Enhanced Nitrogen Removal from Domestic Wastewater by Partial-Denitrification/Anammox in an Anoxic/Oxic Biofilm Reactor
Abstract
1. Introduction
2. Materials and Methods
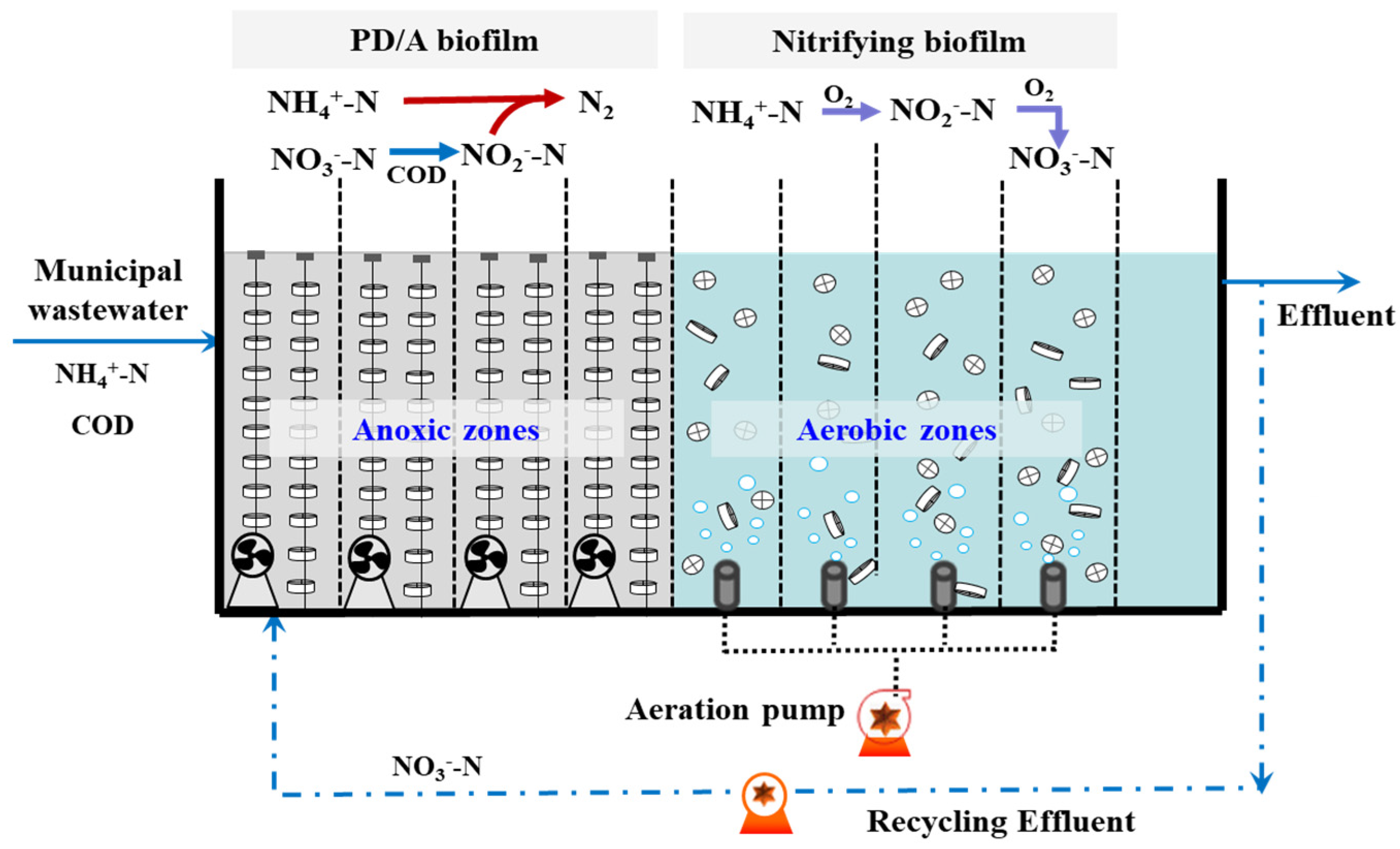
2.1. Reactor and Operation
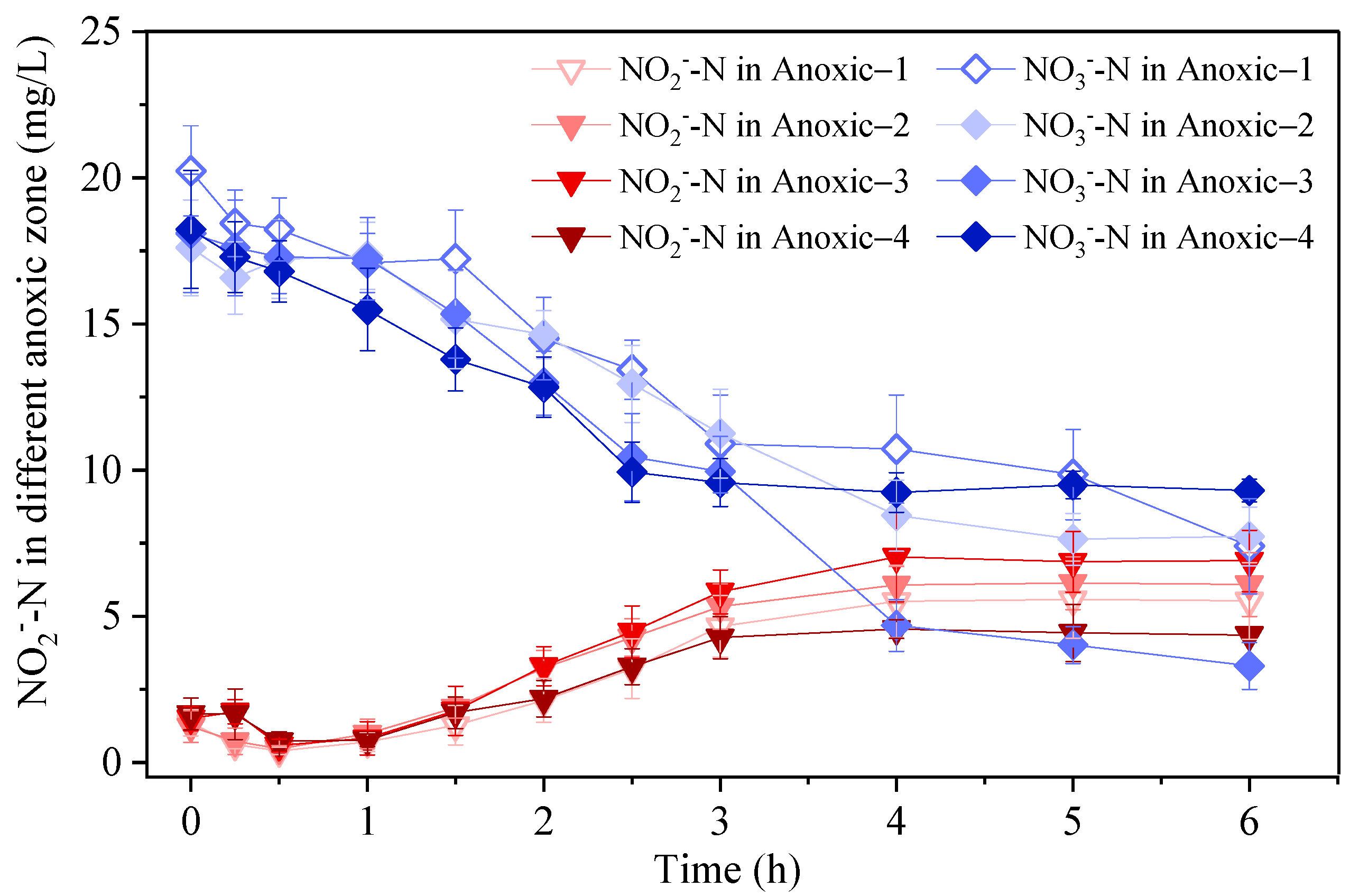
2.2. Batch Tests for NO3−-N Reduction and NO2−-N Accumulation Capacity of Anoxic Biofilms
2.3. Ex-situ Activity of Denitrification Coupling with Anammox
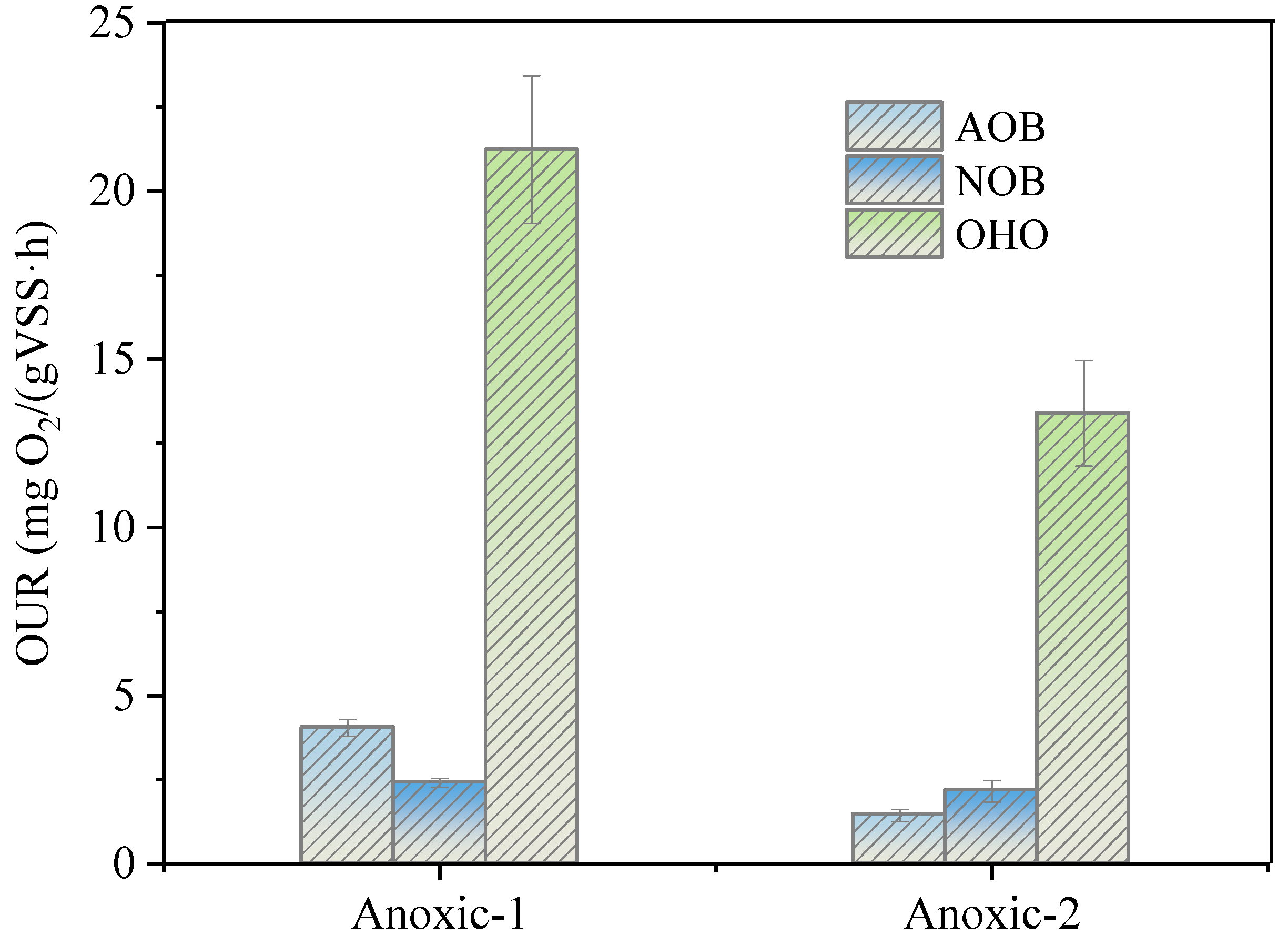
2.4. Oxygen Uptake Rate (OUR) of AOB, NOB, and Ordinary Heterotrophic Organisms (OHO)
2.5. Analytical Methods
2.6. Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.7. Calculations
3. Results and Discussion
3.1. Performance of A/O Biofilm System for Domestic Wastewater Treatment
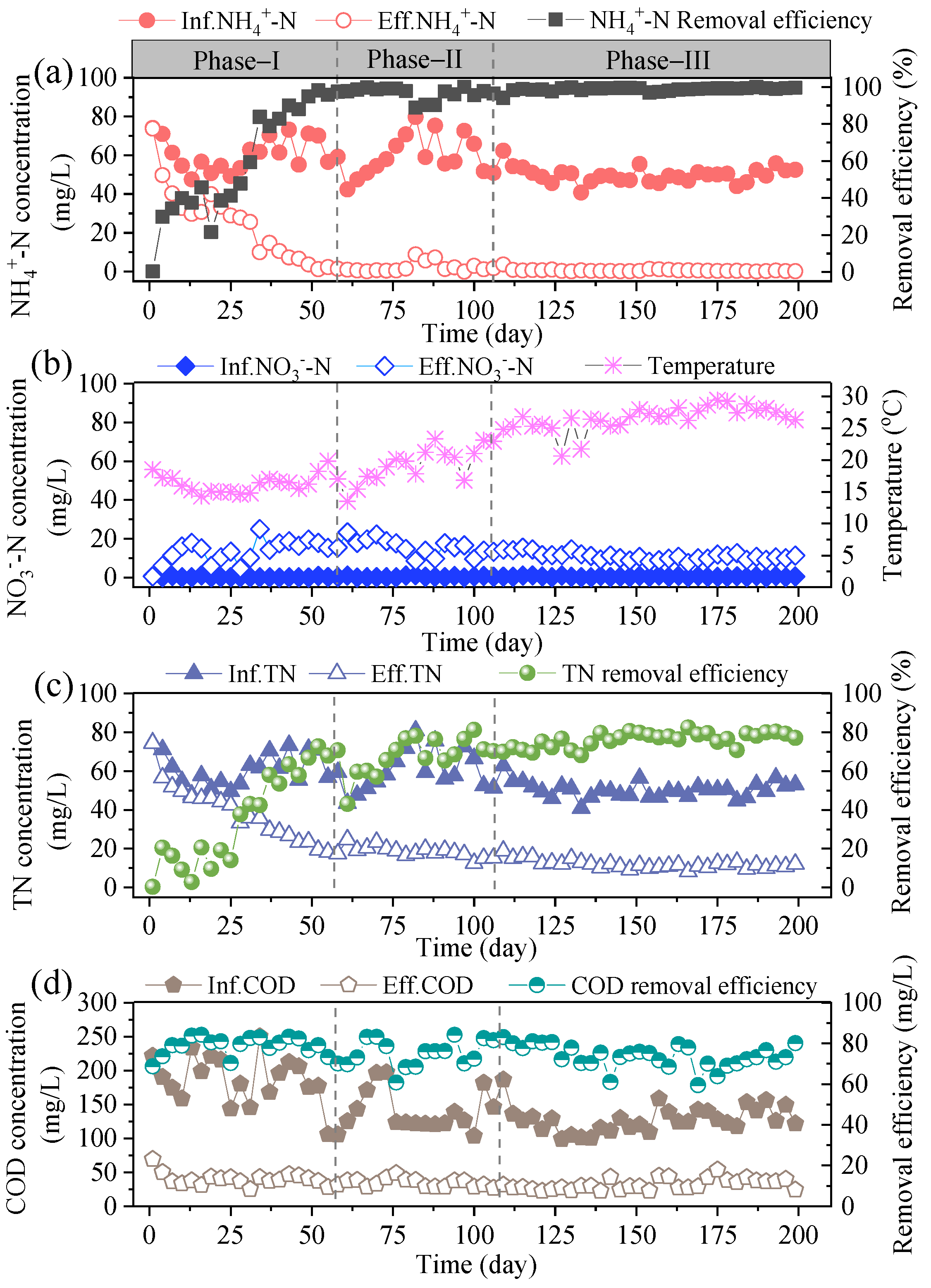
3.1.1. Start-Up and Biofilm Formation
3.1.2. NO2−-N Accumulation in Anoxic Zone
3.2. Development of Partial Denitrification/Anammox in A/O Biofilm System
3.3. Removal Pathway of NH4+-N in Anoxic Stage
3.4. COD Removal during Stable Operation
3.5. Effect of DO on Nitrogen Removal of A/O Biofilm System
3.6. Abundance of Functional Bacteria in PD/A Biofilm
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Deng, L.; Peng, Y.; Li, J.; Gao, R.; Li, W.; Du, R. Enhanced simultaneous nitrogen and phosphorus removal from low COD/TIN domestic wastewater through nitritation-denitritation coupling improved anammox process with an optimal Anaerobic/Oxic/Anoxic strategy. Bioresour. Technol. 2021, 322, 124526. [Google Scholar] [CrossRef] [PubMed]
- Keerio, H.A.; Bae, W.; Park, J.; Kim, M. Substrate uptake, loss, and reserve in ammonia-oxidizing bacteria (AOB) under different substrate availabilities. Process Biochem. 2020, 91, 303–310. [Google Scholar] [CrossRef]
- Ji, B. Towards environment-sustainable wastewater treatment and reclamation by the non-aerated microalgal-bacterial granular sludge process: Recent advances and future directions. Sci. Total Environ. 2022, 806, 150707. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, S.; Feng, X.; Fan, G.; Jetten, M.S.M.; Yin, C. Anammox Bacterial Abundance, Biodiversity and Activity in a Constructed Wetland. Environ. Sci. Technol. 2011, 45, 9951–9958. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Yan, W.; Yu, L.; Zhang, L.; Lay, W.; Zhou, Y. Challenges of THP-AD centrate treatment using partial nitritation-anammox (PN/A)—Inhibition, biomass washout, low alkalinity, recalcitrant and more. Water Res. 2021, 203, 117555. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Liang, Z.; Yang, Y.; Peng, Y.; Li, B.; Wang, S.; Qian, Z. Start-up of single-stage partial nitrification-anammox process treating low-strength swage and its restoration from nitrate accumulation. Bioresour. Technol. 2016, 218, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Zhang, L.; Yu, D.; Zhang, J.; Zhang, W.; Ma, G.; Zhao, X.; Peng, Y. Application of intermittent aeration in nitrogen removal process: Development, advantages and mechanisms. Chem. Eng. J. 2021, 430, 133184. [Google Scholar] [CrossRef]
- Cao, S.; Zhou, Y. New direction in biological nitrogen removal from industrial nitrate wastewater via anammox. Appl. Microbiol. Biotechnol. 2019, 109, 7459–7466. [Google Scholar] [CrossRef]
- Cao, S.; Wang, S.; Peng, Y.; Wu, C.; Du, R.; Gong, L.; Ma, B. Achieving partial denitrification with sludge fermentation liquid as carbon source: The effect of seeding sludge. Bioresour. Technol. 2013, 149, 570–574. [Google Scholar] [CrossRef]
- Du, R.; Peng, Y.; Cao, S.; Wang, S.; Niu, M. Characteristic of nitrous oxide production in partial denitrification process with high nitrite accumulation. Bioresour. Technol. 2016, 203, 341–347. [Google Scholar] [CrossRef]
- Cao, S.; Du, R.; Meng, N.; Li, B.; Ren, N.; Peng, Y. Integrated anaerobic ammonium oxidization with partial denitrification process for advanced nitrogen removal from high-strength wastewater. Bioresour. Technol. 2016, 221, 37. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Peng, Y.; Ji, J.; Shi, L.; Gao, R.; Li, X. Partial denitrification providing nitrite: Opportunities of extending application for anammox. Environ. Int. 2019, 131, 105001. [Google Scholar] [CrossRef]
- Du, R.; Cao, S.; Zhang, H.; Li, X.; Peng, Y. Flexible Nitrite Supply Alternative for Mainstream Anammox: Advances in Enhancing Process Stability. Environ. Sci. Technol. 2020, 54, 6353–6364. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Peng, Y.; Cao, S.; Wang, S.; Wu, C. Advanced nitrogen removal from wastewater by combining anammox with partial denitrification. Bioresour. Technol. 2015, 179, 497–504. [Google Scholar] [CrossRef]
- Ge, H.; Batstone, D.; Keller, J. Evaluation of anaerobic digestion processes for short sludge-age waste activated sludge combined with anammox treatment of digestate liquor. Water Sci. Technol. 2016, 73, 1052–1060. [Google Scholar] [CrossRef][Green Version]
- Winkler, M.K.; Yang, J.; Kleerebezem, R.; Plaza, E.; Trela, J.; Hultman, B.; van Loosdrecht, M.C. Nitrate reduction by organotrophic Anammox bacteria in a nitritation/anammox granular sludge and a moving bed biofilm reactor. Bioresour. Technol. 2012, 114, 217. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, Y.; Li, X.; Du, R. Feasibility of partial-denitrification/ anammox for pharmaceutical wastewater treatment in a hybrid biofilm reactor. Water Res. 2022, 208, 117856. [Google Scholar] [CrossRef]
- Malovanyy, A.; Yang, J.; Trela, J.; Plaza, E. Combination of upflow anaerobic sludge blanket (UASB) reactor and partial nitritation/anammox moving bed biofilm reactor (MBBR) for municipal wastewater treatment. Bioresour. Technol. 2015, 180, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.F.; Shi, Y.; Laureni, M.; Rosenthal, A.; Szivák, I.; Weissbrodt, D.G.; Joss, A.; Buergmann, H.; Johnson, D.R.; Morgenroth, E. Comparing Resistance, Resilience, and Stability of Replicate Moving Bed Biofilm and Suspended Growth Combined Nitritation-Anammox Reactors. Environ. Sci. Technol. 2017, 51, 5108. [Google Scholar] [CrossRef]
- Ferrai, M.; Guglielmi, G.; Andreottola, G. Modelling respirometric tests for the assessment of kinetic and stoichiometric parameters on MBBR biofilm for municipal wastewater treatment. Environ. Modell. Softw. 2010, 25, 626–632. [Google Scholar] [CrossRef]
- Xiao, G.Y.; Ganczarczyk, J. Structural features of biomass in a hybrid MBBR reactor. Environ. Technol. 2006, 27, 289–298. [Google Scholar] [CrossRef]
- Hedegärd, M.; Wik, T. An online method for estimation of degradable substrate and biomass in an aerated activated sludge process. Water Res. 2011, 45, 6308–6320. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.; Melo, L.F.; Purkhold, U.; Wuertz, S.; Wagner, M. Nitrifying and heterotrophic population dynamics in biofilm reactors: Effects of hydraulic retention time and the presence of organic carbon. Water Res. 2002, 36, 469–481. [Google Scholar] [CrossRef]
- Chen, Z.; Qiu, S.; Yu, Z.; Li, M.; Ge, S. Enhanced Secretions of Algal Cell-Adhesion Molecules and Metal Ion-Binding Exoproteins Promote Self-Flocculation of Chlorella sp. Cultivated in Municipal Wastewater. Environ. Sci. Technol. 2021, 55, 11916–11924. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, S.; Wang, W.; Wang, Y.; Zhou, L.; Jiang, B.; Op den Camp, H.J.M.; Risgaard-Petersen, N.; Schwark, L.; Peng, Y.; et al. Hotspots of anaerobic ammonium oxidation at land–freshwater interfaces. Nat. Geosci. 2013, 6, 103–107. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, X.; Miao, Y.; Hu, B. Exploration of rapid start-up of the CANON process from activated sludge inoculum in a sequencing biofilm batch reactor (SBBR). Water Sci. Technol. 2016, 73, 535. [Google Scholar] [CrossRef]
- Andriy, M.; Jozef, T.; Elzbieta, P. Mainstream wastewater treatment in integrated fixed film activated sludge (IFAS) reactor by partial nitritation/anammox process. Bioresour. Technol. 2015, 198, 478–487. [Google Scholar]
- Ma, B.; Qian, W.; Yuan, C.; Yuan, Z.; Peng, Y. Achieving mainstream nitrogen removal through coupling anammox with denitratation. Environ. Sci. Technol. 2017, 51, 8405–8413. [Google Scholar] [CrossRef]
- Gao, R.; Peng, Y.; Li, J.; Liu, Y.; Deng, L.; Li, W.; Kao, C. Mainstream partial denitrification-anammox (PD/A) for municipal sewage treatment from moderate to low temperature: Reactor performance and bacterial structure. Sci. Total Environ. 2022, 806, 150267. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, Y.; Wang, M.; Pan, C.; Li, X.; Peng, Y.; Li, B. Post-endogenous denitrification and phosphorus removal in an alternating anaerobic/oxic/anoxic (AOA) system treating low carbon/nitrogen (C/N) domestic wastewater. Chem. Eng. J. 2018, 339, 450–458. [Google Scholar] [CrossRef]
- Tsuneda, S.; Park, S.; Hayashi, H.; Jung, J.; Hirata, A. Enhancement of nitrifying biofilm formation using selected EPS produced by heterotrophic bacteria. Water Sci. Technol. 2001, 43, 197–204. [Google Scholar] [CrossRef]
- Du, R.; Cao, S.; Wang, S.; Niu, M.; Peng, Y. Performance of partial denitrification (PD)-ANAMMOX process in simultaneously treating nitrate and low C/N domestic wastewater at low temperature. Bioresour. Technol. 2016, 219, 420. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.F.; Melo, L.F.; Vieira, M.J. Influence of medium composition on the characteristics of a denitrifying biofilm formed by Alcaligenes denitrificans in a fluidised bed reactor. Process Biochem. 2002, 37, 837–845. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Peng, Y.; Han, X.; Gan, Y. Nitrogen removal performance and microbial distribution in pilot- and full-scale integrated fixed-biofilm activated sludge reactors based on nitritation-anammox process. Bioresour. Technol. 2015, 196, 448–453. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examinations of Water and Wastewater. In American Water Works Association and Water Environment Federation, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Lima, P.S.; Inacio, A.T.; Moreira, Y.C.; César, D.E.; Dias, R.J.P.; Dezotti, M.; Bassin, J.P. Upgrade of a suspended biomass reactor with limited nitrification to a biofilm system: Addressing critical parameters and performance in different reactor configurations. Biochem. Eng. J. 2021, 170, 107987. [Google Scholar] [CrossRef]
- Ge, S.; Peng, Y.; Wang, S.; Lu, C.; Cao, X.; Zhu, Y. Nitrite accumulation under constant temperature in anoxic denitrification process: The effects of carbon sources and COD/NO3−-N. Bioresour. Technol. 2012, 114, 137–143. [Google Scholar] [CrossRef]
- Shi, L.; Du, R.; Peng, Y. Achieving partial denitrification using carbon sources in domestic wastewater with waste-activated sludge as inoculum. Bioresour. Technol. 2019, 283, 18–27. [Google Scholar] [CrossRef]
- Du, R.; Cao, S.; Niu, M.; Li, B.; Wang, S.; Peng, Y. Performance of partial-denitrification process providing nitrite for anammox in sequencing batch reactor (SBR) and upflow sludge blanket (USB) reactor. Int. Biodeter. Biodegr. 2017, 122, 38–46. [Google Scholar] [CrossRef]
- Gao, R.; Peng, Y.; Li, J.; Du, R.; Yang, L.; Wang, M.; Deng, L. Nitrogen removal from low COD/TIN real municipal sewage by coupling partial denitrification with anammox in mainstream. Chem. Eng. J. 2021, 410, 128221. [Google Scholar] [CrossRef]
- Li, J.; Peng, Y.; Zhang, L.; Liu, J.; Wang, X.; Gao, R.; Pang, L.; Zhou, Y. Quantify the contribution of anammox for enhanced nitrogen removal through metagenomic analysis and mass balance in an anoxic moving bed biofilm reactor. Water Res. 2019, 160, 178–187. [Google Scholar] [CrossRef]
- Ni, S.Q.; Ni, J.Y.; Hu, D.L.; Sung, S. Effect of organic matter on the performance of granular anammox process. Bioresour. Technol. 2012, 110, 701. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Cao, S.; Li, B.; Niu, M.; Wang, S.; Peng, Y. Performance and microbial community analysis of a novel DEAMOX based on partial-denitrification and anammox treating ammonia and nitrate wastewaters. Water Res. 2017, 108, 46–56. [Google Scholar] [CrossRef] [PubMed]


| Concentration | COD (mg/L) | NH4+-N (mg/L) | NO3−-N (mg/L) | TN (mg/L) | C/N | Temperature (°C) |
|---|---|---|---|---|---|---|
| Range | 99–250 | 41–80 | 0–1.1 | 41–81 | 1.5–4.9 | 13.5–29.4 |
| Mean value | 148 ± 37 | 55 ± 9.0 | 0.4 ± 0.3 | 56 ± 9.1 | 2.68 ± 0.7 | 21.8 |
| Specificity | AOB amo A | Nitrospira 16S rRNA | Nitrobacter 16S rRNA | Anammox bacteria 16S rRNA | Total bacteria 16S rRNA |
|---|---|---|---|---|---|
| Former Primer Reverse Primer | amoA-1F amoA-2R | 338F 685R | FGPS872F FGPS1269R | Amx368F Amx820R | 341F 534R |
| Annealing temperature (°C) | 55 | 53 | 51 | 56 | 55 |
| Phase | Time (day) | Influent Concentration (mg/L) | Effluent Concentration (mg/L) | |||||
|---|---|---|---|---|---|---|---|---|
| NH4+-N | NO3−-N | COD | NH4+-N | NO2−-N | NO3−-N | COD | ||
| I | 1–58 | 61 ± 8.4 | 0.25 ± 0.30 | 184 ± 39 | 24 ± 19 | 0.44 ± 0.25 | 13 ± 5.8 | 40 ± 9.3 |
| II | 59–106 | 60 ± 11 | 0.41 ± 0.39 | 141 ± 29 | 2.3 ± 2.7 | 0.41 ± 0.27 | 16 ± 4.3 | 35 ± 6.1 |
| III | 107–199 | 50 ± 4.1 | 0.48 ± 0.30 | 128 ± 19 | 0.64 ± 0.6 | 0.38 ± 0.24 | 11 ± 2.0 | 32 ± 8.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Peng, Y.; Huang, D.; Fan, J.; Du, R. Enhanced Nitrogen Removal from Domestic Wastewater by Partial-Denitrification/Anammox in an Anoxic/Oxic Biofilm Reactor. Processes 2022, 10, 109. https://doi.org/10.3390/pr10010109
Huang Y, Peng Y, Huang D, Fan J, Du R. Enhanced Nitrogen Removal from Domestic Wastewater by Partial-Denitrification/Anammox in an Anoxic/Oxic Biofilm Reactor. Processes. 2022; 10(1):109. https://doi.org/10.3390/pr10010109
Chicago/Turabian StyleHuang, Yu, Yongzhen Peng, Donghui Huang, Jiarui Fan, and Rui Du. 2022. "Enhanced Nitrogen Removal from Domestic Wastewater by Partial-Denitrification/Anammox in an Anoxic/Oxic Biofilm Reactor" Processes 10, no. 1: 109. https://doi.org/10.3390/pr10010109
APA StyleHuang, Y., Peng, Y., Huang, D., Fan, J., & Du, R. (2022). Enhanced Nitrogen Removal from Domestic Wastewater by Partial-Denitrification/Anammox in an Anoxic/Oxic Biofilm Reactor. Processes, 10(1), 109. https://doi.org/10.3390/pr10010109






