Influence of Hydrodynamic Conditions on Precipitation Kinetics of Barium Sulfate in a Multifunctional Reactor
Abstract
:1. Introduction
2. Mathematical Model of the Process
2.1. Kinetic Parameters
- Perfect mixing of the suspension,
- Zero crystal seeds size,
- No aggregation and breakage of crystals,
- McCabe law fulfilled,
- No growth dispersion.
2.2. Hydrodynamic Conditions
3. Materials and Methods
3.1. Stock Solutions
3.2. Crystallization with Chemical Reaction
3.3. Physico-Chemical Properties of Stock and Post-Reaction Solutions
3.4. Solid Product Analysis
4. Results and Discussion
4.1. Relative Supersaturation Determination
4.2. Efficiency and Conversion Degree
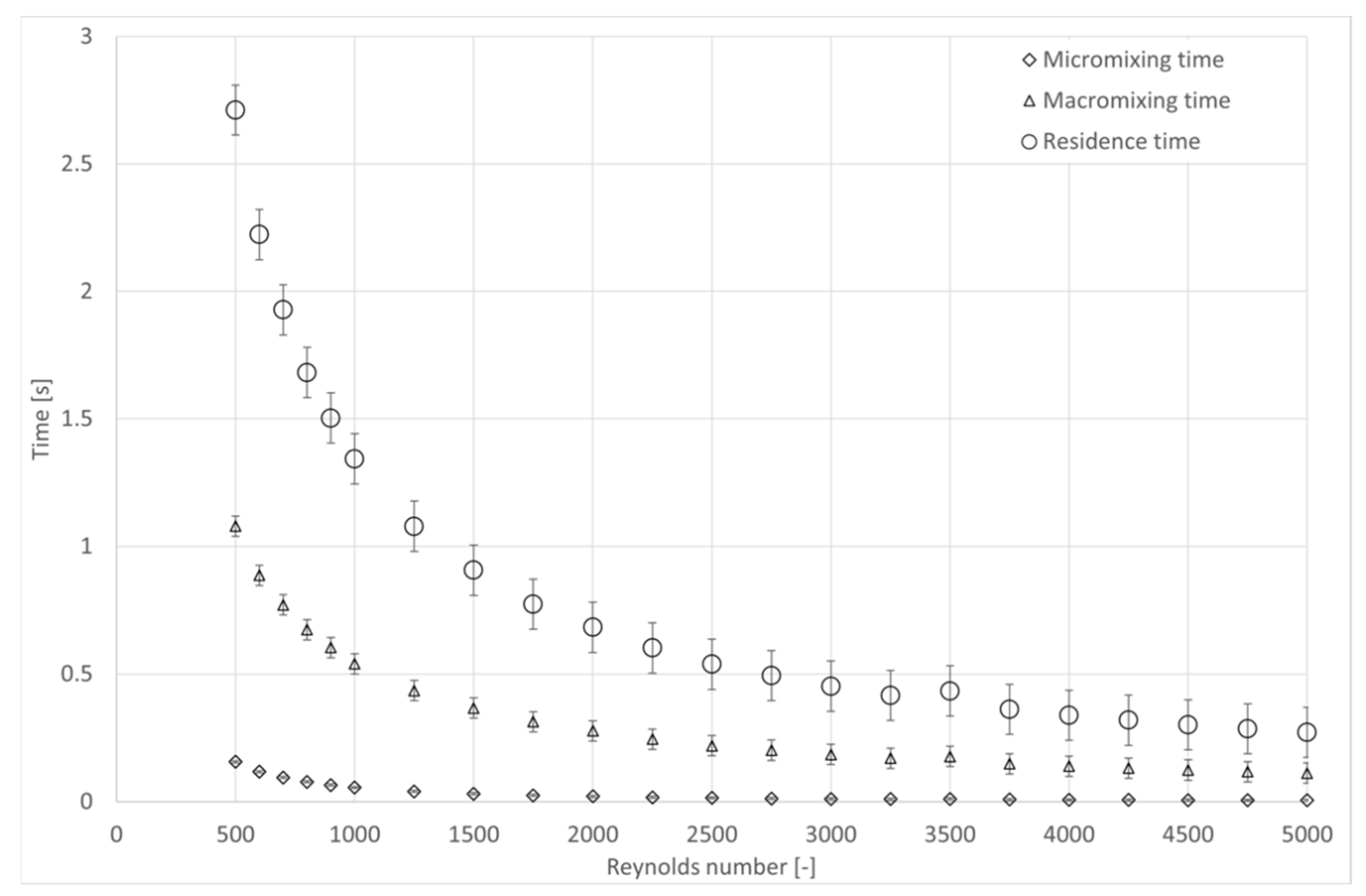
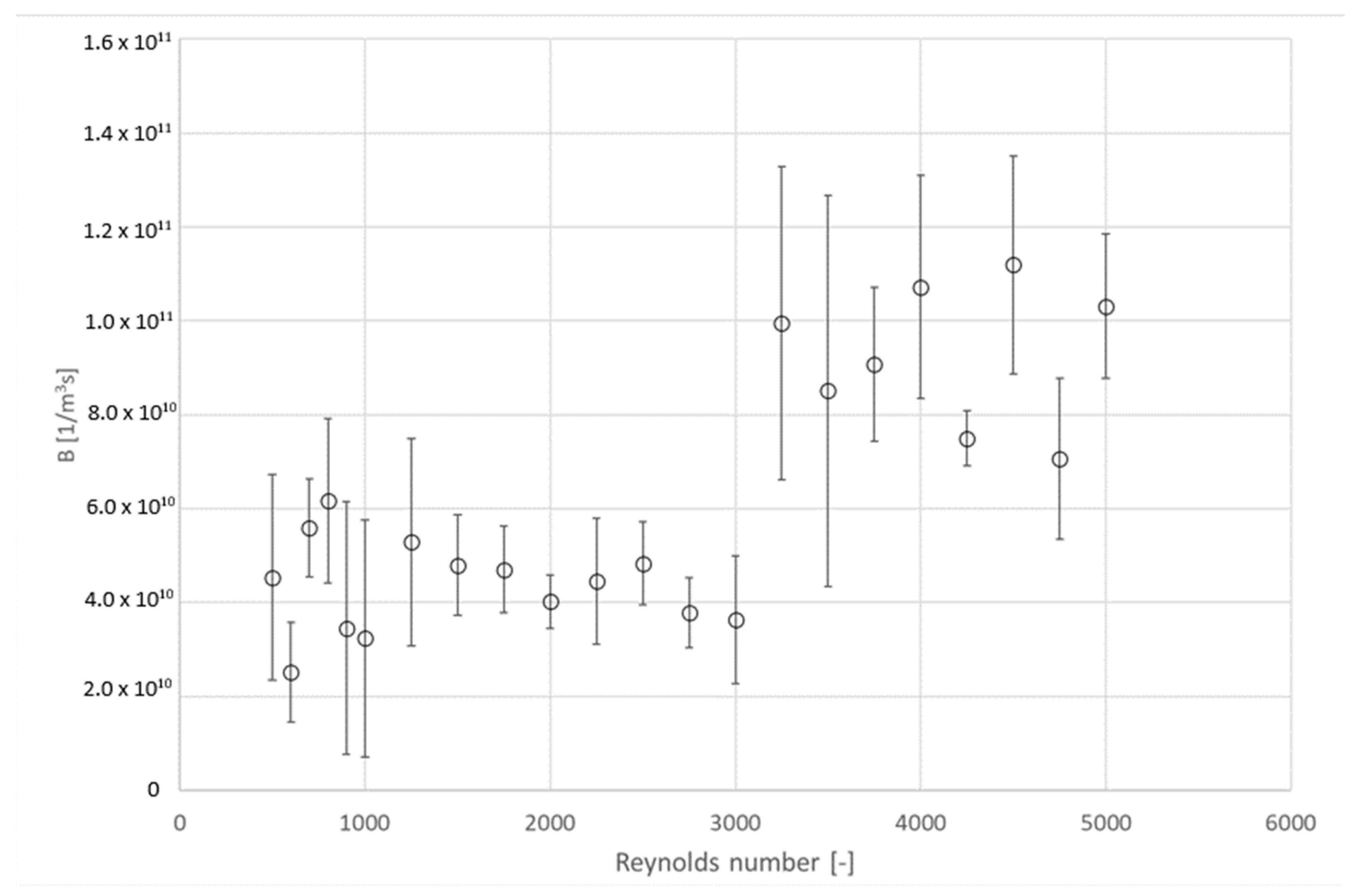
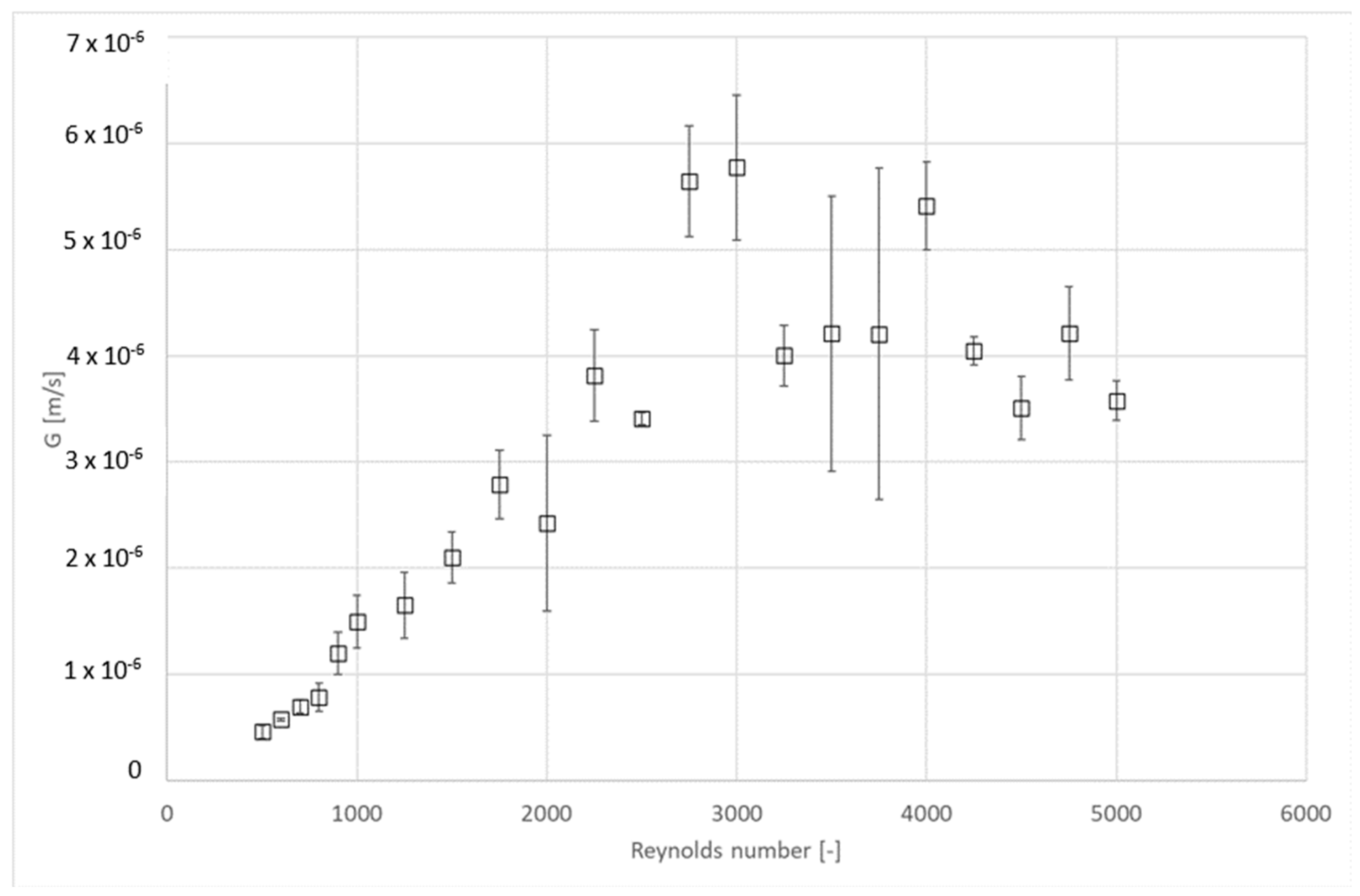
4.3. Influence of Hydrodynamic Conditions on Kinetics
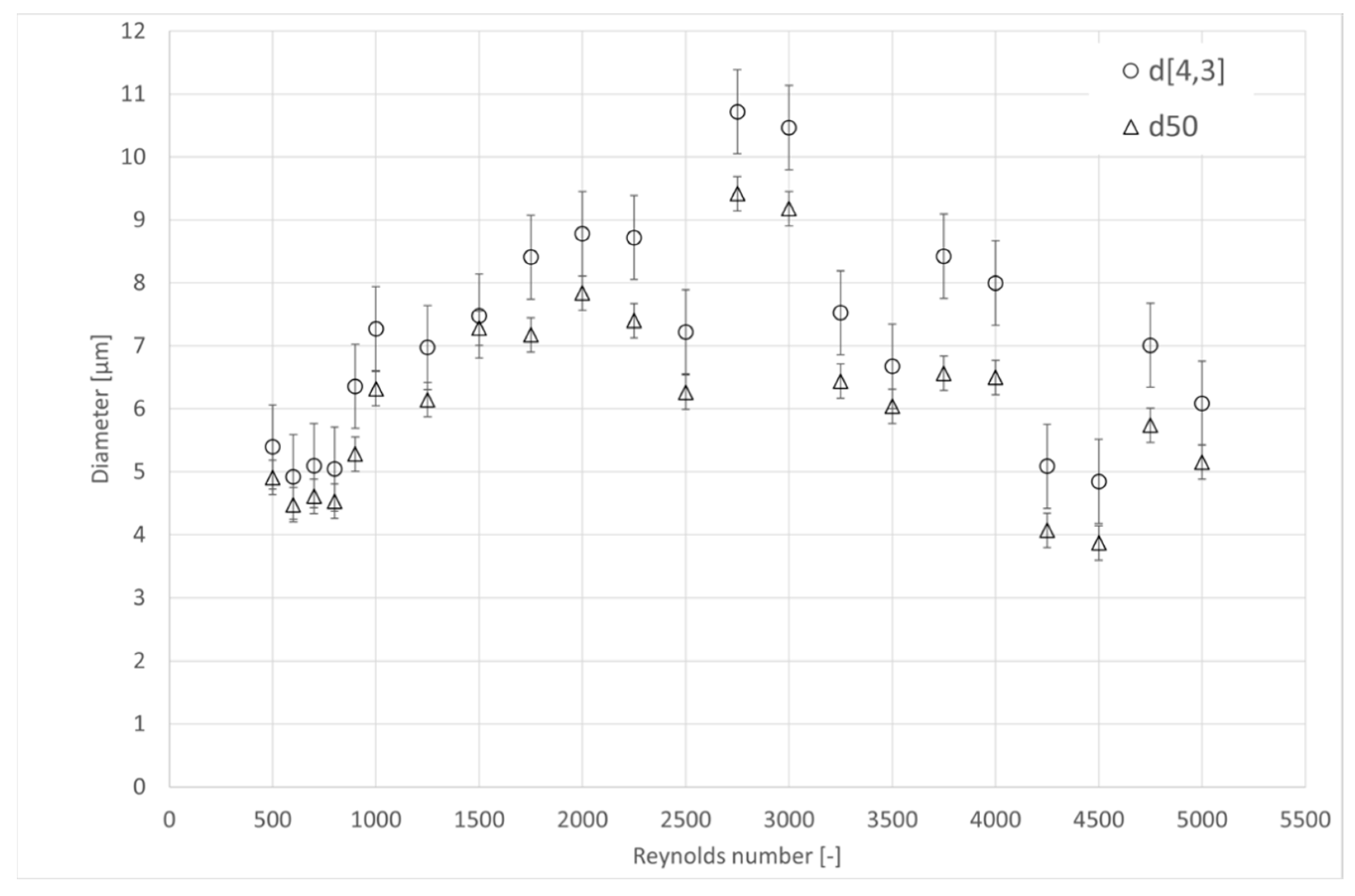
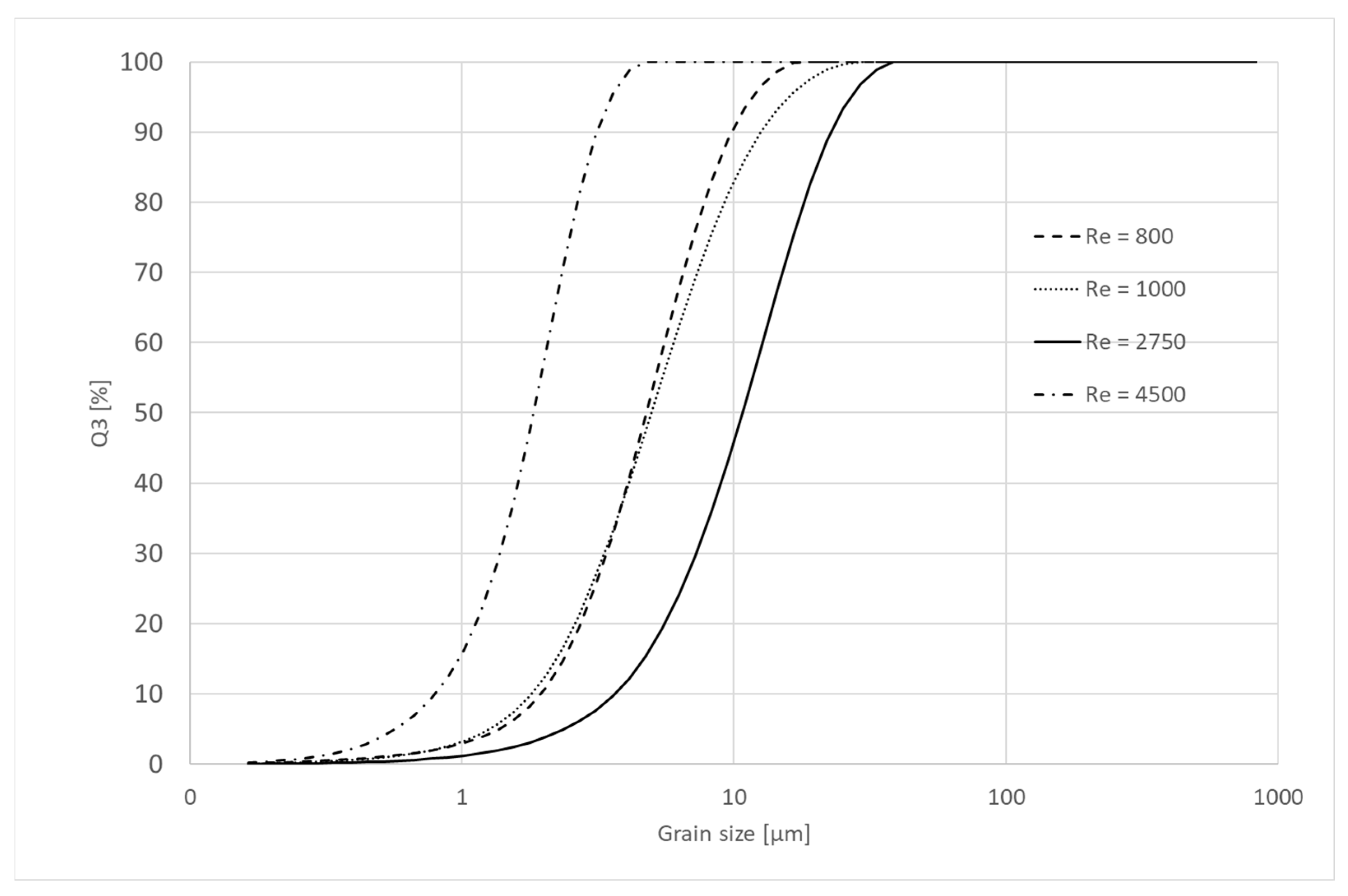
4.4. Microscope Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mullin, J.W. Crystallization, 4th ed.; Knovel; Butterworth Heinemann: Oxford, UK, 2001; ISBN 9780080530116. [Google Scholar]
- Kiss, N.; Brenn, G.; Pucher, H.; Wieser, J.; Scheler, S.; Jennewein, H.; Suzzi, D.; Khinast, J. Formation of O/W emulsions by static mixers for pharmaceuticalapplications. Chem. Eng. Sci. 2011, 66, 5084–5094. [Google Scholar] [CrossRef]
- Ghanem, A.; Lemenand, T.; Della Valle, D.; Peerhossaini, H. Static mixers: Mechanisms, applications, and characterization methods—A review. Chem. Eng. Res. Des. 2014, 92, 205–228. [Google Scholar] [CrossRef]
- Soman, S.S.; Madhuranthakam, C.M.R. Effects of internal geometry modifications on the dispersive anddistributive mixing in static mixers. Chem. Eng. Process. Intensif. 2017, 122, 31–43. [Google Scholar] [CrossRef]
- Thakur, R.K.; Vial, C.; Nigam, K.D.P.; Nauman, E.B.; Djelveh, G. Static Mixers in the Process Industries—A Review. Chem. Eng. Res. Des. 2003, 81, 787–826. [Google Scholar] [CrossRef]
- Laporte, M.; Loisel, C.; Della Valle, D.; Riaublanc, A.; Montillet, A. Flow process conditions to control the void fraction of food foams in static mixers. J. Food Eng. 2014, 128, 119–126. [Google Scholar] [CrossRef]
- Somnuk, K.; Prasit, T.; Prateepchaikul, G. Effects of mixing technologies on continuous methyl ester production: Comparison of using plug flow, static mixer, and ultrasound clamp. Energy Convers. Manag. 2017, 140, 91–97. [Google Scholar] [CrossRef]
- Walica, T.; Kotek, M.; Małysiak, A.; Lemanowicz, M. Static mixers as multifunctional reactors for wastewater treatment Autorzy: In Contemporary Problems of Power Engineering and Environmental Protection; Silesian University of Technology (PolSL): Gliwice, Poland, 2020; ISBN 9788395008795. [Google Scholar]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill Fandbooks, McGraw-Hill: New York, NY, USA, 2003; ISBN 9780070494398. [Google Scholar]
- Ekberg, O.; Bulow, M.; Ekman, S.; Hall, G.; Stading, M.; Wendin, K. Effect of barium sulfate contrast medium on rheology and sensory texture attributes in a model food. Acta Radiol. 2009, 50, 131–138. [Google Scholar] [CrossRef]
- Owen, M. Radiographic, Computed Tomography, and Magnetic Resonance Contrast Media. In Textbook of Veterinary Diagnostic Radiology, 7th ed.; Thrall, D.E., Ed.; W.B. Saunders Ltd.: Philadelphia, PA, USA, 2018; Chapter 6; pp. 96–109. ISBN 9780323482479. [Google Scholar]
- Litt, H.I.; Brody, A.S. BaSO4-Loaded Agarose: A Construction Material for Multimodality Imaging Phantoms. Acad. Radiol. 2001, 8, 377–383. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Gao, C.; Bai, Y.; Liu, B.; Wang, W.; Ma, Y.; Yang, H.; Li, Y. Enhancing effects of radiopaque agent BaSO4 on mechanical and biocompatibility properties of injectable calcium phosphate composite cement. Mater. Sci. Eng. C 2020, 116, 110904. [Google Scholar] [CrossRef] [PubMed]
- Knighton, N.; Bugbee, B. A Mixture of Barium Sulfate and White Paint Is a Low-Cost Substitute Reflectance Standard for Spectralon®; Utah State University: Logan, UT, USA, 2005. [Google Scholar]
- Li, X.; Peoples, J.; Yao, P.; Ruan, X. Ultrawhite BaSO4 Paints and Films for Remarkable Daytime Subambient Radiative Cooling. ACS Appl. Mater. Interfaces 2021, 13, 21733–21739. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.V.; de Souza, A.L.F.; de Lima, P.G.; Wardell, J.L.; Antunes, O.A.C. Stille cross-coupling reaction using Pd/BaSO4 as catalyst reservoir. Appl. Organomet. Chem. 2008, 22, 39–42. [Google Scholar] [CrossRef]
- Neff, J.M. Estimation of bioavailability of metals from drilling mud barite. Integr. Environ. Assess. Manag. 2008, 4, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-W.; Lin, J.; Li, W.-Y.; Hu, Z.-J.; Zhang, Y.-L. Formation of shaped barium sulfate-dye hybrids: Waste dye utilization for eco-friendly treatment of wastewater. Environ. Sci. Pollut. Res. Int. 2010, 17, 78–83. [Google Scholar] [CrossRef]
- Kohandel, F.; Sararoudi, S.S.; Barangi, L. Rheological and mechanical properties of highly filled polyolefin based on PP/barium sulfate. AIP Conf. Proc. 2016, 1713, 120011. [Google Scholar] [CrossRef] [Green Version]
- Aninwene, G.E., II; Webster, T.J. Nano-BaSO4: A Novel Bacteriostatic Polymer Additive. In Proceedings of the 2013 39th Annual Northeast Bioengineering Conference, Syracuse, NY, USA, 5–7 April 2013; pp. 141–142. [Google Scholar]
- Tichapondwa, S.M.; Focke, W.W.; del Fabbro, O.; Gisby, J.; Kelly, C. A Comparative Study of Si-BaSO4 and Si-CaSO4 Pyrotechnic Time-Delay Compositions. J. Energ. Mater. 2016, 34, 342–356. [Google Scholar] [CrossRef] [Green Version]
- Mikhailov, M.; Neshchimenko, V.; Yuryev, S.; Grigorevsky, A.; Lovitskiy, A.; Vashchenkov, I. On the Radiation Stability of BaSO4 Pigment Modified with SiO2 Nanoparticles and Applied for Spacecraft Thermal Control Coatings. Defect Diffus. Forum 2018, 386, 277–282. [Google Scholar] [CrossRef]
- Mikhailov, M.M.; Yuryev, S.A.; Lapin, A.N. Prospects for applying BaSO4 powders as pigments for spacecraft thermal control coatings. Acta Astronaut. 2019, 165, 191–194. [Google Scholar] [CrossRef]
- Parvulescu, A.; De Vos, D.; Jacobs, P. Efficient dynamic kinetic resolution of secondary amines with Pd on alkaline earth salts and a lipase. Chem. Commun. 2005, 42, 5307–5309. [Google Scholar] [CrossRef]
- Andrade, L.H.; Silva, A.V.; Pedrozo, E.C. First dynamic kinetic resolution of selenium-containing chiral amines catalyzed by palladium (Pd/BaSO4) and Candida antartica lipase (CAL-B). Tetrahedron Lett. 2009, 50, 4331–4334. [Google Scholar] [CrossRef]
- Parvulescu, A.N.; Jacobs, P.A.; De Vos, D.E. Palladium Catalysts on Alkaline-Earth Supports for Racemization and Dynamic Kinetic Resolution of Benzylic Amines. Chem. A Eur. J. 2007, 13, 2034–2043. [Google Scholar] [CrossRef]
- Piotrowski, K.; Koralewska, J.; Wierzbowska, B.; Matynia, A. Kinetics of the continuous reaction crystallization of barium sulphate in BaCl2-(NH4)2 SO4-NaCl-H2O system—Neural network model. Pol. J. Chem. Technol. 2009, 11, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Berenjian, A.; Ghasemi, M.R.; Zarghi, A. Preparation of Barium Sulfate Nanoparticles Using Semi-Batch Precipitation. Asian J. Chem. 2011, 23, 491–494. [Google Scholar]
- Fischer, R.B.; Rhinehammer, R.B. Rapid Precipitation of Barium Sulfate. Anal. Chem. 1953, 25, 1544–1548. [Google Scholar] [CrossRef]
- Wojtas, K.; Makowski, Ł.; Orciuch, W. Barium sulfate precipitation in jet reactors: Large eddy simulations, kinetics study and design considerations. Chem. Eng. Res. Des. 2020, 158, 64–76. [Google Scholar] [CrossRef]
- Ronquim, F.M.; Cotrim, M.E.B.; Guilhen, S.N.; Bernardo, A.; Seckler, M.M. Improved barium removal and supersaturation depletion in wastewater by precipitation with excess sulfate. J. Water Process Eng. 2018, 23, 265–276. [Google Scholar] [CrossRef]
- Kieffer, R.; Mangin, D.; Puel, F.; Charcosset, C. Precipitation of barium sulphate in a hollow fiber membrane contactor: Part II The influence of process parameters. Chem. Eng. Sci. 2009, 64, 1885–1891. [Google Scholar] [CrossRef]
- Symeopoulos, B.D.; Koutsoukos, P.G. Spontaneous precipitation of barium sulfate in aqueous solution. J. Chem. Soc. Faraday Trans. 1992, 88, 3063–3066. [Google Scholar] [CrossRef]
- Cafiero, L.M.; Baffi, G.; Chianese, A.; Jachuck, R.J.J. Process Intensification: Precipitation of Barium Sulfate Using a Spinning Disk Reactor. Ind. Eng. Chem. Res. 2002, 41, 5240–5246. [Google Scholar] [CrossRef]
- McCarthy, E.D.; Dunk, W.A.E.; Boodhoo, K.V.K. Application of an intensified narrow channel reactor to the aqueous phase precipitation of barium sulphate. J. Colloid Interface Sci. 2007, 305, 72–87. [Google Scholar] [CrossRef] [Green Version]
- Rehage, H.; Kind, M. The first Damköhler number and its importance for characterizing the influence of mixing on competitive chemical reactions. Chem. Eng. Sci. 2021, 229, 116007. [Google Scholar] [CrossRef]
- Anabaraonye, B.U.; Bentzon, J.R.; Khaliqdad, I.; Feilberg, K.L.; Andersen, S.I.; Walther, J.H. The influence of turbulent transport in reactive processes: A combined numerical and experimental investigation in a Taylor-Couette reactor. Chem. Eng. J. 2021, 421, 129591. [Google Scholar] [CrossRef]
- Judat, B.; Racina, A.; Kind, M. Macro- and Micromixing in a Taylor-Couette Reactor with Axial Flow and Their Influence on the Precipitation of Barium Sulfate. Chem. Eng. Technol. 2004, 27, 287–292. [Google Scholar] [CrossRef]
- Judat, B.; Kind, M. Morphology and internal structure of barium sulfate-derivation of a new growth mechanism. J. Colloid Interface Sci. 2004, 269, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Løge, I.A.; Bentzon, J.R.; Klingaa, C.G.; Walther, J.H.; Anabaraonye, B.U.; Fosbøl, P.L. Scale attachment and detachment: The role of hydrodynamics and surface morphology. Chem. Eng. J. 2022, 430, 132583. [Google Scholar] [CrossRef]
- Besenhard, M.O.; Hohl, R.; Hodzic, A.; Eder, R.J.P.; Khinast, J.G. Modeling a seeded continuous crystallizer for the production of active pharmaceutical ingredients. Cryst. Res. Technol. 2014, 49, 92–108. [Google Scholar] [CrossRef]
- Alvarez, A.J.; Myerson, A.S. Continuous Plug Flow Crystallization of Pharmaceutical Compounds. Cryst. Growth Des. 2010, 10, 2219–2228. [Google Scholar] [CrossRef]
- Synowiec, P.M. Krystalizacja Przemysłowa z Roztworu; WNT: Warsaw, Poland, 2008. [Google Scholar]
- Synowiec, P.M.; Stec, M. Analysis of CaF2 Precipitation Process in the Selected Static Mixers. J. Chem. 2019, 2019, 6728492. [Google Scholar] [CrossRef] [Green Version]
- Ropp, R.C. Group 16 (O, S, Se, Te) Alkaline Earth Compounds. In Encyclopedia of the Alkaline Earth Compounds; Ropp, R.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Chapter 3; pp. 105–197. ISBN 9780444595508. [Google Scholar]
- Mersmann, A.; Braun, B.; Löffelmann, M. Prediction of crystallization coefficients of the population balance. Chem. Eng. Sci. 2002, 57, 4267–4275. [Google Scholar] [CrossRef]
- Stec, M.; Synowiec, P.M. Study of fluid dynamic conditions in the selected static mixers part II-determination of the residence time distribution. Can. J. Chem. Eng. 2017, 95, 2410–2422. [Google Scholar] [CrossRef]
- Ulrich, J. The Crystallization Technology Handbook, 2nd ed.; Marsmann, A., Ed.; Marcel Dekker: New York, NY, USA, 2001; ISBN 0824705289. Cryst. Growth Des.2001, 1, 421. [Google Scholar] [CrossRef]
- Bates, R.L.; Fondy, P.L.; Corpstein, R.R. Examination of Some Geometric Parameters of Impeller Power. Ind. Eng. Chem. Process Des. Dev. 1963, 2, 310–314. [Google Scholar] [CrossRef]


| Parameter | Symbol | Value |
|---|---|---|
| Number of mixing elements | ||
| Inner diameter | ||
| Device length | ||
| Device volume | ||
| Material | PVC |
| Parameter | Symbol | Unit | Value |
|---|---|---|---|
| Ionic activity in solution | 4.68 × 10−6 | ||
| Activity coefficient of Ba2+ | 0.53 | ||
| Activity coefficient of SO42− | 0.53 | ||
| Ionic strength | 0.0245 | ||
| Relative supersaturation | 205 |
| Re | Efficiency (%) | Conversion Degree (%) |
|---|---|---|
| 500 | 91 | 94 |
| 600 | 91.8 | 94.4 |
| 700 | 92.4 | 95 |
| 800 | 93.1 | 95.6 |
| 900 | 93.9 | 96 |
| 1000 | 94.8 | 96.4 |
| 1250 | 95.2 | 97 |
| 1500 | 95.5 | 97 |
| 1750 | 96 | 97.6 |
| 2000 | 96.8 | 98 |
| 2250 | 97.8 | 98.5 |
| 2500 | 99 | 99.4 |
| 2750 | 100 | 100 |
| 3000 | 100 | 100 |
| 3250 | 100 | 100 |
| 3500 | 100 | 100 |
| 3750 | 100 | 100 |
| 4000 | 100 | 100 |
| 4250 | 100 | 100 |
| 4500 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małysiak, A.; Walica, T.; Fronczyk, T.; Lemanowicz, M. Influence of Hydrodynamic Conditions on Precipitation Kinetics of Barium Sulfate in a Multifunctional Reactor. Processes 2022, 10, 146. https://doi.org/10.3390/pr10010146
Małysiak A, Walica T, Fronczyk T, Lemanowicz M. Influence of Hydrodynamic Conditions on Precipitation Kinetics of Barium Sulfate in a Multifunctional Reactor. Processes. 2022; 10(1):146. https://doi.org/10.3390/pr10010146
Chicago/Turabian StyleMałysiak, Agata, Tomasz Walica, Tomasz Fronczyk, and Marcin Lemanowicz. 2022. "Influence of Hydrodynamic Conditions on Precipitation Kinetics of Barium Sulfate in a Multifunctional Reactor" Processes 10, no. 1: 146. https://doi.org/10.3390/pr10010146






