.; Emmenegger, L.; Sasse, T.; Kummer, E.; Jando, J.; Hamie, Q.M.; Meier, C.F.; Hunziker, S.; Forras-Kaufmann, Z.; et al. Human Intestine Luminal ACE2 and Amino Acid Transporter Expression Increased by ACE-Inhibitors. Amino Acids 2015, 47, 693–705. [Google Scholar] [CrossRef] [Green Version]
- Camargo, S.M.R.; Vuille-dit-Bille, R.N.; Mariotta, L.; Ramadan, T.; Huggel, K.; Singer, D.; Götze, O.; Verrey, F. The Molecular Mechanism of Intestinal Levodopa Absorption and Its Possible Implications for the Treatment of Parkinson’s Disease. J. Pharmacol. Exp. Ther. 2014, 351, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Verrey, F.; Singer, D.; Ramadan, T.; Vuille-dit-Bille, R.N.; Mariotta, L.; Camargo, S.M.R. Kidney Amino Acid Transport. Pflügers Arch.-Eur. J. Physiol. 2009, 458, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Maric, S.; Flüchter, P.; Guglielmetti, L.C.; Staerkle, R.F.; Sasse, T.; Restin, T.; Schneider, C.; Holland-Cunz, S.G.; Crenn, P.; Vuille-dit-Bille, R.N. Plasma Citrulline Correlates with Basolateral Amino Acid Transporter LAT4 Expression in Human Small Intestine. Clin. Nutr. 2021, 40, 2244–2251. [Google Scholar] [CrossRef]
- Maric, S.; Restin, T.; Muff, J.L.; Camargo, S.M.; Guglielmetti, L.C.; Holland-Cunz, S.G.; Crenn, P.; Vuille-Dit-bille, R.N. Citrulline, Biomarker of Enterocyte Functional Mass and Dietary Supplement. Metabolism, Transport, and Current Evidence for Clinical Use. Nutrients 2021, 13, 2794. [Google Scholar] [CrossRef]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 Links Amino Acid Malnutrition to Microbial Ecology and Intestinal Inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef]
- DiBaise, J.K.; Young, R.J.; Vanderhoof, J.A. Enteric Microbial Flora, Bacterial Overgrowth, and Short-Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2006, 4, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.G.; Tappenden, K.A.; Winkler, M.F. Short Bowel Syndrome: Highlights of Patient Management, Quality of Life, and Survival. J. Parenter. Enter. Nutr. 2014, 38, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Toskes, P.P.; Giannella, R.A.; Jervis, H.R.; Rout, W.R.; Takeuchi, A. Small Intestinal Mucosal Injury in the Experimental Blind Loop Syndrome: Light- and Electron-Microscopic and Histochemical Studies. Gastroenterology 1975, 68, 1193–1203. [Google Scholar] [CrossRef]
- Pimentel, M.; Saad, R.J.; Long, M.D.; Rao, S.S.C. ACG Clinical Guideline: Small Intestinal Bacterial Overgrowth. Am. J. Gastroenterol. 2020, 115, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M.; Murray, J.A.; Pimentel, M. AGA Clinical Practice Update on Small Intestinal Bacterial Overgrowth: Expert Review. Gastroenterology 2020, 159, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.; Buresi, M.; Lembo, A.; Lin, H.; McCallum, R.; Rao, S.; Schmulson, M.; Valdovinos, M.; Zakko, S.; Pimentel, M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am. J. Gastroenterol. 2017, 112, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Cangemi, D.J.; Lacy, B.E.; Wise, J. Diagnosing Small Intestinal Bacterial Overgrowth: A Comparison of Lactulose Breath Tests to Small Bowel Aspirates. Dig. Dis. Sci. 2021, 66, 2042–2050. [Google Scholar] [CrossRef]
- Frongia, G.; Kessler, M.; Weih, S.; Nickkholgh, A.; Mehrabi, A.; Holland-Cunz, S. Comparison of LILT and STEP Procedures in Children with Short Bowel Syndrome-A Systematic Review of the Literature. J. Pediatr. Surg. 2013, 48, 1794–1805. [Google Scholar] [CrossRef]
- Wilmore, D.W. Growth and Development of an Infant Receiving All Nutrients Exclusively by Vein. J. Am. Med. Assoc. 1968, 203, 860. [Google Scholar] [CrossRef]
- Mihatsch, W.A.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; Desci, T.; Domellöf, M.; Embleton, N.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition. Clin. Nutr. 2018, 37, 2303–2305. [Google Scholar] [CrossRef] [Green Version]
- Joosten, K.; Embleton, N.; Yan, W.; Senterre, T.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Energy. Clin. Nutr. 2018, 37, 2309–2314. [Google Scholar] [CrossRef]
- Mesotten, D.; Joosten, K.; van Kempen, A.; Verbruggen, S.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Carbohydrates. Clin. Nutr. 2018, 37, 2337–2343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Goudoever, J.B.; Carnielli, V.; Darmaun, D.; Sainz de Pipaon, M.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Darmaun, D.; Decsi, T.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Amino Acids. Clin. Nutr. 2018, 37, 2315–2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapillonne, A.; Fidler Mis, N.; Goulet, O.; van den Akker, C.H.P.; Wu, J.; Koletzko, B.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Lipids. Clin. Nutr. 2018, 37, 2324–2336. [Google Scholar] [CrossRef]
- Bronsky, J.; Campoy, C.; Braegger, C.; Braegger, C.; Bronsky, J.; Cai, W.; Carnielli, V.; Darmaun, D.; Decsi, T.; Domellöf, M.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Vitamins. Clin. Nutr. 2018, 37, 2366–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolaček, S.; Puntis, J.W.L.; Hojsak, I.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; Decsi, T.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Venous Access. Clin. Nutr. 2018, 37, 2379–2391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riskin, A.; Picaud, J.C.; Shamir, R.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; Decsi, T.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Standard versus Individualized Parenteral Nutrition. Clin. Nutr. 2018, 37, 2409–2417. [Google Scholar] [CrossRef] [Green Version]
- Hartman, C.; Shamir, R.; Simchowitz, V.; Lohner, S.; Cai, W.; Decsi, T.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Complications. Clin. Nutr. 2018, 37, 2418–2429. [Google Scholar] [CrossRef] [Green Version]
- Jochum, F.; Moltu, S.J.; Senterre, T.; Nomayo, A.; Goulet, O.; Iacobelli, S.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Fluid and Electrolytes. Clin. Nutr. 2018, 37, 2344–2353. [Google Scholar] [CrossRef] [Green Version]
- Domellöf, M.; Szitanyi, P.; Simchowitz, V.; Franz, A.; Mimouni, F.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Iron and Trace Minerals. Clin. Nutr. 2018, 37, 2354–2359. [Google Scholar] [CrossRef] [Green Version]
- Mihatsch, W.; Fewtrell, M.; Goulet, O.; Molgaard, C.; Picaud, J.C.; Senterre, T.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Calcium, Phosphorus and Magnesium. Clin. Nutr. 2018, 37, 2360–2365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, S.; Ksiazyk, J.; Prell, C.; Tabbers, M.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Home Parenteral Nutrition. Clin. Nutr. 2018, 37, 2401–2408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puntis, J.W.L.; Hojsak, I.; Ksiazyk, J.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; Decsi, T.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Organisational Aspects. Clin. Nutr. 2018, 37, 2392–2400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Hernandez, J.; Prajapati, P.; Ogola, G.; Channabasappa, N.; Drews, B.; Piper, H.G. Predicting Time to Full Enteral Nutrition in Children after Significant Bowel Resection. J. Pediatr. Surg. 2017, 52, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Scarlett, Y. Medical Management of Fecal Incontinence. Gastroenterology 2004, 126, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.N. Opioid Analgesics and the Gastrointestinal Tract. Pract. Gastroenterol. 2008, 32, 37–50. [Google Scholar]
- Schiller, L.R. Chronic Diarrhea. Curr. Treat. Options Gastroenterol. 2005, 8, 259–266. [Google Scholar] [CrossRef]
- Thomson, A.B.R.; Sauve, M.D.; Kassam, N.; Kamitakahara, H. Safety of the Long-Term Use of Proton Pump Inhibitors. World J. Gastroenterol. 2010, 16, 2323–2330. [Google Scholar] [CrossRef]
- Lam, J.R.; Schneider, J.L.; Zhao, W.; Corley, D.A. Proton Pump Inhibitor and Histamine 2 Receptor Antagonist Use and Vitamin B12 Deficiency. J. Am. Med. Assoc. 2013, 310, 2435–2442. [Google Scholar] [CrossRef] [Green Version]
- Gillen, D.; Wirz, A.A.; Ardill, J.E.; McColl, K.E.L. Rebound Hypersecretion after Omeprazole and Its Relation to On-Treatment Acid Suppression and Helicobacter Pylori Status. Gastroenterology 1999, 116, 239–247. [Google Scholar] [CrossRef]
- Parrish, C.R. The Clinician’s Guide to Short Bowel Syndrome. Pract. Gastroenterol. 2005, 29, 67–106. [Google Scholar]
- Gatta, L.; Scarpignato, C.; McCallum, R.W.; Lombardo, L.; Pimentel, M.; D’Incà, R.; Gasbarrini, A.; De Stefano, A.; Cerda, E. Systematic Review with Meta-Analysis: Rifaximin Is Effective and Safe for the Treatment of Small Intestine Bacterial Overgrowth. Aliment. Pharmacol. Ther. 2017, 45, 604–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, C.; Qu, C.; Wang, B.; Liang, S.; Zeng, B. Probiotics for Preventing and Treating Small Intestinal Bacterial Overgrowth. J. Clin. Gastroenterol. 2017, 51, 300–311. [Google Scholar] [CrossRef]
- Reddy, V.S.; Patole, S.K.; Rao, S. Role of Probiotics in Short Bowel Syndrome in Infants and Children—A Systematic Review. Nutrients 2013, 5, 679–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.S.; Keam, S.J. Teduglutide: A Review in Short Bowel Syndrome. Drugs 2017, 77, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Kocoshis, S.A.; Merritt, R.J.; Hill, S.; Protheroe, S.; Carter, B.A.; Horslen, S.; Hu, S.; Kaufman, S.S.; Mercer, D.F.; Pakarinen, M.P.; et al. Safety and Efficacy of Teduglutide in Pediatric Patients With Intestinal Failure Due to Short Bowel Syndrome: A 24-Week, Phase III Study. J. Parenter. Enter. Nutr. 2020, 44, 621–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picot, D.; Layec, S.; Seynhaeve, E.; Dussaulx, L.; Trivin, F.; Carsin-Mahe, M. Chyme Reinfusion in Intestinal Failure Related to Temporary Double Enterostomies and Enteroatmospheric Fistulas. Nutrients 2020, 12, 1376. [Google Scholar] [CrossRef]
- Kumpf, V.J.; De Aguilar-Nascimento, J.E.; Diaz-Pizarro Graf, J.I.; Hall, A.M.; McKeever, L.; Steiger, E.; Winkler, M.F.; Compher, C.W. ASPEN-FELANPE Clinical Guidelines. J. Parenter. Enter. Nutr. 2017, 41, 104–112. [Google Scholar] [CrossRef]
- Klek, S.; Forbes, A.; Gabe, S.; Holst, M.; Wanten, G.; Irtun, Ø.; Damink, S.O.; Panisic-Sekeljic, M.; Pelaez, R.B.; Pironi, L.; et al. Management of Acute Intestinal Failure: A Position Paper from the European Society for Clinical Nutrition and Metabolism (ESPEN) Special Interest Group. Clin. Nutr. 2016, 35, 1209–1218. [Google Scholar] [CrossRef]
- Pironi, L.; Corcos, O.; Forbes, A.; Holst, M.; Joly, F.; Jonkers, C.; Klek, S.; Lal, S.; Blaser, A.R.; Rollins, K.E.; et al. Intestinal Failure in Adults: Recommendations from the ESPEN Expert Groups. Clin. Nutr. 2018, 37, 1798–1809. [Google Scholar] [CrossRef] [Green Version]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN Guideline on Clinical Nutrition in the Intensive Care Unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, S.; Cameron, N.R.; Sharma, P.; Bissett, I.P.; O’Grady, G. Chyme Recycling in the Management of Small Bowel Double Enterostomy in Pediatric and Neonatal Populations: A Systematic Review. Clin. Nutr. ESPEN 2020, 37, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gause, C.D.; Hayashi, M.; Haney, C.; Rhee, D.; Karim, O.; Weir, B.W.; Stewart, D.; Lukish, J.; Lau, H.; Abdullah, F.; et al. Mucous Fistula Refeeding Decreases Parenteral Nutrition Exposure in Postsurgical Premature Neonates. J. Pediatr. Surg. 2016, 51, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Cuerda, C.; Pironi, L.; Arends, J.; Bozzetti, F.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. ESPEN Practical Guideline: Clinical Nutrition in Chronic Intestinal Failure. Clin. Nutr. 2021, 40, 5196–5220. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Gonzalez, G.; Kim, H.B. Autologous Intestinal Reconstruction Surgery. Semin. Pediatr. Surg. 2018, 27, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, F.; Andres, A.M.; Lopez-Santamaria, M. Long-Term Results of Surgery for Bowel Lengthening: How Many Transplants Are Avoided, for Which Patients? Curr. Opin. Organ Transplant. 2018, 23, 207–211. [Google Scholar] [CrossRef]
- Pakarinen, M.P. Autologous Intestinal Reconstruction Surgery as Part of Comprehensive Management of Intestinal Failure. Pediatr. Surg. Int. 2015, 31, 453–464. [Google Scholar] [CrossRef]
- Hommel, M.J.; Van Baren, R.; Haveman, J.W. Surgical Management and Autologous Intestinal Reconstruction in Short Bowel Syndrome. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 263–280. [Google Scholar] [CrossRef]
- Kim, H.B.; Fauza, D.; Garza, J.; Oh, J.T.; Nurko, S.; Jaksic, T. Serial Transverse Enteroplasty (STEP): A Novel Bowel Lengthening Procedure. J. Pediatr. Surg. 2003, 38, 425–429. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.B.; Lee, P.W.; Garza, J.; Duggan, C.; Fauza, D.; Jaksic, T. Serial Transverse Enteroplasty for Short Bowel Syndrome: A Case Report. J. Pediatr. Surg. 2003, 38, 881–885. [Google Scholar] [CrossRef]
- Javid, P.J.; Heung, B.K.; Duggan, C.P.; Jaksic, T. Serial Transverse Enteroplasty Is Associated with Successful Short-Term Outcomes in Infants with Short Bowel Syndrome. J. Pediatr. Surg. 2005, 40, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Van Praagh, J.B.; Hofker, H.S.; Haveman, J.W. Comparing Bowel Lengthening Procedures: Which, When, and Why? Curr. Opin. Organ Transplant. 2022, 27, 112–118. [Google Scholar] [CrossRef]
- Cowles, R.A.; Lobritto, S.J.; Stylianos, S.; Brodlie, S.; Smith, L.J.; Jan, D. Serial Transverse Enteroplasty in a Newborn Patient. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 257–260. [Google Scholar] [CrossRef]
- Leung, M.W.Y.; Chan, I.H.Y.; Chao, N.S.Y.; Wong, B.P.Y.; Liu, K.K.W. Serial Transverse Enteroplasty for Short Bowel Syndrome: Hong Kong Experience. Hong Kong Med. J. 2012, 18, 35–39. [Google Scholar] [PubMed]
- Ismail, A.; Alkadhi, A.; Alnagaar, O.; Khirate, A. Serial Transverse Enteroplasty in Intestinal Atresia Management. J. Pediatr. Surg. 2005, 40, 5–6. [Google Scholar] [CrossRef]
- Garnett, G.M.; Kang, K.H.; Jaksic, T.; Woo, R.K.; Puapong, D.P.; Kim, H.B.; Johnson, S.M. First STEPs: Serial Transverse Enteroplasty as a Primary Procedure in Neonates with Congenital Short Bowel. J. Pediatr. Surg. 2014, 49, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Duggan, C.; Piper, H.; Javid, P.J.; Valim, C.; Collier, S.; Kim, H.B.; Jaksic, T. Growth and Nutritional Status in Infants With Short-Bowel Syndrome After the Serial Transverse Enteroplasty Procedure. Clin. Gastroenterol. Hepatol. 2006, 4, 1237–1241. [Google Scholar] [CrossRef]
- Mercer, D.F.; Burnett, T.R.; Hobson, B.D.; Logan, S.J.; Gerhardt, B.K.; Iwansky, S.N.; Quiros-Tejeira, R.E. Repeat Serial Transverse Enteroplasty Leads to Reduction in Parenteral Nutrition in Children with Short Bowel Syndrome. J. Pediatr. Surg. 2021, 56, 733–737. [Google Scholar] [CrossRef]
- Lemoine, C.; Larkin, K.; Brennan, K.; Zoller-Thompson, C.; Cohran, V.; Superina, R. Repeat Serial Transverse Enteroplasty Procedure (ReSTEP): Is It Worth It? J. Pediatr. Surg. 2021, 56, 951–960. [Google Scholar] [CrossRef]
- Mutanen, A.; Barrett, M.; Feng, Y.; Lohi, J.; Rabah, R.; Teitelbaum, D.H.; Pakarinen, M.P. Short Bowel Mucosal Morphology, Proliferation and Inflammation at First and Repeat STEP Procedures. J. Pediatr. Surg. 2019, 54, 511–516. [Google Scholar] [CrossRef] [Green Version]
- Barrett, M.; Demehri, F.R.; Ives, G.C.; Schaedig, K.; Arnold, M.A.; Teitelbaum, D.H. Taking a STEP Back: Assessing the Outcomes of Multiple STEP Procedures. J. Pediatr. Surg. 2017, 52, 69–73. [Google Scholar] [CrossRef]
- Frongia, G.; Majlesara, A.; Saffari, A.; Abbasi, D.S.; Gharabaghi, N.; Okun, J.G.; Thiel, C.; Günther, P.; Vianna, R.; Mehrabi, A. Simultaneous Serial Transverse Enteroplasty (STEP) in Size Mismatch Small Bowel Transplantations. J. Gastrointest. Surg. 2019, 23, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Coletta, R.; Khalil, B.A.; Morabito, A. Short Bowel Syndrome in Children: Surgical and Medical Perspectives. Semin. Pediatr. Surg. 2014, 23, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.; Muto, M.; Belza, C.; De Silva, N.; Avitzur, Y.; Wales, P.W. The Evolution of the Serial Transverse Enteroplasty for Pediatric Short Bowel Syndrome at a Single Institution. J. Pediatr. Surg. 2019, 54, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Nagelkerke, S.C.J.; Poelgeest, M.Y.V.; Wessel, L.M.; Mutanen, A.; Langeveld, H.R.; Hill, S.; Benninga, M.A.; Tabbers, M.M.; Bakx, R. Bowel Lengthening Procedures in Children with Short Bowel Syndrome: A Systematic Review. Eur. J. Pediatr. Surg. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R.A.; Yoeli, D.; Hoeltzel, G.; Moore, H.B.; Prins, K.; Kovler, M.; Goldstein, S.D.; Holland-Cunz, S.G.; Adams, M.; Roach, J.; et al. STEP Improves Long-Term Survival for Pediatric Short Bowel Syndrome Patients: A Markov Decision Analysis. J. Pediatr. Surg. 2020, 55, 1802–1808. [Google Scholar] [CrossRef]
- Bueno, J.; García-Martínez, L.; Redecillas, S.; Segarra, O.; López, M. Long-Term Outcome of Children with Short Bowel Syndrome Treated with a Modification of the STEP Technique Avoiding Mesenteric Defect. Eur. J. Pediatr. Surg. 2021. [Google Scholar] [CrossRef]
- Bueno, J.; Redecillas, S.; García, L.; Lara, A.; Giné, C.; Molino, J.A.; Broto, J.; Segarra, O. Duodenal Lengthening in Short Bowel with Dilated Duodenum. J. Pediatr. Surg. 2015, 50, 493–496. [Google Scholar] [CrossRef]
- Bianchi, A. Intestinal Loop Lengthening-A Technique for Increasing Small Intestinal Length. J. Pediatr. Surg. 1980, 15, 145–151. [Google Scholar] [CrossRef]
- Bianchi, A. Intestinal Lengthening: An Experimental and Clinical Review. J. R. Soc. Med. 1984, 77, 35–41. [Google Scholar]
- Höllwarth, M.E. Surgical Strategies in Short Bowel Syndrome. Pediatr. Surg. Int. 2017, 33, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Coletta, R.; Morabito, A. Non-Transplant Surgical Management of Short Bowel Syndrome in Children: An Overview. Curr. Pediatr. Rev. 2019, 15, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A. Longitudinal Intestinal Lengthening and Tailoring: Results in 20 Children. J. R. Soc. Med. 1997, 90, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A. From the Cradle to Enteral Autonomy: The Role of Autologous Gastrointestinal Reconstruction. Gastroenterology 2006, 130, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A. Experience with Longitudinal Intestinal Lengthening and Tailoring. Eur. J. Pediatr. Surg. 1999, 9, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.R. Isoperistaltic Bowel Lengthening for Short Bowel Syndrome in Children. Am. J. Surg. 1999, 178, 600–603. [Google Scholar] [CrossRef]
- Bueno, J.; Guiterrez, J.; Mazariegos, G.V.; Abu-Elmagd, K.; Madariaga, J.; Ohwada, S.; Kocoshis, S.; Reyes, J. Analysis of Patients with Longitudinal Intestinal Lengthening Procedure Referred for Intestinal Transplantation. J. Pediatr. Surg. 2001, 36, 178–183. [Google Scholar] [CrossRef]
- Aigrain, Y.; Cornet, D.; Cezard, J.P.; Boureau, M. Longitudinal Division of Small Intestine: A Surgical Possibility for Children with the Very Short Bowel Syndrome. Eur. J. Pediatr. Surg. 1985, 40, 233–236. [Google Scholar] [CrossRef]
- Fusaro, F.; Hermans, D.; Wanty, C.; Veyckemans, F.; Pirenne, J.; Reding, R. Post-Serial Transverse Enteroplasty Bowel Redilatation Treated by Longitudinal Intestinal Lengthening and Tailoring Procedure. J. Pediatr. Surg. 2012, 47, e19–e22. [Google Scholar] [CrossRef]
- Weber, T.R.; Powell, M.A. Early Improvement in Intestinal Function after Isoperistaltic Bowel Lengthening. J. Pediatr. Surg. 1996, 31, 61–64. [Google Scholar] [CrossRef]
- Figueroa-Colon, R.; Harris, P.R.; Birdsong, E.; Franklin, F.A.; Georgeson, K.E. Impact of Intestinal Lengthening on the Nutritional Outcome for Children with Short Bowel Syndrome. J. Pediatr. Surg. 1996, 31, 912–916. [Google Scholar] [CrossRef]
- Shah, A.A.; Petrosyan, M.; Franklin, A.L.; Chahine, A.A.; Torres, C.; Sandler, A.D. Autologous Intestinal Reconstruction: A Single Institution Study of the Serial Transverse Enteroplasty (STEP) and the Longitudinal Intestinal Lengthening and Tailoring (LILT). Pediatr. Surg. Int. 2019, 35, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Hosie, S.; Loff, S.; Wirth, H.; Rapp, H.J.; Von Buch, C.; Waag, K.L. Experience of 49 Longitudinal Intestinal Lengthening Procedures for Short Bowel Syndrome. Eur. J. Pediatr. Surg. 2006, 16, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Huskisson, L.J.; Brereton, R.J.; Kiely, E.M.; Spitz, L. Problems with Intestinal Lengthening. J. Pediatr. Surg. 1993, 28, 720–722. [Google Scholar] [CrossRef]
- Thompson, J.S.; Pinch, L.W.; Murray, N.; Vanderhoof, J.A.; Schultz, L.R. Experience with Intestinal Lengthening for the Short-Bowel Syndrome. J. Pediatr. Surg. 1991, 26, 721–724. [Google Scholar] [CrossRef]
- Waag, K.; Hosie, S.; Wessel, L. What Do Children Look Like After Longitudinal Intestinal Lengthening? Eur. J. Pediatr. Surg. 1999, 9, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.S.; Pinch, L.W.; Young, R.; Vanderhoof, J.A. Long-Term Outcome of Intestinal Lengthening. Transplant. Proc. 2000, 32, 1242–1243. [Google Scholar] [CrossRef]
- Reinshagen, K.; Zahn, K.; von Buch, C.; Zoeller, M.; Hagl, C.I.; Ali, M.; Waag, K.L. The Impact of Longitudinal Intestinal Lengthening and Tailoring on Liver Function in Short Bowel Syndrome. Eur. J. Pediatr. Surg. 2008, 18, 249–253. [Google Scholar] [CrossRef]
- Reinshagen, K.; Kabs, C.; Wirth, H.; Hable, N.; Brade, J.; Zahn, K.; Hagl, C.; Jester, I.; Waag, K. Long-Term Outcome in Patients With Short Bowel Syndrome After Longitudinal Intestinal Lengthening and Tailoring. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 573–578. [Google Scholar] [CrossRef]
- Walker, S.R.; Nucci, A.; Yaworski, J.A.; Barksdale, E.M. The Bianchi Procedure: A 20-Year Single Institution Experience. J. Pediatr. Surg. 2006, 41, 113–119. [Google Scholar] [CrossRef]
- Bonnard, A.; Staub, G.; Segura, J.F.; Malbezin, S.; Dorgeret, S.; Aigrain, Y.; De Lagausie, P. Evaluation of Intestinal Absorption after Longitudinal Intestinal Lengthening for Short Bowel Syndrome. J. Pediatr. Surg. 2005, 40, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Alfred Chahine, A.; Ricketts, R.R. A Modification of the Bianchi Intestinal Lengthening Procedure with a Single Anastomosis. J. Pediatr. Surg. 1998, 33, 1292–1293. [Google Scholar] [CrossRef]
- Shun, A.; Thomas, G.; Puppi, J.; La Hei, E.; Langusch, C. Double Barrel Enteroplasty for the Management of Short Bowel Syndrome in Children. Pediatr. Surg. Int. 2021, 37, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Soper, R.T. Isolated Bowel Segment (Model 1): Creation by Myoenteropexy. J. Pediatr. Surg. 1990, 25, 512–513. [Google Scholar] [CrossRef]
- Kimura, K.; Soper, R.T. A New Bowel Elongation Technique for the Short-Bowel Syndrome Using the Isolated Bowel Segment Iowa Models. J. Pediatr. Surg. 1993, 28, 792–794. [Google Scholar] [CrossRef]
- Cserni, T.; Takayasu, H.; Muzsnay, Z.; Varga, G.; Murphy, F.; Folaranmi, S.E.; Rakoczy, G. New Idea of Intestinal Lengthening and Tailoring. Pediatr. Surg. Int. 2011, 27, 1009–1013. [Google Scholar] [CrossRef]
- Coletta, R.; Mussi, E.; Uccheddu, F.; Volpe, Y.; Morabito, A. Preoperative Planning of Spiral Intestinal Lengthening and Tailoring: A Geometrical Approach. Bioengineering 2021, 8, 20. [Google Scholar] [CrossRef]
- Mehrabi, V.; Mehrabi, A.; Jamshidi, S.H.; Pedram, M.S.; Sabagh, M.S.; Jaberansari, N.; Fonouni, H.R.; Sharifi, A.H.; Malekzadeh, R.; Frongia, G. Modified Spiral Intestinal Lengthening and Tailoring for Short Bowel Syndrome. Surg. Innov. 2016, 23, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Cserni, T.; Varga, G.; Erces, D.; Kaszaki, J.; Boros, M.; Laszlo, A.; Murphy, F.; Földvari, A.; Morabito, A.; Bianchi, A.; et al. Spiral Intestinal Lengthening and Tailoring-First in Vivo Study. J. Pediatr. Surg. 2013, 48, 1907–1913. [Google Scholar] [CrossRef]
- Alberti, D.; Boroni, G.; Giannotti, G.; Parolini, F.; Armellini, A.; Morabito, A.; Bianchi, A. “Spiral Intestinal Lenghtening and Tailoring (SILT)” for a Child with Severely Short Bowel. Pediatr. Surg. Int. 2014, 30, 1169–1172. [Google Scholar] [CrossRef]
- Coletta, R.; Aldeiri, B.; Morabito, A. Institutional Experience with Spiral Intestinal Lengthening and Tailoring. Eur. J. Pediatr. Surg. 2018, 29, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Cserni, T.; Biszku, B.; Guthy, I.; Dicso, F.; Szaloki, L.; Folaranmi, S.; Murphy, F.; Rakoczy, G.; Bianchi, A.; Morabito, A. The First Clinical Application of the Spiral Intestinal Lengthening and Tailoring (Silt) in Extreme Short Bowel Syndrome. J. Gastrointest. Surg. 2014, 18, 1852–1857. [Google Scholar] [CrossRef] [PubMed]
- Alberti, D.; Righetti, L.; Bianchi, A.; de’Angelis, G.L.; Boroni, G. Transverse Flap Duodenoplasty (TFD): A New Technique in Autologous Bowel Reconstructive Surgery. Pediatr. Surg. Int. 2018, 34, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Int, P.S. Autologous Gastro-Intestinal Reconstruction: The Composite Ileo-Colic Loop. Pediatric Surg. Int. 1996, 11, 248–251. [Google Scholar]
- Printz, H.; Schlenzka, R.; Reguadt, P.; Tscherny, M.; Wagner, A.C.C.; Eissele, R.; Rothmund, M.; Arnold, R.; Göke, B. Small Bowel Lengthening by Mechanical Distraction. Digestion 1997, 58, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Safford, S.D.; Freemerman, A.J.; Safford, K.M.; Bentley, R.; Skinner, M.A. Longitudinal Mechanical Tension Induces Growth in the Small Bowel of Juvenile Rats. Gut 2005, 54, 1085–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, H.; Sun, X.; Yang, H.; Nose, K.; Somara, S.; Bitar, K.N.; Owyang, C.; Okawada, M.; Teitelbaum, D.H. Distraction-Induced Intestinal Enterogenesis: Preservation of Intestinal Function and Lengthening after Reimplantation into Normal Jejunum. Ann. Surg. 2012, 255, 302–310. [Google Scholar] [CrossRef] [Green Version]
- Luntz, J.; Brei, D.; Teitelbaum, D.; Spencer, A. Mechanical Extension Implants for Short-Bowel Syndrome. Proc. SPIE Int. Soc. Opt. Eng. 2006, 6173, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Shekherdimian, S.; Panduranga, M.K.; Carman, G.P.; Dunn, J.C.Y. The Feasibility of Using an Endoluminal Device for Intestinal Lengthening. J. Pediatr. Surg. 2010, 45, 1575–1580. [Google Scholar] [CrossRef]
- Huynh, N.; Dubrovsky, G.; Rouch, J.D.; Scott, A.; Stelzner, M.; Shekherdimian, S.; Dunn, J.C.Y. Feasibility and Scalability of Spring Parameters in Distraction Enterogenesis in a Murine Model. J. Surg. Res. 2017, 215, 219–224. [Google Scholar] [CrossRef]
- Portelli, K.I.; Park, J.B.; Taylor, J.S.; Thomas, A.L.; Stelzner, M.; Martin, M.G.; Dunn, J.C.Y. Intestinal Adaptation Following Spring Insertion into a Roux Limb in Mice. J. Pediatr. Surg. 2021, 56, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Rouch, J.D.; Huynh, N.; Chiang, E.; Shekherdimian, S.; Lee, S.L.; Wu, B.M.; Dunn, J.C.Y. Mechanical Lengthening in Multiple Intestinal Segments In-Series. J. Pediatr. Surg. 2016, 51, 957–959. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.; Rouch, J.D.; Scott, A.; Chiang, E.; Wu, B.M.; Shekherdimian, S.; Dunn, J.C.Y. Spring-Mediated Distraction Enterogenesis in-Continuity. J. Pediatr. Surg. 2016, 51, 1983–1987. [Google Scholar] [CrossRef] [PubMed]
- Stark, R.; Panduranga, M.; Carman, G.; Dunn, J.C.Y. Development of an Endoluminal Intestinal Lengthening Capsule. J. Pediatr. Surg. 2012, 47, 136–141. [Google Scholar] [CrossRef]
- Sullins, V.F.; Scott, A.; Wagner, J.P.; Steinberger, D.; Lee, S.L.; Wu, B.M.; Dunn, J.C.Y. Intestinal Lengthening in an Innovative Rodent Surgical Model. J. Pediatr. Surg. 2014, 49, 1791–1794. [Google Scholar] [CrossRef]
- Dubrovsky, G.; Huynh, N.; Thomas, A.L.; Shekherdimian, S.; Dunn, J.C.Y. Double Plication for Spring-Mediated Intestinal Lengthening of a Defunctionalized Roux Limb. J. Pediatr. Surg. 2018, 53, 1806–1810. [Google Scholar] [CrossRef]
- Sullins, V.F.; Wagner, J.P.; Suwarnasarn, A.T.; Lee, S.L.; Wu, B.M.; Dunn, J.C.Y. A Novel Biodegradable Device for Intestinal Lengthening. J. Pediatr. Surg. 2014, 49, 109–113. [Google Scholar] [CrossRef]
- Diyaolu, M.; Thomas, A.L.; Wood, L.S.; Taylor, J.; Dunn, J.C. Mesenteric Neovascularization during Spring-Mediated Intestinal Lengthening. J. Pediatr. Surg. 2021, 56, 5–10. [Google Scholar] [CrossRef]
- Huynh, N.; Dubrovsky, G.; Rouch, J.D.; Scott, A.; Chiang, E.; Nguyen, T.; Wu, B.M.; Shekherdimian, S.; Krummel, T.M.; Dunn, J.C.Y. Three-Dimensionally Printed Surface Features to Anchor Endoluminal Spring for Distraction Enterogenesis. PLoS ONE 2018, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Negoro, S.; Kato, D.-I.; Ohki, T.; Yasuhira, K.; Kawashima, Y.; Nagai, K.; Takeo, M.; Shibata, N.; Kamiya, K.; Shigeta, Y. Structural and Functional Characterization of Nylon Hydrolases. In Enzymatic Plastic Degradation, 1st ed.; Elsevier Inc.: Munich, Germany, 2021; Volume 648, ISBN 9780128220122. [Google Scholar]
- Demehri, F.R.; Wong, P.M.; Freeman, J.J.; Fukatsu, Y.; Teitelbaum, D.H. A Novel Double-Balloon Catheter Device for Fully Endoluminal Intestinal Lengthening. Pediatr. Surg. Int. 2014, 30, 1223–1229. [Google Scholar] [CrossRef]
- Demehri, F.R.; Utter, B.; Freeman, J.J.; Fukatsu, Y.; Luntz, J.; Brei, D.; Teitelbaum, D.H. Development of an Endoluminal Intestinal Attachment for a Clinically Applicable Distraction Enterogenesis Device. J. Pediatr. Surg. 2016, 51, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Demehri, F.R.; Freeman, J.J.; Fukatsu, Y.; Luntz, J.; Teitelbaum, D.H. Development of an Endoluminal Intestinal Lengthening Device Using a Geometric Intestinal Attachment Approach. Surgery 2015, 158, 802–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubrovsky, G.; Huynh, N.; Thomas, A.L.; Shekherdimian, S.; Dunn, J.C. Intestinal Lengthening via Multiple In-Continuity Springs. J. Pediatr. Surg. 2019, 54, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.D.; Carter, R.; Hinshaw, D.B. Segmental Reversal of Small Intestine After Massive Bowel Resection. J. Am. Med. Assoc. 1962, 182, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Kurz, R.; Sauer, H. Treatment and Metabolic Findings in Extreme Short-Bowel Syndrome with 11 Cm Jejunal Remnant. J. Pediatr. Surg. 1983, 18, 257–263. [Google Scholar] [CrossRef]
- Panis, Y.; Messing, B.; Rivet, P.; Coffin, B.; Hautefeuille, P.; Matuchansky, C.; Rambaud, J.C.; Valleur, P. Segmental Reversal of the Small Bowel as an Alternative to Intestinal Transplantation in Patients with Short Bowel Syndrome. Ann. Surg. 1997, 225, 401–407. [Google Scholar] [CrossRef]
- Beyer-Berjot, L.; Joly, F.; Maggiori, L.; Corcos, O.; Bouhnik, Y.; Bretagnol, F.; Panis, Y. Segmental Reversal of the Small Bowel Can End Permanent Parenteral Nutrition Dependency. Ann. Surg. 2012, 256, 739–745. [Google Scholar] [CrossRef]
- Pigot, F.; Messing, B.; Chaussade, S.; Pfeiffer, A.; Pouliquen, X.; Jian, R. Severe Short Bowel Syndrome with a Surgically Reversed Small Bowel Segment. Dig. Dis. Sci. 1990, 35, 137–144. [Google Scholar] [CrossRef]
- Lloyd, D.A. Antiperistaltic Colonic Interposition Following Massive Small Bowel Resection in Rats. J. Pediatr. Surg. 1981, 16, 64–69. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Narasimharao, K.L.; Usha Rani, V. Morphological and Functional Changes in the Gut after Massive Small Bowel Resection and Colon Interposition in Rhesus Monkeys. Digestion 1984, 29, 47–54. [Google Scholar] [CrossRef]
- Garcia, V.F.; Templeton, J.M.; Eichelberger, M.R.; Koop, C.E.; Vinograd, I. Colon Interposition for the Short Bowel Syndrome. J. Pediatr. Surg. 1981, 16, 994–995. [Google Scholar] [CrossRef]
- Trinkle, J.K.; Bryant, L.R. Reversed Colon Segment in an Infant with Massive Small Bowel Resection: A Case Report. J. Kentucky Med. Assoc. 1967, 65, 1090–1091. [Google Scholar]
- Hutcher, N.E.; Mendez-Picon, G.; Salzberg, A.M. Prejejunal Transposition of Colon to Prevent the Development of Short Bowel Syndrome in Puppies with 90 per Cent Small Intestine Resection. J. Pediatr. Surg. 1973, 8, 771–777. [Google Scholar] [CrossRef]
- King, D.R.; Anvari, M.; Jamieson, G.G.; King, J.M. Does the Colon Adopt Small Bowel Features in a Small Bowel Environment? Aust. N. Z. J. Surg. 1996, 66, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Glick, P.L.; de Lorimier, A.A.; Scott Adzick, N.; Harrison, M.R. Colon Interposition: An Adjuvant Operation for Short-Gut Syndrome. J. Pediatr. Surg. 1984, 19, 719–725. [Google Scholar] [CrossRef]
- Georgeson, K.; Halpin, D.; Figueroa, R.; Vincente, Y.; Hardin, W. Sequential Intestinal Lengthening Procedures for Refractory Short Bowel Syndrome. J. Pediatr. Surg. 1994, 29, 316–321. [Google Scholar] [CrossRef]
- Zurita, M.; Raurich, J.M.; Ramírez, A.; Gil, J.; Darder, J. A New Neovalve Type in Short Bowel Syndrome Surgery. Rev. Española Enferm. Dig. 2004, 96, 108. [Google Scholar] [CrossRef] [Green Version]
- Shafiekhani, M.; Azadeh, N.; Ashrafzadeh, K.; Esmaeili, M.; Nikoupour, H. Serial Transverse Enteroplasty and Nipple Valve Construction, Two Life Saving Techniques for Patients with Short Bowel Syndrome, a Report of 5 Cases. BMC Surg. 2021, 21, 446. [Google Scholar] [CrossRef]
- Mackby, M.J.; Richards, V.; Gilfillan, R.S.; Floridia, R. Methods of Increasing the Efficiency of Residual Small Bowel Segments. A Preliminary Study. Am. J. Surg. 1965, 109, 32–38. [Google Scholar] [CrossRef]
- Poth, E.J. Use of Gastrointestinal Reversal in Surgical Procedures. Am. J. Surg. 1969, 118, 893–899. [Google Scholar] [CrossRef]
- Camprodon, R.; Guerrero, J.A.; Salva, J.A.; Jornet, J. Shortened Small Bowel Syndrome. Mackby’s Operation. Am. J. Surg. 1975, 129, 585–586. [Google Scholar] [CrossRef]
- Thompson, J.S. Surgical Therapy for the Short Bowel Syndrome. J. Surg. Res. 1985, 39, 81–91. [Google Scholar] [CrossRef]
- Bianchi, A. Autologous Gastrointestinal Reconstruction for Short Bowel Syndrome. Br. J. Hosp. Med. 2007, 68, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Vernon, A.H.; Georgeson, K.E. Surgical Options for Short Bowel Syndrome. Semin. Pediatr. Surg. 2001, 10, 91–98. [Google Scholar] [CrossRef]
- de Lorimier, A.A.; Harrison, M.R. Intestinal Plication in the Treatment of Atresia. J. Pediatr. Surg. 1983, 18, 734–737. [Google Scholar] [CrossRef]
- Kimura, K.; Perdzynski, W.; Soper, R.T. Elliptical Seromuscular Resection for Tapering the Proximal Dilated Bowel in Duodenal or Jejunal Atresia. J. Pediatr. Surg. 1996, 31, 1405–1406. [Google Scholar] [CrossRef]
- Cruz, R.J. Modified Antimesenteric Tapering Enteroplasty: An Alternative Technique for the Treatment of Dysfunctional Anastomosis in Patients with Short Bowel. Dis. Colon Rectum 2021, 9, e520–e525. [Google Scholar] [CrossRef]
- Lillehei, R.C.; Goott, B.; Miller, F.A. The Physiological Response of the Small Bowel of the Dog to Ischemia Including Prolonged in Vitro Preservation of the Bowel with Successful Replacement and Survival. Ann. Surg. 1959, 150, 543–560. [Google Scholar] [CrossRef]
- Starzl, T.E.; Kaupp, H.A. Mass Homotransplantation Of Abdominal Organs In Dogs. Surg. Forum 1960, 11, 28–30. [Google Scholar]
- Reyes, J.D. Intestinal Transplantation: An Unexpected Journey. J. Pediatr. Surg. 2014, 49, 13–18. [Google Scholar] [CrossRef]
- Goulet, O.; Revillon, Y.; Brousse, N.; Jan, D.; Canion, D.; Rambaud, C.; Cerf-Bensussan, N.; Buisson, C.; Hubert, P.; de Potter, S.; et al. Successful Small Bowel Transplantation In An Infant. Transplantation 1992, 53, 940–942. [Google Scholar] [CrossRef] [PubMed]
- Deltz, E.; Schroeder, P.; Gebhardt, H.; Gundlach, M.; Engemann, R.; Timmermann, W. First Successful Clinical Small Intestine Transplantation. Tactics and Surgical Technic. Chirurg 1989, 60, 235–239. [Google Scholar] [PubMed]
- Kesseli, S.; Sudan, D. Small Bowel Transplantation. Surg. Clin. N. Am. 2019, 99, 103–116. [Google Scholar] [CrossRef]
- Gürkan, A. Advances in Small Bowel Transplantation. Turk. J. Surg. 2017, 33, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.; Abu-Elmagd, K.; Mazariegos, G.; Vianna, R.; Langnas, A.; Mangus, R.; Farmer, D.G.; Lacaille, F.; Iyer, K.; Fishbein, T. Intestinal Transplant Registry Report: Global Activity and Trends. Am. J. Transplant. 2015, 15, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, C.S.; Kaufman, S.S.; Fishbein, T.M. Inclusion of the Colon in Intestinal Transplantation. Curr. Opin. Organ Transplant. 2011, 16, 312–315. [Google Scholar] [CrossRef]
- Reyes, J.; Tzakis, A.G.; Todo, S.; Nour, B.; Starzl, T.E. Small Bowel and Liver/Small Bowel Transplantation in Children. Semin. Pediatr. Surg. 1993, 2, 289–300. [Google Scholar]
- Raghu, V.K.; Beaumont, J.L.; Everly, M.J.; Venick, R.S.; Lacaille, F.; Mazariegos, G.V. Pediatric Intestinal Transplantation: Analysis of the Intestinal Transplant Registry. Pediatr. Transplant. 2019, 23, e13580. [Google Scholar] [CrossRef]
- Levin, D. Bench to Bedside: Approaches for Engineered Intestine, Esophagus, and Colon. Gastroenterol. Clin. N. Am. 2019, 48, 607–623. [Google Scholar] [CrossRef]
- Meran, L.; Massie, I.; Campinoti, S.; Weston, A.E.; Gaifulina, R.; Tullie, L.; Faull, P.; Orford, M.; Kucharska, A.; Baulies, A.; et al. Engineering Transplantable Jejunal Mucosal Grafts Using Patient-Derived Organoids from Children with Intestinal Failure. Nat. Med. 2020, 26, 1593–1601. [Google Scholar] [CrossRef]
- Goulet, O.; Baglin-Gobet, S.; Talbotec, C.; Fourcade, L.; Colomb, V.; Sauvat, F.; Jais, J.P.; Michel, J.L.; Jan, D.; Ricour, C. Outcome and Long-Term Growth after Extensive Small Bowel Resection in the Neonatal Period: A Survey of 87 Children. Eur. J. Pediatr. Surg. 2005, 15, 95–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tullie, L.; Jones, B.C.; De Coppi, P.; Li, V.S.W. Building Gut from Scratch—Progress and Update of Intestinal Tissue Engineering. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Wengerter, B.C.; Emre, G.; Park, J.Y.; Geibel, J. Three-Dimensional Printing in the Intestine. Clin. Gastroenterol. Hepatol. 2016, 14, 1081–1085. [Google Scholar] [CrossRef]
- Zakhem, E.; Raghavan, S.; Suhar, R.A.; Bitar, K.N. Bioengineering and Regeneration of Gastrointestinal Tissue: Where Are We Now and What Comes Next? Expert Opin. Biol. Ther. 2019, 19, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Bernier-Latmani, J.; Petrova, T.V. Intestinal Lymphatic Vasculature: Structure, Mechanisms and Functions. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 510–526. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Huang, Y.; Li, K.; Fan, Y.; Xie, H.; Li, X. Small Intestinal Submucosa: Superiority, Limitations and Solutions, and Its Potential to Address Bottlenecks in Tissue Repair. J. Mater. Chem. B 2019, 7, 5038–5055. [Google Scholar] [CrossRef]
- Qin, H.H.; Dunn, J.C.Y. Small Intestinal Submucosa Seeded with Intestinal Smooth Muscle Cells in a Rodent Jejunal Interposition Model. J. Surg. Res. 2011, 171, e21–e26. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.K.; Badylak, S.F. Small Bowel Tissue Engineering Using Small Intestinal Submucosa as a Scaffold. J. Surg. Res. 2001, 99, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Q.; Watanabe, Y.; Toki, A. Experimental Assessment of Small Intestinal Submucosa as a Small Bowel Graft in a Rat Model. J. Pediatr. Surg. 2003, 38, 1596–1601. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Watanabe, Y.; Noda, T.; Yoshida, A.; Oyama, T.; Toki, A. Morphologic Evaluation of Regenerated Small Bowel by Small Intestinal Submucosa. J. Pediatr. Surg. 2005, 40, 1898–1902. [Google Scholar] [CrossRef]
- Nakao, M.; Ueno, T.; Oga, A.; Kuramitsu, Y.; Nakatsu, H.; Oka, M. Proposal of Intestinal Tissue Engineering Combined with Bianchi’s Procedure. J. Pediatr. Surg. 2015, 50, 573–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirbilek, S.; Kanmaz, T.; Özardali, I.; Edali, M.N.; Yücesan, S. Using Porcine Small Intestinal Submucosa in Intestinal Regeneration. Pediatr. Surg. Int. 2003, 19, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, G.Y.; Xiao, Y.P.; Fan, L.Y.; Wang, Q. The Biomechanical Behavior and Host Response to Porcine-Derived Small Intestine Submucosa, Pericardium and Dermal Matrix Acellular Grafts in a Rat Abdominal Defect Model. Biomaterials 2011, 32, 7086–7095. [Google Scholar] [CrossRef] [PubMed]
- Hussey, G.S.; Keane, T.J.; Badylak, S.F. The Extracellular Matrix of the Gastrointestinal Tract: A Regenerative Medicine Platform. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Saldin, L.T.; Cramer, M.C.; Velankar, S.S.; White, L.J.; Badylak, S.F. Extracellular Matrix Hydrogels from Decellularized Tissues: Structure and Function. Acta Biomater. 2017, 49, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDevitt, C.A.; Wildey, G.M.; Cutrone, R.M. Transforming Growth Factor-Β1 in a Sterilized Tissue Derived from the Pig Small Intestine Submucosa. J. Biomed. Mater. Res.-Part A 2003, 67, 637–640. [Google Scholar] [CrossRef]
- Hussey, G.S.; Cramer, M.C.; Badylak, S.F. Extracellular Matrix Bioscaffolds for Building Gastrointestinal Tissue. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nowocin, A.K.; Southgate, A.; Shurey, S.; Sibbons, P.; Gabe, S.M.; Ansari, T. The Development and Implantation of a Biologically Derived Allograft Scaffold. J. Tissue Eng. Regen. Med. 2016, 10, 140–148. [Google Scholar] [CrossRef]
- Zakhem, E.; Elbahrawy, M.; Orlando, G.; Bitar, K.N. Successful Implantation of an Engineered Tubular Neuromuscular Tissue Composed of Human Cells and Chitosan Scaffold. Surgery 2015, 158, 1598–1608. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Khalil, H.A.; Lei, N.Y.; Wang, Q.; Wang, K.; Wu, B.M.; Dunn, J.C.Y. Bioengineering Functional Smooth Muscle with Spontaneous Rhythmic Contraction in Vitro. Sci. Rep. 2018, 8, 13544. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, K.; Solorzano-Vargas, R.S.; Lin, P.-Y.; Walthers, C.M.; Thomas, A.-L.; Martín, M.G.; Dunn, J.C.Y. Bioengineered Intestinal Muscularis Complexes with Long-Term Spontaneous and Periodic Contractions. PLoS ONE 2018, 13, e0195315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Guo, C.; Manousiouthakis, E.; Wang, X.; Cairns, D.M.; Roh, T.T.; Du, C.; Kaplan, D.L. Bi-Layered Tubular Microfiber Scaffolds as Functional Templates for Engineering Human Intestinal Smooth Muscle Tissue. Adv. Funct. Mater. 2020, 30, 2000543. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Gilmont, R.R.; Bitar, K.N. Neuroglial Differentiation of Adult Enteric Neuronal Progenitor Cells as a Function of Extracellular Matrix Composition. Biomaterials 2013, 34, 6649–6658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbani, L.; Camilli, C.; Phylactopoulos, D.-E.; Crowley, C.; Natarajan, D.; Scottoni, F.; Maghsoudlou, P.; McCann, C.J.; Pellegata, A.F.; Urciuolo, A.; et al. Multi-Stage Bioengineering of a Layered Oesophagus with in Vitro Expanded Muscle and Epithelial Adult Progenitors. Nat. Commun. 2018, 9, 4286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olayanju, A.; Jones, L.; Greco, K.; Goldring, C.E.; Ansari, T. Application of Porcine Gastrointestinal Organoid Units as a Potential in Vitro Tool for Drug Discovery and Development. J. Appl. Toxicol. 2019, 39, 4–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, G.; Manfrin, A.; Lutolf, M.P. Progress and Potential in Organoid Research. Nat. Rev. Genet. 2018, 19, 671–687. [Google Scholar] [CrossRef]
- Sugimoto, S.; Kobayashi, E.; Fujii, M.; Ohta, Y.; Arai, K.; Matano, M.; Ishikawa, K.; Miyamoto, K.; Toshimitsu, K.; Takahashi, S.; et al. An Organoid-Based Organ-Repurposing Approach to Treat Short Bowel Syndrome. Nature 2021, 592, 99–104. [Google Scholar] [CrossRef]
- Grant, C.N.; Mojica, S.G.; Sala, F.G.; Ryan Hill, J.; Levin, D.E.; Speer, A.L.; Barthel, E.R.; Shimada, H.; Zachos, N.C.; Grikscheit, T.C. Human and Mouse Tissue-Engineered Small Intestine Both Demonstrate Digestive and Absorptive Function. Am. J. Physiol.-Gastrointest. Liver Physiol. 2015, 308, G664–G677. [Google Scholar] [CrossRef] [Green Version]
- Grover, H.; Spatarelu, C.-P.; De’De’, K.; Zhao, S.; Yang, K.; Shrike Zhang, Y.; Chen, Z. Vascularization in 3D Printed Tissues: Emerging Technologies to Overcome Longstanding Obstacles. AIMS Cell Tissue Eng. 2018, 2, 163–184. [Google Scholar] [CrossRef]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization Strategies for Tissue Engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Xu, Z.; Ren, J. Small Intestinalization of Colon Using Ileum Organoids. Trends Cell Biol. 2021, 31, 517–519. [Google Scholar] [CrossRef] [PubMed]




| Children | Adults | |
|---|---|---|
| Congenital | Jejunal atresia Ileal atresia | - |
| Acquired (extensive small bowel resection) | Necrotizing enterocolitis Malrotation with midgut volvulus Gastroschisis Extensive aganglionosis Trauma | Crohn’s disease Catastrophic mesenteric events (arterial embolism, venous thrombosis) Volvulus Trauma Adhesive obstruction Malignancies Radiation enteritis |
| Functional SBS in severe malabsorption (bowel length intact) | Chronic intestinal pseudo-obstruction syndrome Refractory sprue Radiation enteritis Congenital villous atrophy | |
| Method | First Description (Year) | Advantages | Disadvantages | Technical Difficulty * | Human Models | Success Rate | Evidence |
|---|---|---|---|---|---|---|---|
| Procedures increasing small intestinal length | |||||||
| Serial transverse enteroplasty procedure | 2003 [79] | Technically easy, reSTEP possible | Bleeding from staples might be difficult to control | + | Several | 45% PN weanings | Case series |
| Longitudinal intestinal lengthening and tailoring procedure | 1980 [99] | A lot of experience, avoidance of staplers | Manipulation of mesentery, three anastomoses, technically demanding, no second LILT after primary LILT possible | +++ | Several | 169 of 324 (52%) weanings from PN | Case series |
| Modification of LILTs with one anastomosis | 1991 [106] | Only one anastomosis needed | Manipulation of mesentery, technically demanding, no second LILT after primary LILT possible, lack of experience | +++ | Two patients with no reported outcomes | Unknown | Case reports |
| Double barrel enteroplasty | 2008 [123] | Less traction on the mesenteric vessels than LILT | Manipulation of mesentery, technically demanding, no second LILT after primary LILT possible, lack of experience | +++ | Ten patients from a single institution | 5/10 weanings after 39 months, no deaths | Case series |
| Kimura Iowa procedure | 1990 [124] | Possible if mesenteric blood supply is not mobile enough for other approaches | Technically demanding, requires at least two operations, lack of experience | ++++ | One patient | 50–60% of daily caloric intake via the enteric route after 18 months | Case report |
| Spiral intestinal lengthening and tailoring | 2011 [126] | Less mesenteric manipulation of mesentery than LILT, only limited bowel dilation necessary | Mesenteric manipulation, long anastomosis, limited lengthening, low evidence | +++ | <10 cases | No major complications after a median follow-up of 26 months | Case series |
| Transverse flap duodenoplasty | 2018 [133] | Possible for duodenum | Limited lengthening, low evidence, technically demanding, possible injury to Ampulla of Vater | +++ | One 2-month-old child | Enteral feeding accounting for 54% of caloric intake | Case report |
| Composite ileo-colic loop | 1996 [134] | Enables LILT procedure in non-dilated small bowel | Technically demanding, requires at least two operations, lack of experience, colon needs to be present | ++++ | Only pig models | / | Animal models |
| Mechanical distraction | 1997 [135] | Increases length and surface area without the need for dilated bowel | Use of foreign material, one- or two-stage procedure, spring may obstruct or perforate small bowel | ++ | Pig and rodent models | / | Animal models |
| Small bowel transplantation | 1959 [179] | Last choice, does not depend on bowel length/dilation | Immunosupression, high mortality, technically demanding | ++++ | Several | Patient survival rates were reported as 76%, 56% and 43% at 1, 5 and 10 years | Case series |
| Procedures slowing down intestinal transit without bowel lengthening | |||||||
| Antiperistaltic small intestinal segment | 1962 [155] | Increases transit time, technically easy | Small effect, low evidence | + | Several | 38 adult patients, 17 weanings (45%), deaths 16% after 5 years follow-up | Case series |
| Colon interposition | 1984 [166] | Technically easy, may be used in cases with very short, small bowel | Colon needs to be present, low evidence, three anastomoses | + | Six infants | 3/6 weaning from PN | Case series |
| Intestinal valves and sphincters | 1994 [167] | Dilation of small intestine with possibility to perform LILT/STEP/other lengthening procedures later | Intestinal stasis and bacterial overgrowth, low evidence, possible intestinal obstruction | + | Few cases | Acceptable surgical outcome | Case reports |
| Recirculating bowel loops | 1965 [170] | Increases transit time, technically easy | Small effect, low evidence, intestinal stasis and bacterial overgrowth | ++ | Three adult cases | Outcomes were reported as successful and favor- able | Case reports |
| Procedures improving small intestinal motility without bowel lengthening | |||||||
| Tailoring and plication | 1983 [176] | Improvement of motility, technically easy | Low evidence, needs to be weighed against lengthening procedures (LILT, STEP, etc.) | + | Twelve infants | Mucosa was retained, no obstructions were observed and most intestinal segments appeared normal | Case series |
| Modified antimesenteric tapering enteroplasty | 2021 [178] | Reduces stasis, technically easy | long anastomosis, low evidence | + | In four adult patients | 3/4 were able to wean of PN | Case series |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muff, J.L.; Sokolovski, F.; Walsh-Korb, Z.; Choudhury, R.A.; Dunn, J.C.Y.; Holland-Cunz, S.G.; Vuille-dit-Bille, R.N. Surgical Treatment of Short Bowel Syndrome—The Past, the Present and the Future, a Descriptive Review of the Literature. Children 2022, 9, 1024. https://doi.org/10.3390/children9071024
Muff JL, Sokolovski F, Walsh-Korb Z, Choudhury RA, Dunn JCY, Holland-Cunz SG, Vuille-dit-Bille RN. Surgical Treatment of Short Bowel Syndrome—The Past, the Present and the Future, a Descriptive Review of the Literature. Children. 2022; 9(7):1024. https://doi.org/10.3390/children9071024
Chicago/Turabian StyleMuff, Julian L., Filipp Sokolovski, Zarah Walsh-Korb, Rashikh A. Choudhury, James C. Y. Dunn, Stefan G. Holland-Cunz, and Raphael N. Vuille-dit-Bille. 2022. "Surgical Treatment of Short Bowel Syndrome—The Past, the Present and the Future, a Descriptive Review of the Literature" Children 9, no. 7: 1024. https://doi.org/10.3390/children9071024
APA StyleMuff, J. L., Sokolovski, F., Walsh-Korb, Z., Choudhury, R. A., Dunn, J. C. Y., Holland-Cunz, S. G., & Vuille-dit-Bille, R. N. (2022). Surgical Treatment of Short Bowel Syndrome—The Past, the Present and the Future, a Descriptive Review of the Literature. Children, 9(7), 1024. https://doi.org/10.3390/children9071024






