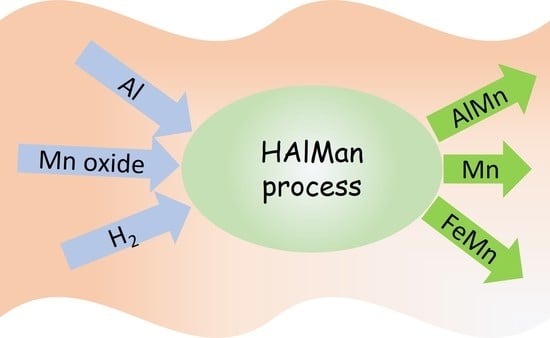
A Sustainable Process to Produce Manganese and Its Alloys through Hydrogen and Aluminothermic Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
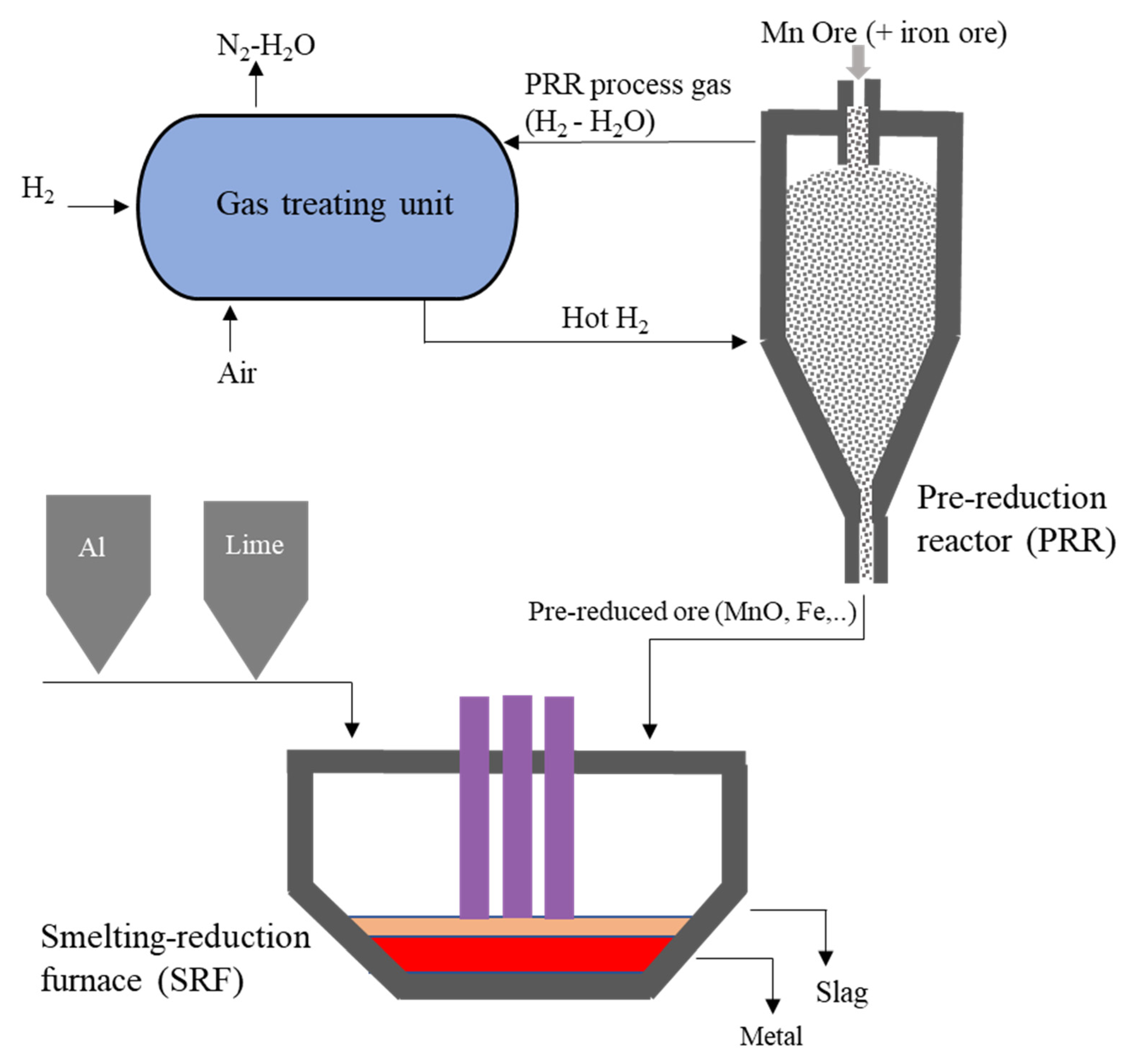
2.2. Pre-Reduction by Hydrogen
2.3. Smelting-Aluminothermic Reduction
2.4. Mass and Energy Balance
3. Experimental Results
4. Discussion
4.1. Material Flow and Process Thermochemistry
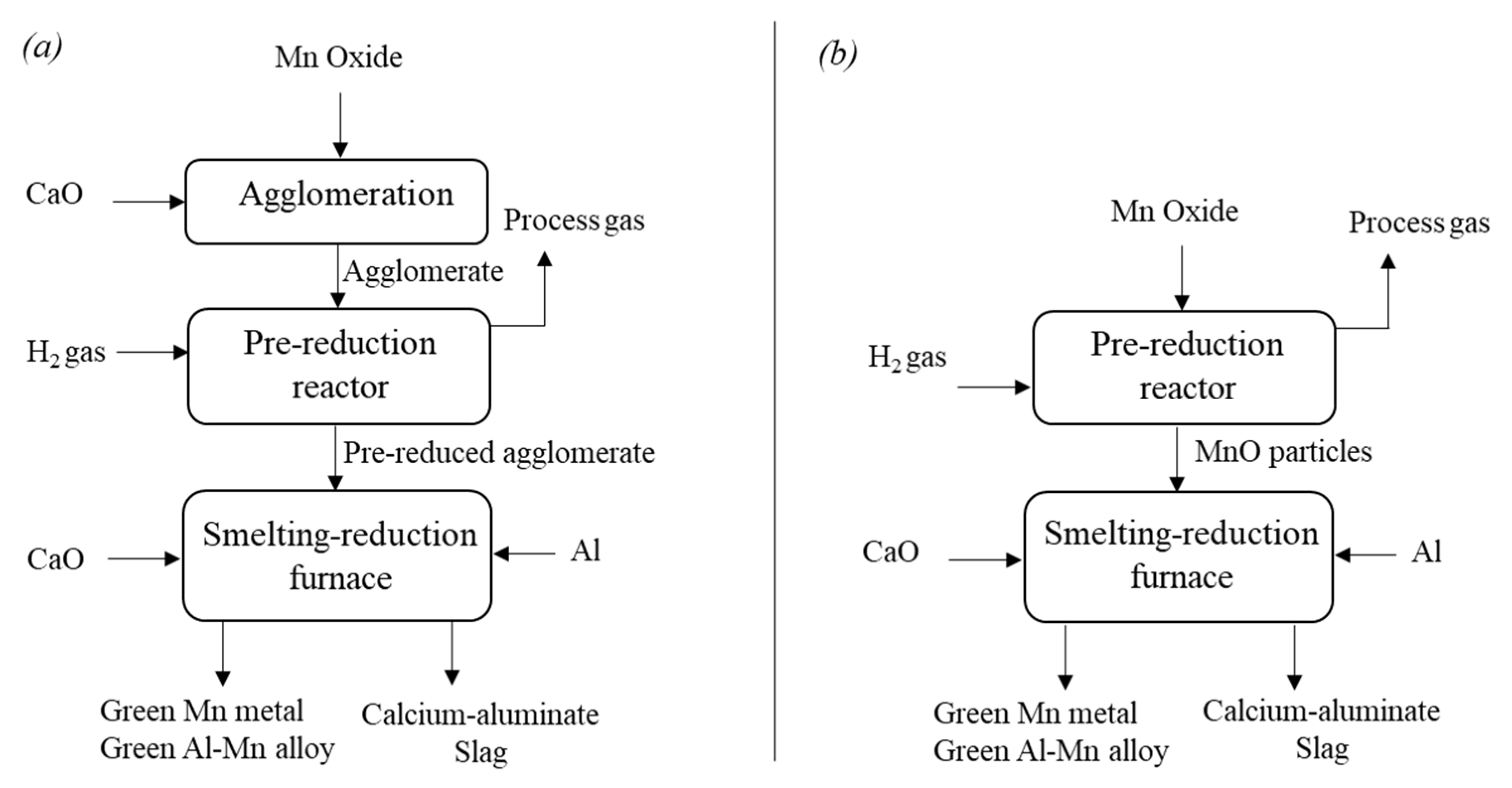
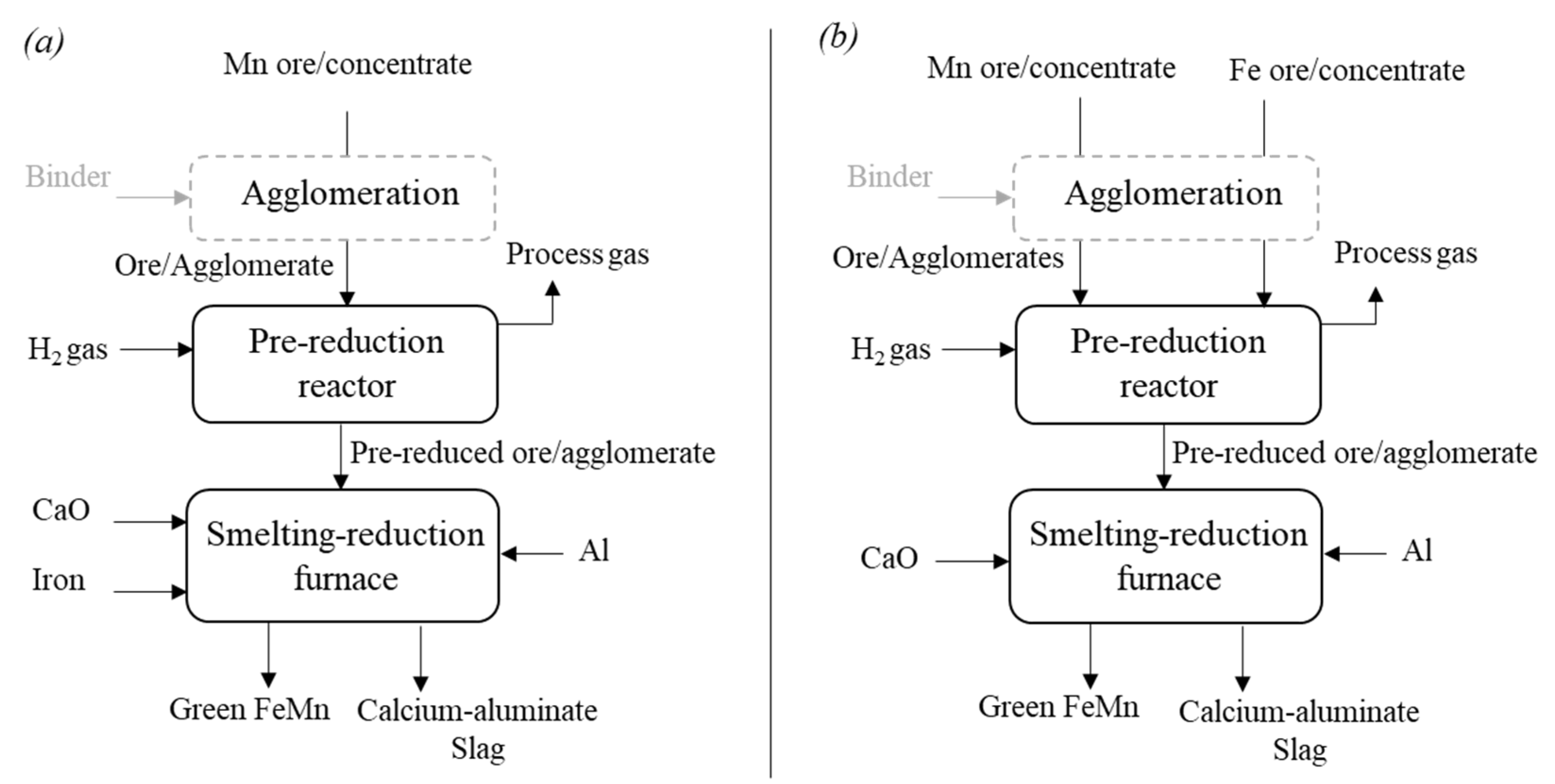
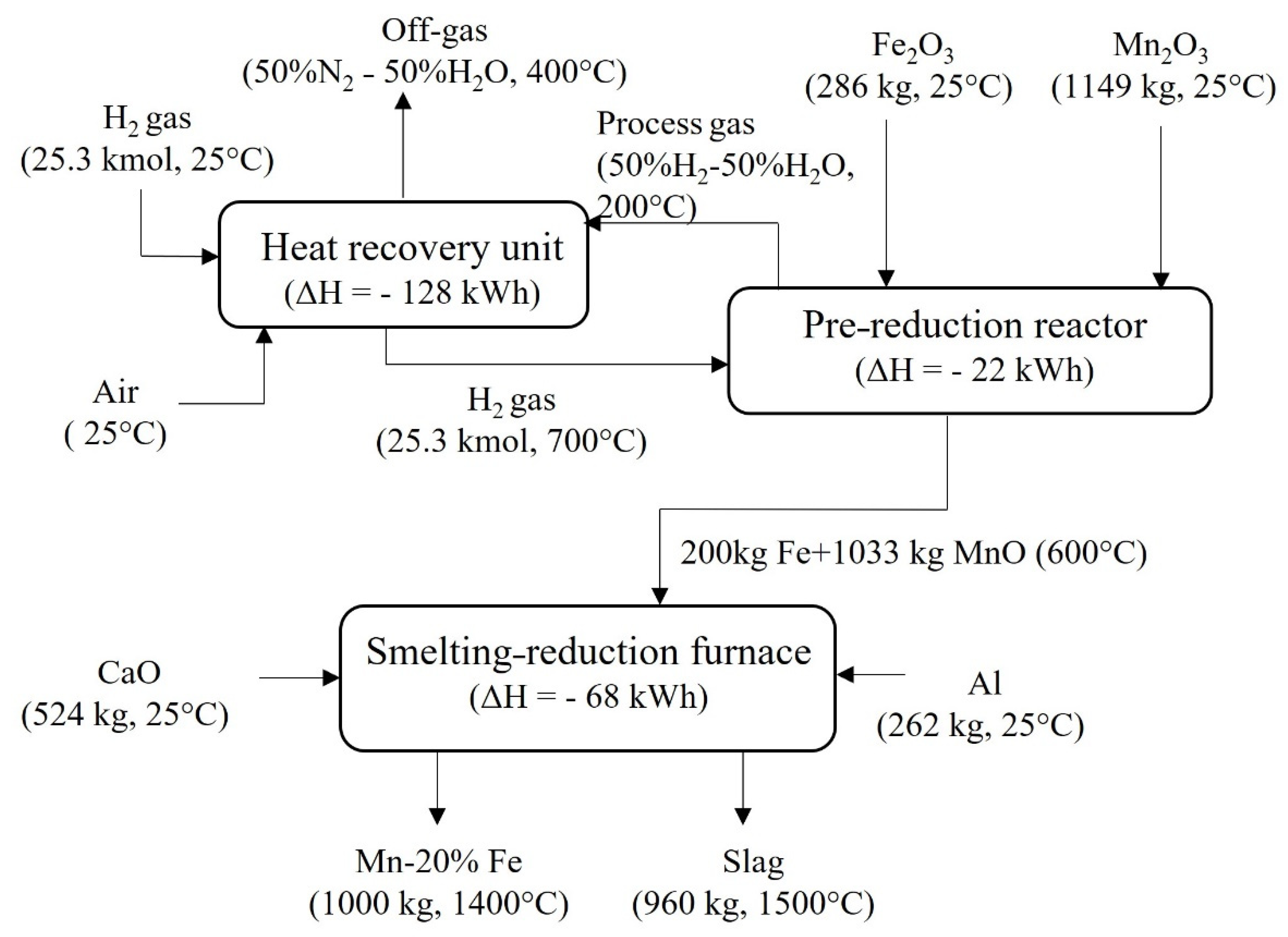
4.1.1. Production of Green Mn and Green AlMn Alloys
4.1.2. Production of Green FeMn
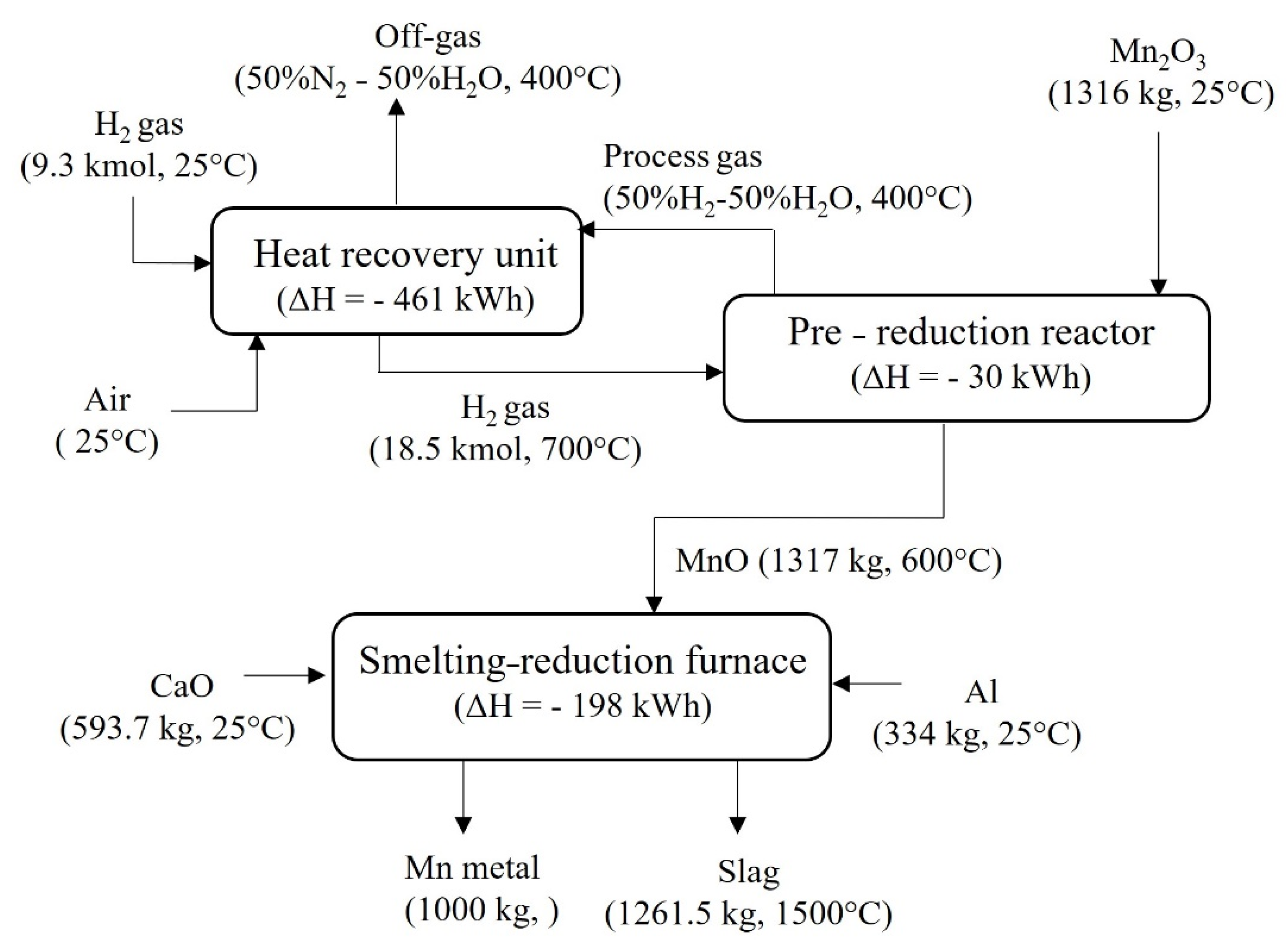
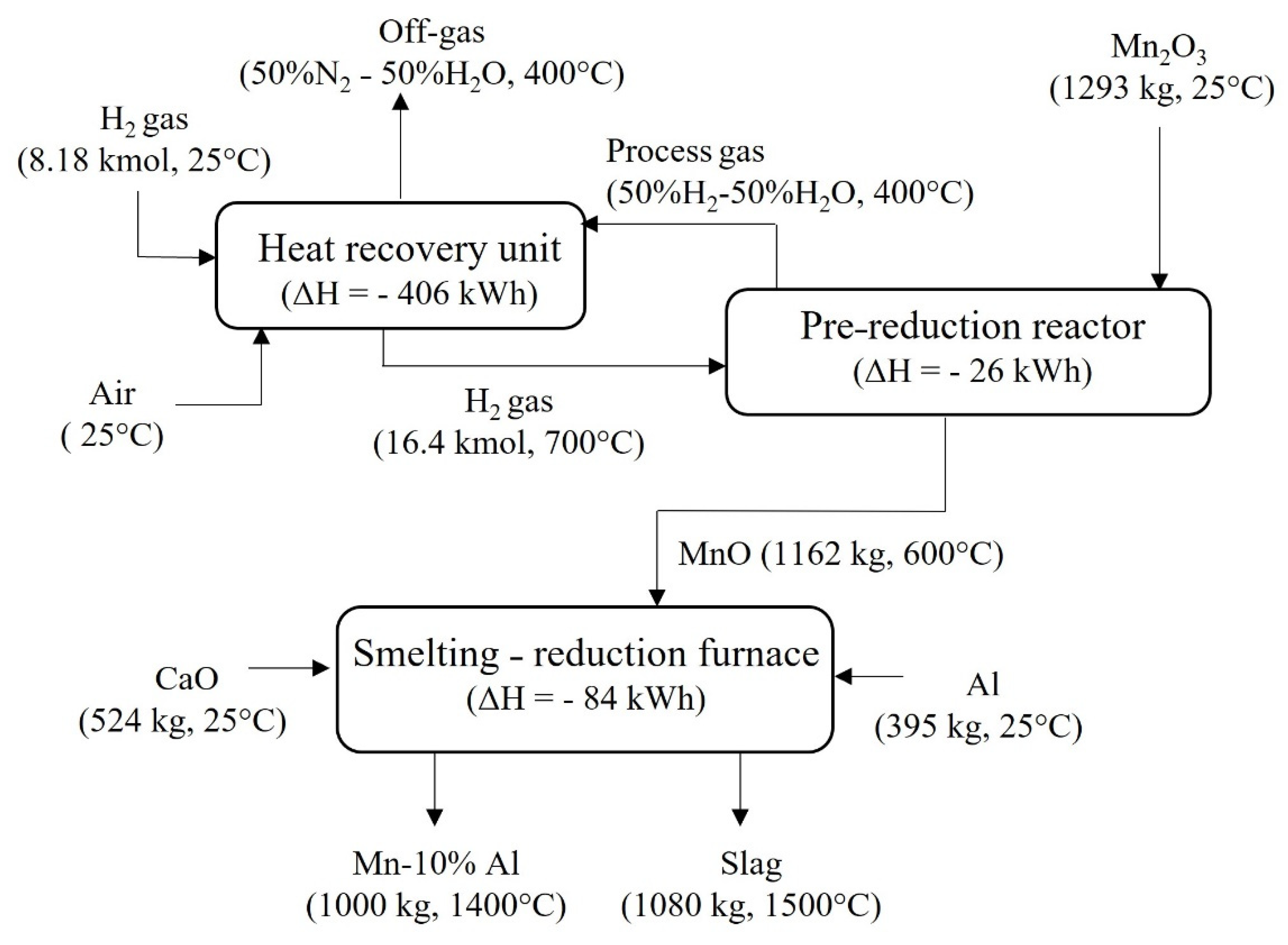
4.2. Mass and Energy Balances
4.2.1. Green Mn Metal Production
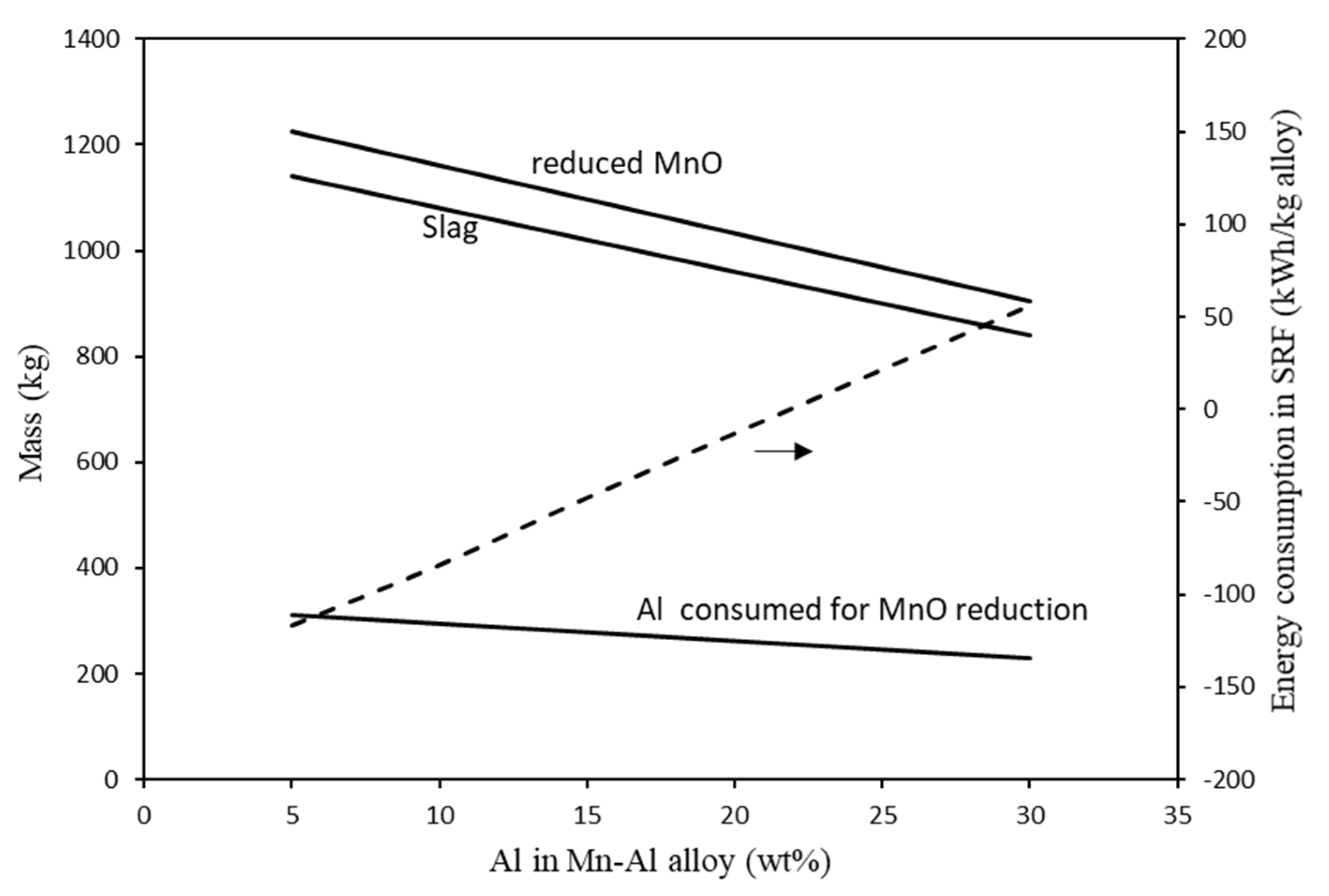
4.2.2. Green AlMn Alloys Production
4.2.3. Green FeMn Production
4.3. Process Sustainability
4.3.1. Materials and By-Products
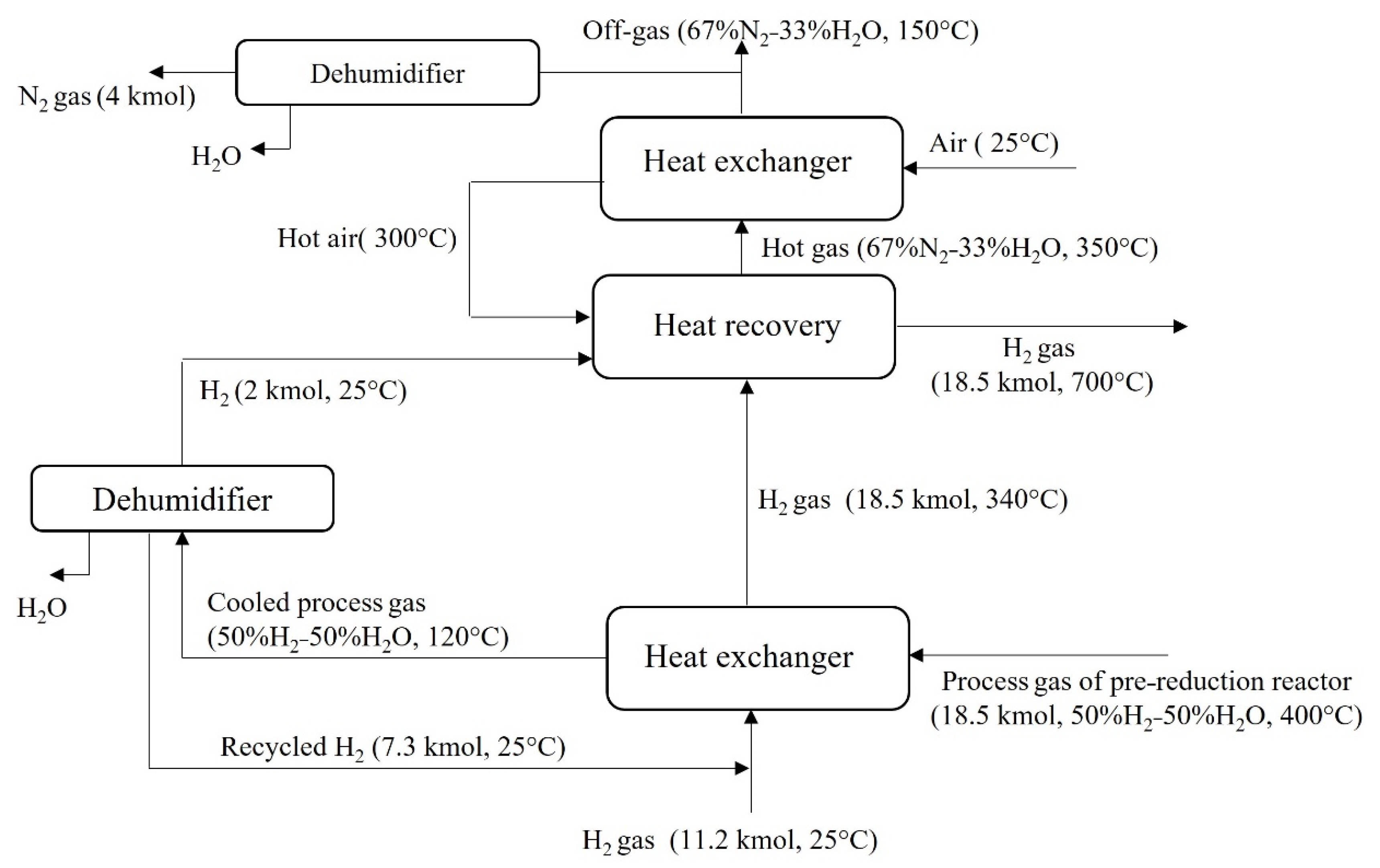
4.3.2. Hydrogen Looping
4.3.3. Energy Consumption
4.3.4. CO2 Emission
5. Conclusions
- The HAlMan process is flexible and can be implemented to produce Mn metal, a variety of AlMn alloys with different Al contents, and carbon-free FeMn alloys.
- The HAlMan process has three main steps for the pre-reduction of Mn ore/oxides to MnO, smelting-aluminothermic reduction of the pre-reduced ore, and a gas treatment unit to do hydrogen looping and hydrogen heating for the pre-reduction reactor.
- The process is quite flexible to use different raw materials such as manganese and iron ores, iron scrap, Al scrap, Al dross, HCFeMn slag, etc.
- Hydrogen recovery from the pre-reduction reactor process gas is very important and about 78% of the hydrogen in this gas can be looped and it can supply 40–50% of the reactor feed gas.
- Total energy consumption in the HAlMan process is low; below 1500 kWh/t Mn metal, and below 1400 kWh/t AlMn alloys. Energy consumption is decreased with increasing Al content of the alloy.
- Energy consumption to produce Green FeMn is in the range 1000–1700 kWh/t, depending on iron source, which is significantly lower than current commercial processes for FeMn alloys production.
- CO2 emission from the HAlMan process is insignificant; Green FeMn production by this process instead of the current commercial technologies will prevent the emission of about 1.5 t CO2/t FeMn.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Manganese Institute. IMNI Statistics Report 2020. Available online: https://www.manganese.org/ (accessed on 10 November 2021).
- Olsen, S.E.; Tangstad, M.; Lindstad, T. Production of Manganese Ferroalloys; Tapir Academic Press: Trondheim, Norway, 2007. [Google Scholar]
- Safarian, J. Duplex Process to Produce Ferromanganese and Direct Reduced Iron by Natural Gas. ACS Sustain. Chem. Eng. 2021, 9, 5010–5026. [Google Scholar] [CrossRef]
- Cheraghi, A.; Yoozbashizadeh, H.; Safarian, J. Gaseous Reduction of Manganese Ores: A Review and Theoretical Insight. Miner. Process. Extr. Met. Rev. 2020, 41, 198–215. [Google Scholar] [CrossRef]
- Kudyba, A.; Aktar, S.; Johansen, I.; Safarian, J. Aluminothermic Reduction of Manganese Oxide from Selected MnO-Containing Slags. Materials 2021, 14, 356. [Google Scholar] [CrossRef] [PubMed]
- Barner, H.E.; Mantell, C.L. Kinetics of Hydrogen Reduction of Manganese Dioxide. Ind. Eng. Chem. Process. Des. Dev. 1968, 7, 285–294. [Google Scholar] [CrossRef]
- De Bruijn, T.; Soerawidjaja, T.; De Jongt, W.; Van Den Berg, P. Modelling of the reduction of manganese oxides with hydrogen. Chem. Eng. Sci. 1980, 35, 1591–1599. [Google Scholar] [CrossRef]
- Zaki, M.; Hasan, M.; Pasupulety, L.; Kumari, K. Thermochemistry of manganese oxides in reactive gas atmospheres: Probing redox compositions in the decomposition course MnO2 → MnO. Thermochim. Acta 1997, 303, 171–181. [Google Scholar] [CrossRef]
- Terayama, K.; Shimazaki, T. Effect of hydrogen on the reduction kinetics of manganese oxide at high temperatures by new EGA method. Netsu Sokutei 2000, 27, 13–18. [Google Scholar]
- El-Gawad, H.H.A.; Ahmed, M.; El-Hussiny, N.; Shalabi, M. Reduction of low grade Egyptian manganese ore via hydrogen at 800 C–950 C. Open Access Libr. J. 2014, 1, e427. [Google Scholar] [CrossRef]
- El-Hussiny, N.; El-Gawad, H.H.A.; Mohamed, F.; Shalabi, M. Pelletization and reduction of Egyptian low grade manganese ore pellets via hydrogen at 750–950 °C. Int. J. Sci. Eng. Res. 2015, 6, 339–346. [Google Scholar]
- Jiaxing, S.; Tao, G.; Wen, D.; Yiming, M.; Xiang, F.; Hao, W. Study on Thermal Chemical Reaction of Al/MnO2 Thermite. IOP Conf. Ser. Earth Environ. Sci. 2018, 186, 012046. [Google Scholar] [CrossRef]
- Sarangi, B.; Ray, H.S. Kinetics of Aluminothermic Reduction of MnO2 and Fe2O3: A Thermoanalytical Investigation. ISIJ Int. 1996, 36, 1135–1141. [Google Scholar] [CrossRef]
- Bhoi, B.; Murthy, B.V.R.; Datta, P.; Jouhari, A.K. Studies on Aluminothermic Reduction of Manganese ore for Ferro-Manganese Making. In Ferro Alloy Industries in the Liberalised Economy; Vatsh, A.K., Singh, S.D., Gas, N.G., Ramachandrarao, P., Eds.; NML: Jainshedpur, India, 1997; pp. 66–70. [Google Scholar]
- Sorensen, B.; Gaal, S.; Ringdalen, E.; Tangstad, M.; Kononov, R.; Ostrovski, O. Phase compositions of manganese ores and their change in the process of calcination. Int. J. Miner. Process. 2010, 94, 101–110. [Google Scholar] [CrossRef]
- Cheraghi, A.; Becker, H.; Eftekhari, H.; Yoozbashizadeh, H.; Safarian, J. Characterization and calcination behavior of a low-grade manganese ore. Mater. Today Commun. 2020, 25, 101382. [Google Scholar] [CrossRef]
- Azof, F.I.; Safarian, J. Leaching kinetics and mechanism of slag produced from smelting-reduction of bauxite for alumina recovery. Hydrometall. 2020, 195, 105388. [Google Scholar] [CrossRef]
- Azof, F.I.; Yang, Y.; Panias, D.; Kolbeinsen, L.; Safarian, J. Leaching characteristics and mechanism of the synthetic calcium-aluminate slags for alumina recovery. Hydrometallurgy 2019, 185, 273–290. [Google Scholar] [CrossRef]
- Bertuccioli, L.; Chan, A.; Hart, D.; Lehner, F.; Madden, B.; Standen, E. Study on Development of Water Electrolysis in the EU, E4tech Sàrl with Element Energy Ltd., for the Fuel Cells and Hydrogen Joint Undertaking. 2014. Available online: https://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/reference/referencespapers.aspx?referenceid=2141466 (accessed on 15 November 2021).
- Stafekk, I.; Scammen, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the globalenergy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar]
- Ahmed, A.; Haifa, H.; El-Fawakhry, M.K.; El-Faramawy, H.; Eissa, M. Parameters Affecting Energy Consumption for Producing High Carbon Ferromanganese in a Closed Submerged Arc Furnace. J. Iron Steel Res. Int. 2014, 21, 666–672. [Google Scholar] [CrossRef]
- Lee, Y.E.; Kolbeinsen, L. Kinetics of oxygen rfining process for ferromanganese alloys. ISIJ Int. 2005, 45, 1282–1290. [Google Scholar] [CrossRef][Green Version]
- You, B.-D.; Lee, B.-W.; Pak, J.-J. Manganese loss during the oxygen refining of high-carbon ferromanganese melts. Met. Mater. 1999, 5, 497–502. [Google Scholar] [CrossRef]

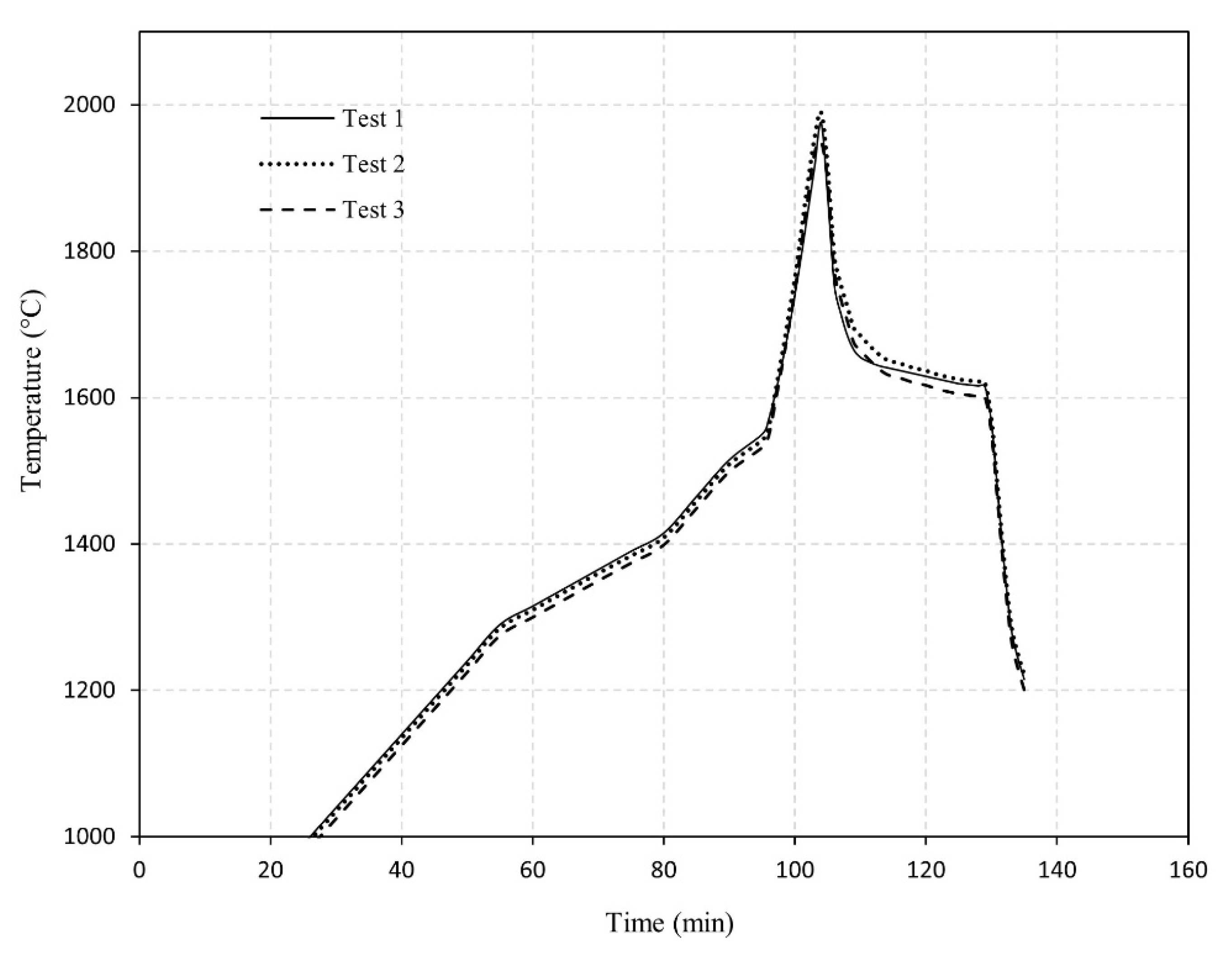
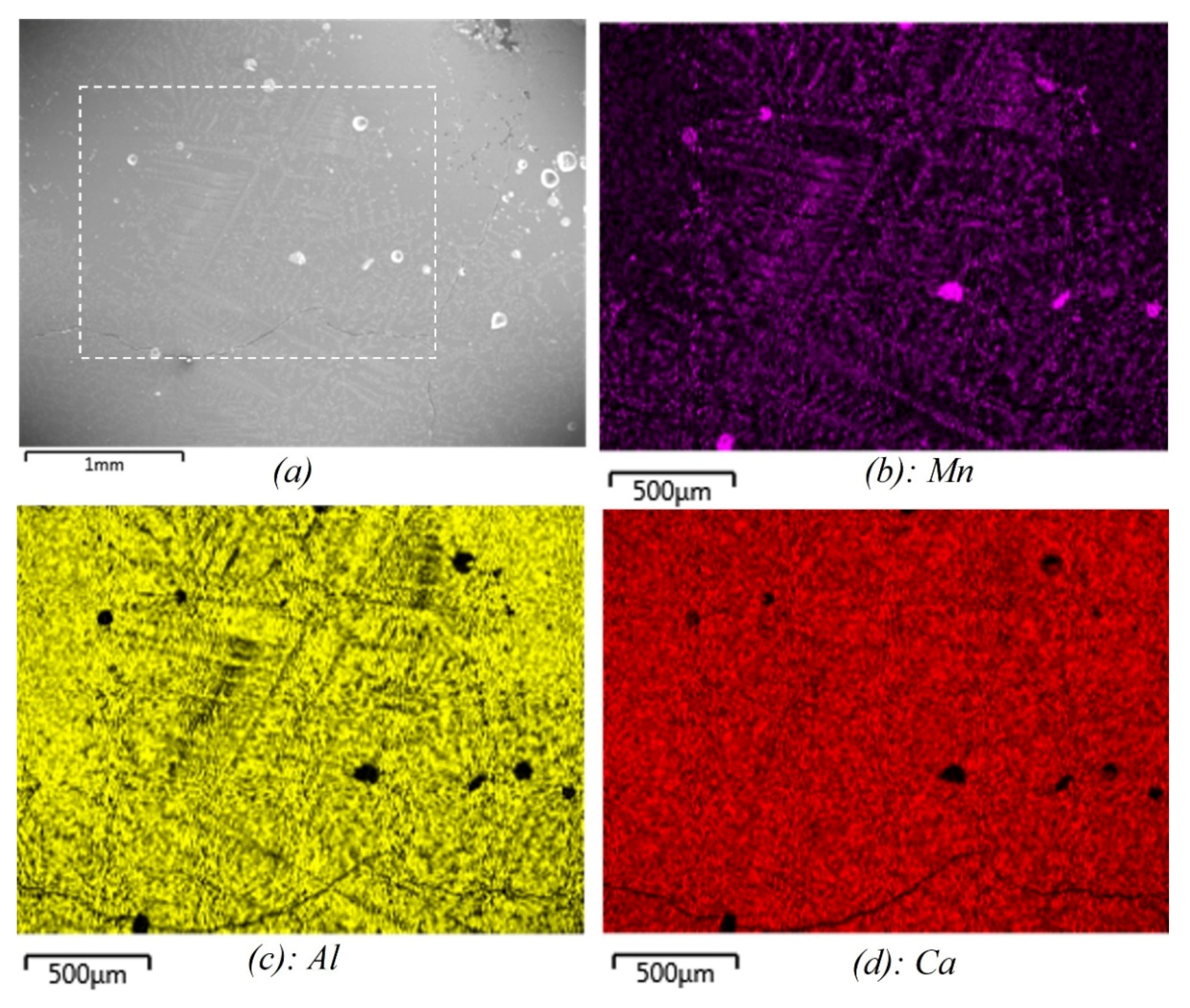


| Metal | ||||||
| Component: | Mn | Al | Fe | Si | Ca | Mg |
| Test 1 | 98.6 | - | 1.2 | 0.2 | - | 0.01 |
| Test 2 | 92.1 | 6.0 | 0.9 | 0.6 | 0.5 | - |
| Test 3 | 79.0 | - | 20.7 | 0.3 | - | - |
| Slag | ||||||
| Component: | MnO | Al2O3 | FeO | SiO2 | CaO | MgO |
| Test 1 | 20.3 | 38.9 | - | 0.9 | 39.2 | 0.7 |
| Test 2 | 0.02 | 49.2 | - | 0.5 | 49.5 | 0.5 |
| Test 3 | 20.1 | 38.5 | 0.03 | 0.8 | 40.0 | 0.6 |
| Metal Product | H2 Reactor Feed Gas | H2 Looped | H2 Burned for Heat Recovery | Net H2 Input of HAlMan Process |
|---|---|---|---|---|
| Mn metal | 18.5 | 7.3 | 2 | 11.2 |
| Mn−10%Al | 16.4 | 6.4 | 1.8 | 10 |
| Mn−20%Al | 14.6 | 5.7 | 1.6 | 8.9 |
| Mn−30%Al | 12.7 | 5.0 | 1.4 | 7.7 |
| Mn−20%Fe, iron scrap use | 14.6 | 5.7 | 1.6 | 8.9 |
| Mn−20%Fe, Fe2O3 use | 25.3 | 9.9 | 2.8 | 15.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safarian, J. A Sustainable Process to Produce Manganese and Its Alloys through Hydrogen and Aluminothermic Reduction. Processes 2022, 10, 27. https://doi.org/10.3390/pr10010027
Safarian J. A Sustainable Process to Produce Manganese and Its Alloys through Hydrogen and Aluminothermic Reduction. Processes. 2022; 10(1):27. https://doi.org/10.3390/pr10010027
Chicago/Turabian StyleSafarian, Jafar. 2022. "A Sustainable Process to Produce Manganese and Its Alloys through Hydrogen and Aluminothermic Reduction" Processes 10, no. 1: 27. https://doi.org/10.3390/pr10010027
APA StyleSafarian, J. (2022). A Sustainable Process to Produce Manganese and Its Alloys through Hydrogen and Aluminothermic Reduction. Processes, 10(1), 27. https://doi.org/10.3390/pr10010027