Reaction Monitoring by Ultrasounds in a Pseudohomogeneous Medium: Triglyceride Ethanolysis for Biodiesel Production
Abstract
:1. Introduction
2. Materials and Methods
3. Results
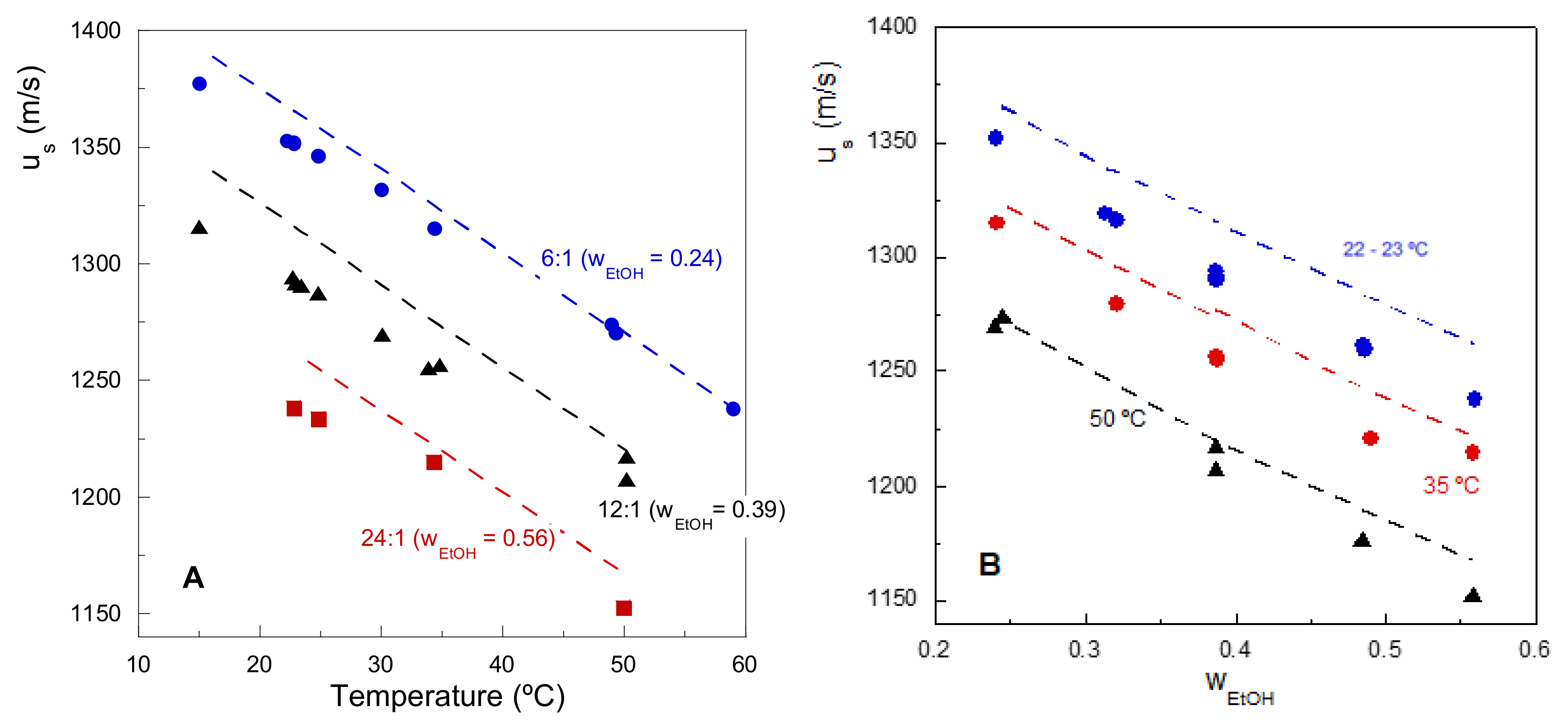
3.1. Experimental System Behavior
3.2. Conversion Estimation from us
3.2.1. Conversion Estimation through an Algebraic Correlation
3.2.2. Conversion Estimation through the Contribution of Components in the Mixture to us
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pensieri, S.; Bozzano, R. Active and Passive Acoustic Methods for In-situ Monitoring of the Ocean Status. In Advances in Underwater Acoustics; InTechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Doubek, M.; Vacek, V.; Hallewell, G.; Pearson, B. Speed-of-sound based sensors for environmental monitoring. In Proceedings of the 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar] [CrossRef]
- Foster, F.S.; Pavlin, C.J.; Harasiewicz, K.A.; Christopher, D.A.; Turnbull, D.H. Advances in ultrasound biomicroscopy. Ultrasound Med. Biol. 2000, 26, 1–27. [Google Scholar] [CrossRef]
- Hossain, M.Z. Monitoring of the Sound Velocity Variation in Rat Muscle and a Novel Approach to Quantify Muscle Injury. 2021. Available online: https://www.researchgate.net/publication/356664534_Monitoring_of_the_sound_velocity_variation_in_rat_muscle_and_a_novel_approach_to_quantify_muscle_injury (accessed on 17 December 2021).
- Ghazipour, H.; Gutiérrez, A.; Mohammad-Aghaie, D.; Alavianmher, M.M.; Hosseini, S.M.; Aparicio, S. Insights on biodiesel blends with alkanol solvents. J. Mol. Liq. 2021, 332, 115864. [Google Scholar] [CrossRef]
- Katunin, A.; Dragan, K.; Dziendzikowski, M. Damage identification in aircraft composite structures: A case study using various non-destructive testing techniques. Compos. Struct. 2015, 127, 1–9. [Google Scholar] [CrossRef]
- Rose, J.L. An Introduction to Ultrasonic Guided Waves. In Proceedings of the 4th Middle East NDT Conference and Exhibition; Kingdom of Bahrain, Manama, Bahrain, 9–13 December 2007. [Google Scholar]
- Dwivedi, K.; Vyas, V.; Tripathi, I.P. Evaluation of Molar Sound Velocity and Molar Compressibility for Binary System of 1-Butanol + Hexadecane and 1- Butanol + Squalane. Int. J. Pharm. Chem. 2016, 6, 92–99. [Google Scholar]
- Umadevi, M.; Kesavasamy, R.; Rathina, K.; Mahalakshmi, R. Studies on liquid-liquid interactions of some ternary mixtures by density, viscosity and ultrasonic speed measurements. J. Mol. Liq. 2016, 219, 820–828. [Google Scholar] [CrossRef]
- Simić, Z.V.; Kijevčanin, M.L.; Radović, I.R.; Grilc, M.; Ivaniš, G.R. Thermodynamic and transport properties of biomass-derived furfural, furfuryl alcohol and their mixtures. Energies 2021, 14, 7769. [Google Scholar] [CrossRef]
- Nikam, P.S.; Mahale, T.R.; Hasan, M. Molecular interactions in binary mixtures of ethyl acetate with 1-pentanol, 1-hexanol, 3,5,5 trimethyl hexanol, 1-octanol and 1-decanol at 298.15, 303.15 and 308.15K: An ultrasonic study. Indian J. Pure Appl. Phys. 1999, 37, 92–96. [Google Scholar]
- Lamberti, N.; Ardia, L.; Albanese, D.; Di Matteo, M. An ultrasound technique for monitoring the alcoholic wine fermentation. Ultrasonics 2009, 49, 94–97. [Google Scholar] [CrossRef]
- Resa, P.; Elvira, L.; De Espinosa, F.M. Concentration control in alcoholic fermentation processes from ultrasonic velocity measurements. Food Res. Int. 2004, 37, 587–594. [Google Scholar] [CrossRef]
- Awad, T.S. Ultrasonic studies of the crystallization behavior of two palm fats O/W emulsions and its modification. Food Res. Int. 2004, 37, 579–586. [Google Scholar] [CrossRef]
- Svilainis, L.; Rodriguez, A.; Liaukonis, D.; Chaziachmetovas, A. Assessment of ultrasound velocity application for chemical process monitoring. Elektronika Elektrotechnika 2015, 21, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Zabala, S.; Arzamendi, G.; Reyero, I.; Gandia, L.M. Monitoring of the methanolysis reaction for biodiesel production by off-line and on-line refractive index and speed of sound measurements. Fuel 2014, 121, 157–164. [Google Scholar] [CrossRef]
- Van Gerpen J, K.G. 4—Biodiesel Production. In The Biodiesel Handbook, 2nd ed.; Gerhard Knothe, J.K., Van Gerpen, J., Eds.; AOCS Press: Champaign, IL, USA, 2010; pp. 31–96. ISBN 978-1-893997-62-2. [Google Scholar]
- Knothe, G. Biodiesel and renewable diesel: A comparison. Prog. Energy Combust. Sci. 2010, 36, 364–373. [Google Scholar] [CrossRef]
- Santacesaria, E.; Vicente, G.M.; Di Serio, M.; Tesser, R. Main technologies in biodiesel production: State of the art and future challenges. Catal. Today 2012, 195, 2–13. [Google Scholar] [CrossRef]
- Santori, G.; Di Nicola, G.; Moglie, M.; Polonara, F. A review analyzing the industrial biodiesel production practice starting from vegetable oil refining. Appl. Energy 2012, 92, 109–132. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, B.D.; Kegl, B.; Marković, S.D.; Mitrović, M.S. Determining the speed of sound, density, and bulk modulus of rapeseed oil, biodiesel, and diesel fuel. Therm. Sci. 2013, 16, 505–514. [Google Scholar] [CrossRef]
- Lopes, A.F.G.; Talavera-Prieto, M.D.C.; Ferreira, A.G.M.; Santos, J.B.; Santos, M.J.; Portugal, A.T.G. Speed of sound in pure fatty acid methyl esters and biodiesel fuels. Fuel 2014, 116, 242–254. [Google Scholar] [CrossRef] [Green Version]
- Tat, M.E.; Van Gerpen, J.H. Measurement of Biodiesel Speed of Sound and Its Impact on Injection Timing; Final Report No. NREL/SR-510-31462; National Renewable Energy Laboratory, U.S. Department of Energy Laboratory: Golden, CO, USA, 2003.
- Samuel, O.D.; Okwu, M.O.; Amosun, S.T.; Verma, T.N.; Afolalu, S.A. Production of fatty acid ethyl esters from rubber seed oil in hydrodynamic cavitation reactor: Study of reaction parameters and some fuel properties. Ind. Crops Prod. 2019, 141, 111658. [Google Scholar] [CrossRef]
- Stamenković, O.S.; Veličković, A.V.; Veljković, V.B. The production of biodiesel from vegetable oils by ethanolysis: Current state and perspectives. Fuel 2011, 90, 3141–3155. [Google Scholar] [CrossRef]
- Arzamendi, G.; Arguiñarena, E.; Campo, I.; Gandía, L.M. Monitoring of biodiesel production: Simultaneous analysis of the transesterification products using size-exclusion chromatography. Chem. Eng. J. 2006, 122, 31–40. [Google Scholar] [CrossRef]
- Reyero, I. Avances en la Producción de Biodiésel: Etanolisis y Nuevos Catalizadores Heterogéneos. Ph.D. Thesis, Universidad Pública de Navarra, Pamplona, Spain, 2014. [Google Scholar]
- Chen, X.; Li, Z.; Chun, Y.; Yang, F.; Xu, H.; Wu, X. Effect of the Formation of Diglycerides/Monoglycerides on the Kinetic Curve in Oil Transesterification with Methanol Catalyzed by Calcium Oxide. ACS Omega 2020, 5, 4646–4656. [Google Scholar] [CrossRef]
- Borovinskaya, E.; Ritter, E.; Reschetilowski, W. Optimization of base catalyzed ethanolysis of vegetable oils in microreactors using design of experiments. Appl. Sci. 2020, 10, 1624. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, T.; Kubo, M.; Shibasaki-Kitakawa, N.; Yonemoto, T. Is excess methanol addition required to drive transesterification of triglyceride toward complete conversion? Energy Fuels 2009, 23, 6163–6167. [Google Scholar] [CrossRef]
- Reyero, I.; Arzamendi, G.; Zabala, S.; Gandia, L.M. Kinetics of the NaOH-catalyzed transesterification of sunflower oil with ethanol to produce biodiesel. Fuel Process. Technol. 2015, 129, 147–155. [Google Scholar] [CrossRef]
- Nomoto, O. Empirical Formula for Sound Velocity in Liquid Mixtures. J. Phys. Soc. Jpn. 1958, 13, 1528–1532. [Google Scholar] [CrossRef]
- Meister, E.C. Measurement of the Temperature and Concentration Dependent Sound Velocity in Ethanol-Water Liquid Mixtures. In Physikalisch-Chemisches Praktikum; ETH: Zurich, Switzerland, 2015. [Google Scholar]
- Rode, S.; Bhandakkar, V.D.; Chimankar, O.P. Theoretical Evaluation of Ultrasonic Velocity in Binary Liquid Mixtures at 5 MHz for Temperature 298 K. IOSR J. Appl. Phys. 2016, 8, 10–14. [Google Scholar]
- Junjie, Z. Calculation of Ultrasonic Velocity in Binary Liquid Mixtures of Benzene. J. China Univ. Sci. Technol. 1984, 14, 298–299. [Google Scholar]
- Van Dael, W. Thermodynamic properties and the velocity of sound. In Experimental Thermodynamics, Experimental Thermodynamics of Non-Reacting Systems; Le Neindre, B., Vodar, B., Eds.; Butterworths: London, UK, 1975; Volume 2, pp. 527–575. ISBN 0-408-70566-3. [Google Scholar]
- Rarokar, N.R.; Menghani, S.; Kerzare, D.; Khedekar, P.B. Progress in Synthesis of Monoglycerides for Use in Food and Pharmaceuticals. J. Exp. Food Chem. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Nitbani, F.O.; Tjitda, P.J.P.; Nurohmah, B.A.; Wogo, H.E. Preparation of fatty acid and monoglyceride from vegetable oil. J. Oleo Sci. 2020, 69, 277–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Parameter | Value |
|---|---|
| u0,Tref (m/s) | 1452.7 |
| aT (m/s/°C) | −3.5 |
| aw (m/s) | −373.8 |
| aX (m/s) | 6.36 |
| aTw (m/s) | −0.029 |
| aXw (m/s) | 43.7 |
| aXT (m/s) | −0.032 |
| Si | si,0 | si,1 | si,2 | si,3 | si,4 | si,5 |
|---|---|---|---|---|---|---|
| DG; 6:1 | 1.0000 | 1.0000 | 1.0000 | −0.0000 | 0.0000 | 0.0000 |
| DG; 12:1 | −1.4984 | −2.1438 | −2.31120 | 0.5376 | 1.6094 | 1.7145 |
| DG; 24:1 | 1.4828 | 3.4650 | 3.23220 | −3.9000 | −8.8995 | −8.2972 |
| GL; 6:1 | −1.0327 | −3.7899 | −1.87140 | 14.600 | 24.861 | 22.322 |
| GL; 12:1 | 1.0900 | 2.8252 | −0.01086 | −22.700 | −31.158 | −27.827 |
| GL; 24:1 | −1.0421 | −1.3587 | −0.03736 | 12.500 | 14.569 | 13.0670 |
| ui,T = a − b(T-20) | a (m/s) | b (m/s/°C) |
|---|---|---|
| Oil | 1430 ± 3 | 3.5 ± 0.1 |
| Diglycerides, monoglycerides | 1490 ± 10 | |
| Ethyl esters | 1396 ± 4 | |
| Ethanol | 1144 ± 2 | |
| Glycerol | 1921.3 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyero, I.; Gandía, L.M.; Arzamendi, G. Reaction Monitoring by Ultrasounds in a Pseudohomogeneous Medium: Triglyceride Ethanolysis for Biodiesel Production. Processes 2022, 10, 12. https://doi.org/10.3390/pr10010012
Reyero I, Gandía LM, Arzamendi G. Reaction Monitoring by Ultrasounds in a Pseudohomogeneous Medium: Triglyceride Ethanolysis for Biodiesel Production. Processes. 2022; 10(1):12. https://doi.org/10.3390/pr10010012
Chicago/Turabian StyleReyero, Inés, Luis M. Gandía, and Gurutze Arzamendi. 2022. "Reaction Monitoring by Ultrasounds in a Pseudohomogeneous Medium: Triglyceride Ethanolysis for Biodiesel Production" Processes 10, no. 1: 12. https://doi.org/10.3390/pr10010012







