Adaptation of a High-Pressure Liquid Chromatography System for the Measurement of Viscosity
Abstract
:1. Introduction
2. Materials and Methods
2.1. High Pressure Liquid Chromatography (HPLC)
2.2. Materials
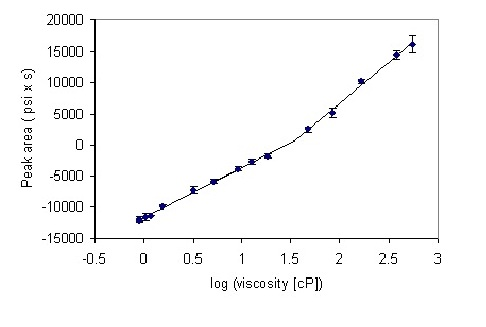
3. Results and Discussion
3.1. Optimization of Mobile Phase
3.2. Digital Removal of Pump Pulse Spikes


| % (w/w) | B | D | η25 °C (cP) |
|---|---|---|---|
| 0 | 64.526 | 0.096595 | 0.89 * |
| 10 | 64.526 | 0.094838 | 1.06 |
| 20 | 63.716 | 0.093106 | 1.42 |
| 30 | 62.710 | 0.090872 | 1.99 |
| 40 | 61.528 | 0.088231 | 2.95 |
| 50 | 60.012 | 0.084710 | 4.71 |
| 60 | 57.988 | 0.079863 | 8.41 |
| 65 | 56.804 | 0.077022 | 11.8 |
| 67 | 56.256 | 0.075692 | 13.7 |
| 70 | 55.355 | 0.073466 | 17.4 |
| 75 | 53.650 | 0.069230 | 27.0 |
| 80 | 51.654 | 0.064246 | 45.0 |
| 85 | 49.415 | 0.058684 | 80.4 |
| 90 | 46.630 | 0.051654 | 160 |
| 91 | 46.037 | 0.050186 | 187 |
| 92 | 45.333 | 0.048385 | 221 |
| 93 | 44.796 | 0.047127 | 260 |
| 94 | 44.206 | 0.045713 | 308 |
| 95 | 43.503 | 0.043944 | 367 |
| 96 | 42.864 | 0.042414 | 440 |
| 97 | 42.166 | 0.040700 | 531 |
| 98 | 41.435 | 0.038918 | 648 |
| 99 | 40.742 | 0.037274 | 794 |
| 100 | 40.075 | 0.035730 | 976 |

3.3. Measurement of Vaccine and Protein Samples

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dogan, H.; Kokini, J.L. Handbook of Food Engineering, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 1–124. [Google Scholar]
- Connelly, R.K.; Kokini, J.L. Mixing simulation of a viscous Newtonian liquid in a twin sigma blade mixer. AIChE J. 2006, 52, 3383–3393. [Google Scholar] [CrossRef]
- Landauer, O.; Mateescu, C.; Iulian, O.; Geana, D. Models for the viscosity of liquid mixtures—Application to the binary-system water-dioxan. Rev. Roum. Chim. 1987, 32, 1129–1139. [Google Scholar]
- Hayduk, W.; Cheng, S.C. Review of relation between diffusivity and solvent viscosity in dilute liquid solutions. Chem. Eng. Sci. 1971, 26, 635–646. [Google Scholar] [CrossRef]
- Monkos, K. Determination of some hydrodynamic parameters of ovine serum albumin solutions using viscometric measurements. J. Biol.Phys. 2005, 31, 219–232. [Google Scholar] [CrossRef]
- Salinas, B.A.; Sathish, H.A.; Bishop, S.M.; Harn, N.; Carpenter, J.F.; Randolph, T.W. Understanding and modulating opalescence and viscosity in a monoclonal antibody formulation. J. Pharm. Sci. 2010, 99, 82–93. [Google Scholar] [CrossRef]
- Shire, S.J.; Shahrokh, Z.; Liu, J. Challenges in the development of high protein concentration formulations. J. Pharm. Sci. 2004, 93, 1390–1402. [Google Scholar] [CrossRef]
- Saluja, A.; Badkar, A.V.; Zeng, D.L.; Nema, S.; Kalonia, D.S. Application of high-frequency rheology measurements for analyzing protein–protein interactions in high protein concentration solutions using a model monoclonal antibody (IgG2). J. Pharm. Sci. 2006, 95, 1967–1983. [Google Scholar] [CrossRef]
- Chari, R.; Jerath, K.; Badkar, A.V.; Kalonia, D.S. Long- and short-range electrostatic interactions affect the rheology of highly concentrated antibody solutions. Pharm. Res. 2009, 26, 2607–2618. [Google Scholar] [CrossRef]
- Zimmer, H.; Osterr, A. Viscosity and viscosity measurements. Chem. U. Tech. Ztg. 1932, 50, 47–50. [Google Scholar]
- Bee, J.S.; Stevenson, J.L.; Mehta, B.; Svitel, J.; Pollastrini, J.; Platz, R.; Freund, E.; Carpenter, J.F.; Randolph, T.W. Response of a concentrated monoclonal antibody formulation to high shear. Biotechnol. Bioeng. 2009, 103, 936–943. [Google Scholar] [CrossRef]
- Reardon, P.T.; Graham, A.L.; Feng, S.; Chawla, V.; Admuthe, R.S.; Mondy, L.A. Non-Newtonian end effects in falling ball viscometry of concentrated suspensions. Rheol. Acta 2007, 46, 413–424. [Google Scholar]
- He, F.; Becker, G.W.; Litowski, J.R.; Narhi, L.O.; Brems, D.N.; Razinkov, V.I. High-throughput dynamic light scattering method for measuring viscosity of concentrated protein solutions. Anal. Biochem. 2010, 399, 141–143. [Google Scholar] [CrossRef]
- Sharma, V.K.; Kalonia, D.S. Effect of vacuum drying on protein-mannitol interactions: The physical state of mannitol and protein structure in the dried state. AAPS Pharm. Sci. Tech. 2004, 5. [Google Scholar] [CrossRef]
- Carpenter, J.F.; Randolph, T.W.; Jiskoot, W.; Crommelin, D.J.A.; Middaugh, C.R.; Winter, G.; Fan, Y.-X.; Kirshner, S.; Verthelyi, D.; Kozlowski, S.; et al. Overlooking subvisible particles in therapeutic protein products: Gaps that may compromise product quality. J. Pharm. Sci. 2009, 98, 1201–1205. [Google Scholar] [CrossRef]
- Chang, N.-S. Formula for the viscosity of a glycerol−water mixture. Ind. Eng. Chem. Res. 2008, 47, 3285–3288. [Google Scholar] [CrossRef]
- Dow-Chemical-Company. OPTIM Glycerine. Available online: http://www.dow.com/glycerine/resources/table18.htm (accessed on 12 February 2014).
- Lide, D.R. Viscosity in CRC Handbook of Chemistry and Physics, 78th ed.; Lide, D.R., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 6–200. [Google Scholar]
- Monkos, K.; Turczynski, B. A comparative study on viscosity of human, bovine and pig IgG immunoglobulins in aqueous solutions. Int. J. Biol. Macromol. 1999, 26, 155–159. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gregory, S.; Mach, H. Adaptation of a High-Pressure Liquid Chromatography System for the Measurement of Viscosity. Chromatography 2014, 1, 55-64. https://doi.org/10.3390/chromatography1020055
Gregory S, Mach H. Adaptation of a High-Pressure Liquid Chromatography System for the Measurement of Viscosity. Chromatography. 2014; 1(2):55-64. https://doi.org/10.3390/chromatography1020055
Chicago/Turabian StyleGregory, Sonia, and Henryk Mach. 2014. "Adaptation of a High-Pressure Liquid Chromatography System for the Measurement of Viscosity" Chromatography 1, no. 2: 55-64. https://doi.org/10.3390/chromatography1020055
APA StyleGregory, S., & Mach, H. (2014). Adaptation of a High-Pressure Liquid Chromatography System for the Measurement of Viscosity. Chromatography, 1(2), 55-64. https://doi.org/10.3390/chromatography1020055