A Case of X-Linked Hypophosphatemic Rickets with Dentin Dysplasia in Mandibular Third Molars
Abstract
:1. Introduction
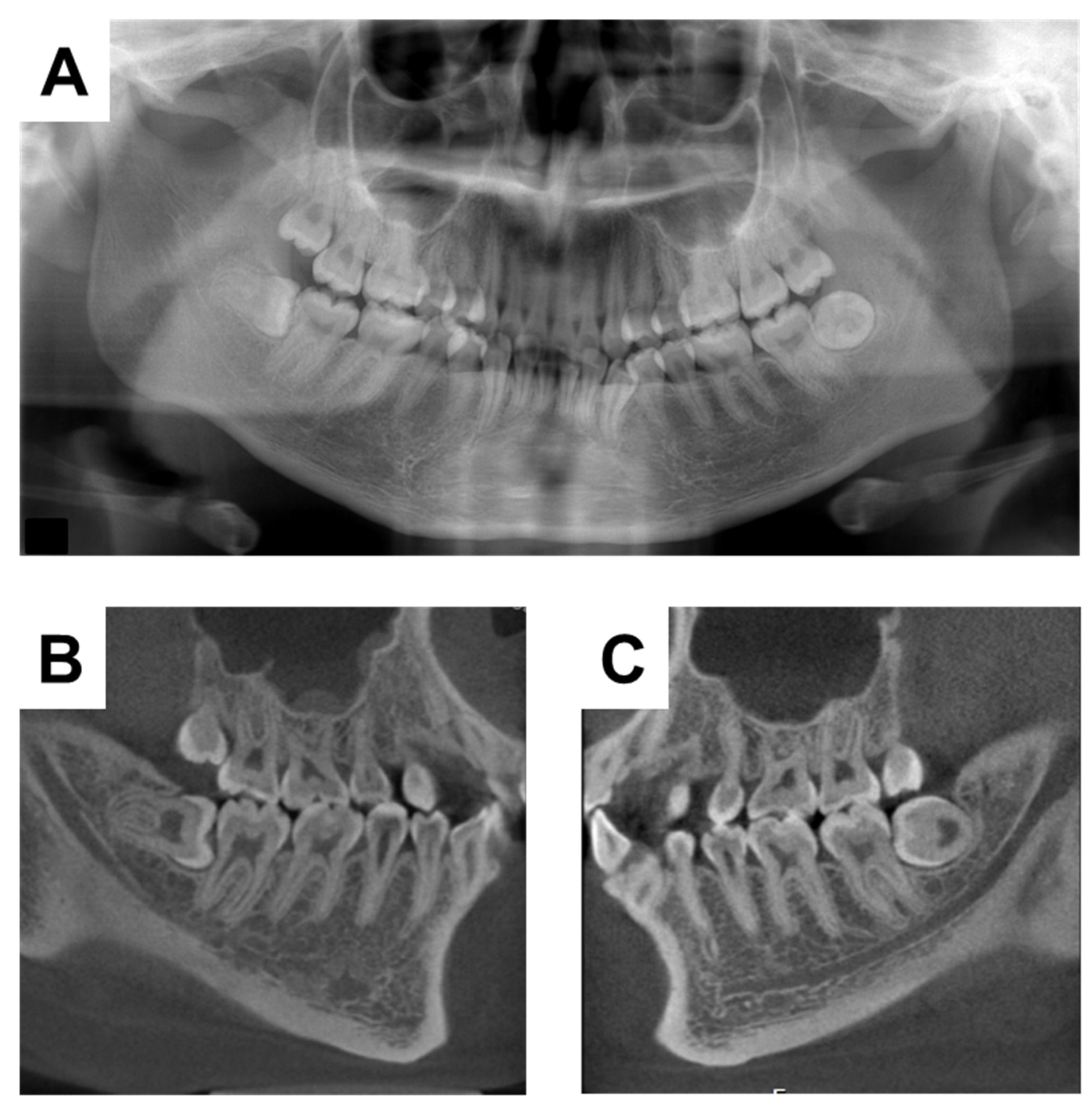
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beck-Nielsen, S.S.; Brock-Jacobsen, B.; Gram, J.; Brixen, K.; Jensen, T.K. Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur. J. Endocrinol. 2009, 160, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Endo, I.; Fukumoto, S.; Ozono, K.; Namba, N.; Inoue, D.; Okazaki, R.; Yamauchi, M.; Sugimoto, T.; Minagawa, M.; Michigami, T.; et al. Nationwide survey of fibroblast growth factor 23 (FGF23)-related hypophosphatemic diseases in Japan: Prevalence, biochemical data and treatment. Endocr. J. 2015, 62, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Rafaelsen, S.; Johansson, S.; Ræder, H.; Bjerknes, R. Hereditary hypophosphatemia in Norway: A retrospective population-based study of genotypes, phenotypes, and treatment complications. Eur. J. Endocrinol. 2016, 174, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Baroncelli, G.I.; Mora, S. X-Linked Hypophosphatemic Rickets: Multisystemic Disorder in Children Requiring Multidisciplinary Management. Front. Endocrinol. 2021, 12, 688309. [Google Scholar] [CrossRef]
- Al Juraibah, F.; Al Amiri, E.; Al Dubayee, M.; Al Jubeh, J.; Al Kandari, H.; Al Sagheir, A.; Al Shaikh, A.; Beshyah, S.A.; Deeb, A.; Habeb, A.; et al. Diagnosis and management of X-linked hypophosphatemia in children and adolescent in the Gulf Cooperation Council countries. Arch. Osteoporos. 2021, 16, 52. [Google Scholar] [CrossRef]
- Onishi, T.; Ogawa, T.; Hayashibara, T.; Hoshino, T.; Okawa, R.; Ooshima, T. Hyper-expression of osteocalcin mRNA in odontoblasts of Hyp mice. J. Dent. Res. 2005, 84, 84–88. [Google Scholar] [CrossRef]
- Zhang, H.; Chavez, M.B.; Kolli, T.N.; Tan, M.H.; Fong, H.; Chu, E.Y.; Li, Y.; Ren, X.; Watanabe, K.; Kim, D.G.; et al. Dentoalveolar Defects in the Hyp Mouse Model of X-linked Hypophosphatemia. J. Dent. Res. 2020, 99, 419–428. [Google Scholar] [CrossRef]
- Murayama, T.; Iwatsubo, R.; Akiyama, S.; Amano, A.; Morisaki, I. Familial hypophosphatemic vitamin D-resistant rickets: Dental findings and histologic study of teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000, 90, 310–316. [Google Scholar] [CrossRef]
- Wato, K.; Okawa, R.; Matayoshi, S.; Ogaya, Y.; Nomura, R.; Nakano, K. X-linked hypophosphatemia diagnosed after identification of dental symptoms. Pediatr. Dent. J. 2020, 30, 115–119. [Google Scholar] [CrossRef]
- Sabandal, M.M.; Robotta, P.; Bürklein, S.; Schäfer, E. Review of the dental implications of X-linked hypophosphataemic rickets (XLHR). Clin. Oral Investig. 2015, 19, 759–768. [Google Scholar] [CrossRef]
- Goodman, J.R.; Gelbier, M.J.; Bennett, J.H.; Winter, G.B. Dental problems associated with hypophosphataemic vitamin D resistant rickets. Int. J. Paediatr. Dent. 1998, 8, 19–28. [Google Scholar] [CrossRef]
- Chaussain-Miller, C.; Sinding, C.; Wolikow, M.; Lasfargues, J.J.; Godeau, G.; Garabédian, M. Dental abnormalities in patients with familial hypophosphatemic vitamin D-resistant rickets: Prevention by early treatment with 1-hydroxyvitamin D. J. Pediatr. 2003, 142, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Baroncelli, G.I.; Angiolini, M.; Ninni, E.; Galli, V.; Saggese, R.; Giuca, M.R. Prevalence and pathogenesis of dental and periodontal lesions in children with X-linked hypophosphatemic rickets. Eur. J. Paediatr. Dent. 2006, 7, 61–66. [Google Scholar]
- Baroncelli, G.I.; Zampollo, E.; Manca, M.; Toschi, B.; Bertelloni, S.; Michelucci, A.; Isola, A.; Bulleri, A.; Peroni, D.; Giuca, M.R. Pulp chamber features, prevalence of abscesses, disease severity, and PHEX mutation in X-linked hypophosphatemic rickets. J. Bone Miner. Metab. 2021, 39, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Masatomi, Y.; Nakajima, Y.; Shintani, S.; Moriwaki, Y.; Sobue, S.; Ooshima, T. The occurrence of interglobular dentin in incisors of hypophosphatemic mice fed a high-calcium and high-phosphate diet. J. Dent. Res. 1992, 71, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Masatomi, Y.; Nakagawa, Y.; Kanamoto, Y.; Sobue, S.; Ooshima, T. Effects of serum phosphate level on formation of incisor dentine in hypophosphatemic mice. J. Oral Pathol. Med. 1996, 25, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Lira Dos Santos, E.J.; Chavez, M.B.; Tan, M.H.; Mohamed, F.F.; Kolli, T.N.; Foster, B.L.; Liu, E.S. Effects of Active Vitamin D or FGF23 Antibody on Hyp Mice Dentoalveolar Tissues. J. Dent. Res. 2021, 100, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Biosse Duplan, M.; Coyac, B.R.; Bardet, C.; Zadikian, C.; Rothenbuhler, A.; Kamenicky, P.; Briot, K.; Linglart, A.; Chaussain, C. Phosphate and Vitamin D Prevent Periodontitis in X-Linked Hypophosphatemia. J. Dent. Res. 2017, 96, 388–395. [Google Scholar] [CrossRef]
- Fukumoto, S.; Ozono, K.; Michigami, T.; Minagawa, M.; Okazaki, R.; Sugimoto, T.; Takeuchi, Y.; Matsumoto, T. Pathogenesis and diagnostic criteria for rickets and osteomalacia--proposal by an expert panel supported by the Ministry of Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research, and the Japan Endocrine Society. J. Bone Miner. Metab. 2015, 33, 467–473. [Google Scholar] [CrossRef]
- Kuremoto, K.; Okawa, R.; Matayoshi, S.; Kokomoto, K.; Nakano, K. Estimation of dental age based on the developmental stages of permanent teeth in Japanese children and adolescents. Sci. Rep. 2022, 12, 3345. [Google Scholar] [CrossRef]
- Bitzan, M.; Goodyer, P.R. Hypophosphatemic Rickets. Pediatr. Clin. N. Am. 2019, 66, 179–207. [Google Scholar] [CrossRef] [PubMed]
- Holm, I.A.; Nelson, A.E.; Robinson, B.G.; Mason, R.S.; Marsh, D.J.; Cowell, C.T.; Carpenter, T.O. Mutational analysis and genotype-phenotype correlation of the PHEX gene in X-linked hypophosphatemic rickets. J. Clin. Endocrinol. Metab. 2001, 86, 3889–3899. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, E.; Zou, M.; Al-Rijjal, R.A.; Baitei, E.Y.; Hammami, S.; Bircan, I.; Akçurin, S.; Meyer, B.; Shi, Y. Novel and de novo PHEX mutations in patients with hypophosphatemic rickets. Bone 2013, 52, 286–291. [Google Scholar] [CrossRef] [PubMed]





| Item | Abbreviation | Value | Standard Value |
|---|---|---|---|
| Inorganic phosphorus | IP | 2.1 mg/dL | 2.9–4.8 mg/dL |
| Calcium | Ca | 10.1 mg/dL | 8.6–10.3 mg/dL |
| Alkaline phosphatase | ALP | 113 U/L | 38–113 U/L |
| Aspartate aminotransferase | AST | 26 U/L | ≦40 |
| Alanine aminotransferase | ALT | 35 U/L | ≦40 |
| Albmin | Alb | 4.4 g/dL | 3.6–4.7 g/dL |
| Urea nitrogen | UN | 14 mg/dL | 7–22 mg/dL |
| Creatinin | Cre | 0.57 mg/dL | 0.5–0.9 mg/dL |
| e glomerular filtration rate creat | eGFR creat | 112.2 | |
| 25-hydroxyvitamin D3 | 25-(OH)D3 | 14 ng/mL | 20–60 ng/mL |
| 1,25-dihydroxyvitamin D3 | 1,25-(OH)2D3 | 61 pg/mL | 61 pg/mL |
| Parathyroid hormone | PTH | 85.8 pg/mL | <20 |
| Fibrosis 4 index | FiB4 index | 0.38 |
| Upper | 7.0 | 6.8 | 6.9 | 6.8 | 7.2 | 6.7 | 6.8 | 6.8 | 5.6 | 6.8 | 6.8 | 6.9 | 6.8 | 7.0 |
| Tooth | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Lower | 6.2 | 6.3 | 6.4 | 5.4 | 5.6 | 5.7 | 6.5 | 6.5 | 6.4 | 5.6 | 5.4 | 6.4 | 6.3 | 6.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okawa, R.; Hamada, M.; Takagi, M.; Matayoshi, S.; Nakano, K. A Case of X-Linked Hypophosphatemic Rickets with Dentin Dysplasia in Mandibular Third Molars. Children 2022, 9, 1304. https://doi.org/10.3390/children9091304
Okawa R, Hamada M, Takagi M, Matayoshi S, Nakano K. A Case of X-Linked Hypophosphatemic Rickets with Dentin Dysplasia in Mandibular Third Molars. Children. 2022; 9(9):1304. https://doi.org/10.3390/children9091304
Chicago/Turabian StyleOkawa, Rena, Masakazu Hamada, Misato Takagi, Saaya Matayoshi, and Kazuhiko Nakano. 2022. "A Case of X-Linked Hypophosphatemic Rickets with Dentin Dysplasia in Mandibular Third Molars" Children 9, no. 9: 1304. https://doi.org/10.3390/children9091304
APA StyleOkawa, R., Hamada, M., Takagi, M., Matayoshi, S., & Nakano, K. (2022). A Case of X-Linked Hypophosphatemic Rickets with Dentin Dysplasia in Mandibular Third Molars. Children, 9(9), 1304. https://doi.org/10.3390/children9091304






