Diels–Alder Cycloadditions of Bio-Derived Furans with Maleimides as a Sustainable «Click» Approach towards Molecular, Macromolecular and Hybrid Systems
Abstract
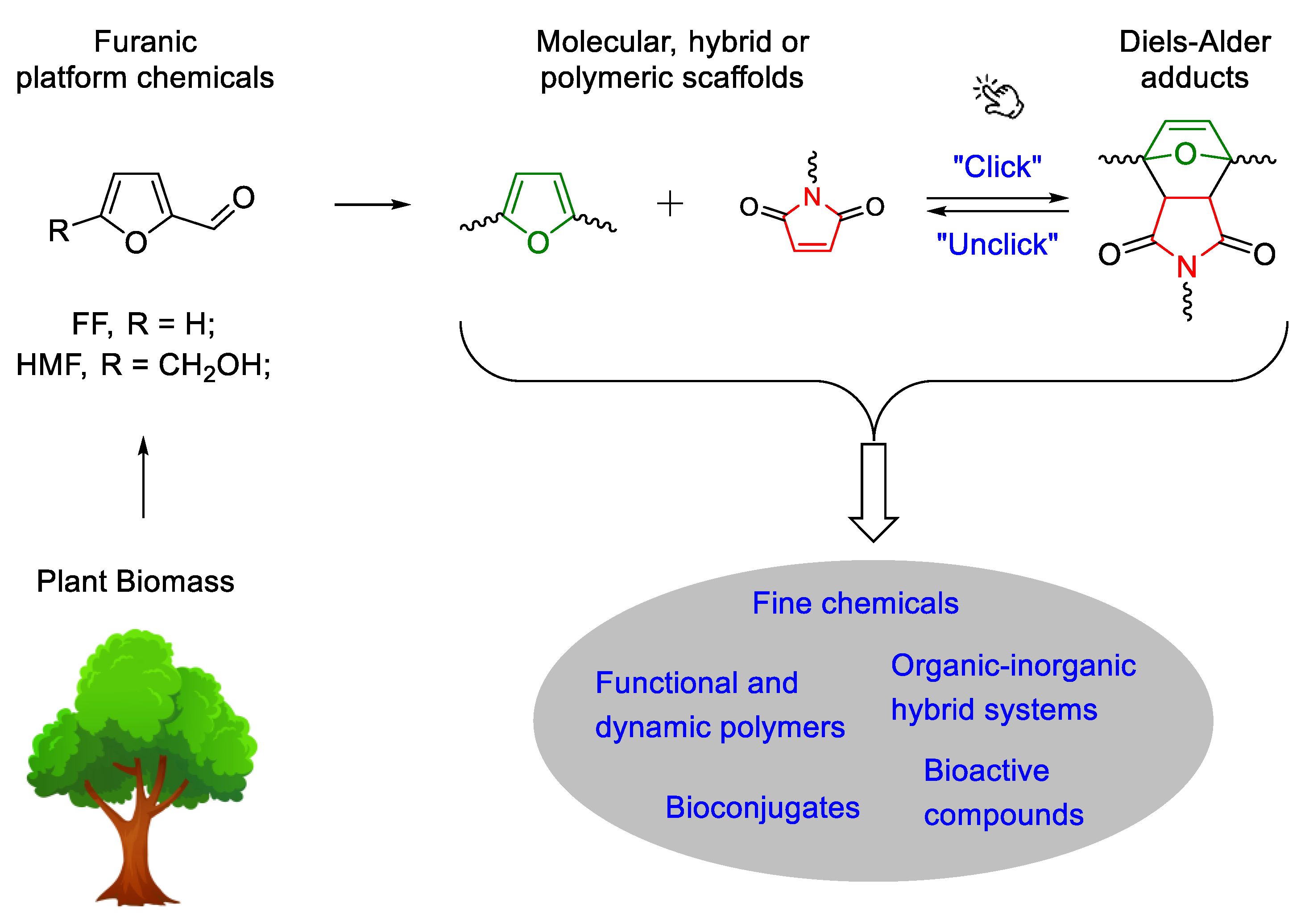
:1. Introduction
2. Application of fmDA “Click” Reaction for Synthesis of Functional Fine Chemicals
3. Application of a fmDA “Click” Approach for the Development of Dynamic Molecular, Biomolecular and Organic-Inorganic Hybrid Systems
4. Application of fmDA Cycloaddition for the Preparation of Functional or Dynamic Polymers
4.1. Synthesis of Dynamic Linear Polymers Using the fmDA “Click” Reaction
4.2. Synthesis of Cross-Linked Dynamers Using the fmDA “Click” Reaction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-MF | 2-methylfuran |
| BAMF | 2,5-bis(acetoxymethyl)furan |
| BHMF | 2,5-bis(hydroxymethyl)furan |
| BMI | 4,4’-bis(maleimido)diphenylmethane |
| Bn | benzyl |
| CAN | covalent adapfigure network |
| DA | Diels–Alder |
| DFT | density functional theory |
| DMF | 2,5-dimethylfuran |
| FA | furfuryl alcohol |
| FF | furfural |
| fmDA | furan/maleimide Diels–Alder |
| HMF | 5-(hydroxymethyl)furfural |
| HOMO | highest occupied molecular orbital |
| LUMO | lowest unoccupied molecular orbital |
| N.d. | not determined |
| NMR | nuclear magnetic resonance |
| NP | nanoparticle |
| PDI | polydispersity index |
| rDA | retro-Diels–Alder |
| ROMP | ring-opening metathesis polymerization |
| RT | room temperature |
| TFA | trifluoroacetic acid |
| THF | tetrahydrofuran |
| Ts | tosyl |
References
- Hou, Q.; Qi, X.; Zhen, M.; Qian, H.; Nie, Y.; Bai, C.; Zhang, S.; Bai, X.; Ju, M. Biorefinery roadmap based on catalytic production and upgrading 5-hydroxymethylfurfural. Green Chem. 2021, 23, 119–231. [Google Scholar] [CrossRef]
- Galkin, K.I.; Ananikov, V.P. The Increasing Value of Biomass: Moving From C6 Carbohydrates to Multifunctionalized Building Blocks via 5-(hydroxymethyl)furfural. ChemistryOpen 2020, 9, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Paone, E.; Rodriguez-Padron, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Galkin, K.I.; Ananikov, V.P. When Will 5-Hydroxymethylfurfural, the “Sleeping Giant” of Sustainable Chemistry, Awaken? ChemSusChem 2019, 12, 2976–2982. [Google Scholar] [CrossRef]
- Mika, L.T.; Cséfalvay, E.; Németh, A. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef]
- Zhu, J.; Yin, G. Catalytic Transformation of the Furfural Platform into Bifunctionalized Monomers for Polymer Synthesis. ACS Catal. 2021, 11, 10058–10083. [Google Scholar] [CrossRef]
- Kucherov, F.A.; Romashov, L.V.; Galkin, K.I.; Ananikov, V.P. Chemical Transformations of Biomass-Derived C6-Furanic Platform Chemicals for Sustainable Energy Research, Materials Science, and Synthetic Building Blocks. ACS Sustain. Chem. Eng. 2018, 6, 8064–8092. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Wang, T. Furfural: A Promising Platform Compound for Sustainable Production of C4 and C5 Chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M.; Carvalho, A.J.; Trovatti, E. Progress of Polymers from Renewable Resources: Furans, Vegetable Oils, and Polysaccharides. Chem. Rev. 2016, 116, 1637–1669. [Google Scholar] [CrossRef]
- Van Putten, R.J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Geng, Z.; Shin, J.J.; Xi, Y.; Hawker, C.J. Click chemistry strategies for the accelerated synthesis of functional macromolecules. J. Polym. Sci. 2021, 59, 963–1042. [Google Scholar] [CrossRef]
- Arslan, M.; Acik, G.; Tasdelen, M.A. The emerging applications of click chemistry reactions in the modification of industrial polymers. Polym. Chem. 2019, 10, 3806–3821. [Google Scholar] [CrossRef]
- Tasdelen, M.A. Diels–Alder “click” reactions: Recent applications in polymer and material science. Polym. Chem. 2011, 2, 2133–2145. [Google Scholar] [CrossRef]
- Settle, A.E.; Berstis, L.; Rorrer, N.A.; Román-Leshkov, Y.; Beckham, G.T.; Richards, R.M.; Vardon, D.R. Heterogeneous Diels–Alder catalysis for biomass-derived aromatic compounds. Green Chem. 2017, 19, 3468–3492. [Google Scholar] [CrossRef]
- Galkin, K.I.; Ananikov, V.P. Intermolecular Diels-Alder Cycloadditions of Furfural-Based Chemicals from Renewable Resources: A Focus on the Regio- and Diastereoselectivity in the Reaction with Alkenes. Int. J. Mol. Sci. 2021, 22, 11856. [Google Scholar] [CrossRef]
- Cioc, R.C.; Lutz, M.; Pidko, E.A.; Crockatt, M.; van der Waal, J.C.; Bruijnincx, P.C.A. Direct Diels–Alder reactions of furfural derivatives with maleimides. Green Chem. 2021, 23, 367–373. [Google Scholar] [CrossRef]
- Cioc, R.C.; Smak, T.J.; Crockatt, M.; van der Waal, J.C.; Bruijnincx, P.C.A. Furoic acid and derivatives as atypical dienes in Diels-Alder reactions. Green Chem. 2021, 23, 5503–5510. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A.; Carvalho, A.J.F.; Trovatti, E.; Kramer, R.K.; Lacerda, T.M. Macromolecular materials based on the application of the Diels-Alder reaction to natural polymers and plant oils. Eur. J. Lipid Sci. Technol. 2018, 120, 1700091. [Google Scholar] [CrossRef]
- Gandini, A. The furan/maleimide Diels–Alder reaction: A versatile click–unclick tool in macromolecular synthesis. Prog. Polym. Sci. 2013, 38, 1–29. [Google Scholar] [CrossRef]
- Gevrek, T.N.; Sanyal, A. Furan-containing polymeric Materials: Harnessing the Diels-Alder chemistry for biomedical applications. Eur. Polym. J. 2021, 153, 110514. [Google Scholar] [CrossRef]
- Kucherov, F.A.; Romashov, L.V.; Averochkin, G.M.; Ananikov, V.P. Biobased C6-Furans in Organic Synthesis and Industry: Cycloaddition Chemistry as a Key Approach to Aromatic Building Blocks. ACS Sustain. Chem. Eng. 2021, 9, 3011–3042. [Google Scholar] [CrossRef]
- Ravasco, J.; Gomes, R.F.A. Recent Advances on Diels-Alder-Driven Preparation of Bio-Based Aromatics. ChemSusChem 2021, 14, 3047. [Google Scholar] [CrossRef]
- Naz, F.; Wu, Y.; Zhang, N.; Yang, Z.; Yu, C. Anticancer Attributes of Cantharidin: Involved Molecular Mechanisms and Pathways. Molecules 2020, 25, 3279. [Google Scholar] [CrossRef] [PubMed]
- Puerto Galvis, C.E.; Vargas Mendez, L.Y.; Kouznetsov, V.V. Cantharidin-based small molecules as potential therapeutic agents. Chem. Biol. Drug Des. 2013, 82, 477–499. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.P.; Dong, J.; Cai, H.; Wang, W. Cantharidin as an antitumor agent: A retrospective review. Curr. Med. Chem. 2013, 20, 159–166. [Google Scholar] [CrossRef]
- Hart, M.E.; Chamberlin, A.R.; Walkom, C.; Sakoff, J.A.; McCluskey, A. Modified norcantharidins; synthesis, protein phosphatases 1 and 2A inhibition, and anticancer activity. Bioorg. Med. Chem. Lett. 2004, 14, 1969–1973. [Google Scholar] [CrossRef]
- Baba, Y.; Hirukawa, N.; Tanohira, N.; Sodeoka, M. Structure-based design of a highly selective catalytic site-directed inhibitor of Ser/Thr protein phosphatase 2B (calcineurin). J. Am. Chem. Soc. 2003, 125, 9740–9749. [Google Scholar] [CrossRef]
- McCluskey, A.; Ackland, S.P.; Bowyer, M.C.; Baldwin, M.L.; Garner, J.; Walkom, C.C.; Sakoff, J.A. Cantharidin analogues: Synthesis and evaluation of growth inhibition in a panel of selected tumour cell lines. Bioorg. Chem. 2003, 31, 68–79. [Google Scholar] [CrossRef]
- Galkin, K.I.; Kucherov, F.A.; Markov, O.N.; Egorova, K.S.; Posvyatenko, A.V.; Ananikov, V.P. Facile Chemical Access to Biologically Active Norcantharidin Derivatives from Biomass. Molecules 2017, 22, 2210. [Google Scholar] [CrossRef] [Green Version]
- Salvati, M.E.; Balog, A.; Wei, D.D.; Pickering, D.; Attar, R.M.; Geng, J.; Rizzo, C.A.; Hunt, J.T.; Gottardis, M.M.; Weinmann, R.; et al. Identification of a novel class of androgen receptor antagonists based on the bicyclic-1H-isoindole-1,3(2H)-dione nucleus. Bioorg. Med. Chem. Lett. 2005, 15, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Weber, R.; Twieg, R.J. Improved synthesis of DCDHF fluorophores with maleimide functional groups. Tetrahedron Lett. 2006, 47, 7213–7217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daeffler, C.S.; Miyake, G.M.; Li, J.; Grubbs, R.H. Partial Kinetic Resolution of Oxanorbornenes by Ring-Opening Metathesis Polymerization with a Chiral Ruthenium Initiator. ACS Macro Lett. 2014, 3, 102–104. [Google Scholar] [CrossRef] [Green Version]
- Elduque, X.; Sanchez, A.; Sharma, K.; Pedroso, E.; Grandas, A. Protected Maleimide Building Blocks for the Decoration of Peptides, Peptoids, and Peptide Nucleic Acids. Bioconjug. Chem. 2013, 24, 832–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucherov, F.A.; Galkin, K.I.; Gordeev, E.G.; Ananikov, V.P. Efficient route for the construction of polycyclic systems from bioderived HMF. Green Chem. 2017, 19, 4858–4864. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Huber, G.W.; Dumesic, J.A. Chemical-Switching Strategy for Synthesis and Controlled Release of Norcantharimides from a Biomass-Derived Chemical. ChemSusChem 2020, 13, 5213–5219. [Google Scholar] [CrossRef]
- Liu, P.; Yasir, M.; Kilbinger, A.F.M. Catalytic Living Ring Opening Metathesis Polymerisation: The Importance of Ring Strain in Chain Transfer Agents. Angew. Chem. Int. Ed. 2019, 58, 15278–15282. [Google Scholar] [CrossRef] [Green Version]
- Yasir, M.; Liu, P.; Markwart, J.C.; Suraeva, O.; Wurm, F.R.; Smart, J.; Lattuada, M.; Kilbinger, A.F.M. One-Step Ring Opening Metathesis Block-Like Copolymers and their Compositional Analysis by a Novel Retardation Technique. Angew. Chem. Int. Ed. 2020, 59, 13597–13601. [Google Scholar] [CrossRef]
- Froidevaux, V.; Borne, M.; Laborbe, E.; Auvergne, R.; Gandini, A.; Boutevin, B. Study of the Diels–Alder and retro-Diels–Alder reaction between furan derivatives and maleimide for the creation of new materials. RSC Adv. 2015, 5, 37742–37754. [Google Scholar] [CrossRef]
- Clavier, H.; Broggi, J.; Nolan, S.P. Ring-Rearrangement Metathesis (RRM) Mediated by Ruthenium-Indenylidene Complexes. Eur. J. Org. Chem. 2010, 2010, 937–943. [Google Scholar] [CrossRef]
- Román, E.; Gil, M.; Luque-Agudo, V.; Serrano, J. Expeditious ‘On-Water’ Cycloaddition between N-Substituted Maleimides and Furans. Synlett 2014, 25, 2179–2183. [Google Scholar] [CrossRef]
- Canadell, J.; Fischer, H.; De With, G.; van Benthem, R.A.T.M. Stereoisomeric effects in thermo-remendable polymer networks based on Diels-Alder crosslink reactions. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3456–3467. [Google Scholar] [CrossRef]
- Fan, B.; Trant, J.F.; Hemery, G.; Sandre, O.; Gillies, E.R. Thermo-responsive self-immolative nanoassemblies: Direct and indirect triggering. Chem. Commun. 2017, 53, 12068–12071. [Google Scholar] [CrossRef] [Green Version]
- Taimoory, S.M.; Sadraei, S.I.; Fayoumi, R.A.; Nasri, S.; Revington, M.; Trant, J.F. Preparation and Characterization of a Small Library of Thermally-Labile End-Caps for Variable-Temperature Triggering of Self-Immolative Polymers. J. Org. Chem. 2018, 83, 4427–4440. [Google Scholar] [CrossRef] [PubMed]
- Heath, W.H.; Palmieri, F.; Adams, J.R.; Long, B.K.; Chute, J.; Holcombe, T.W.; Zieren, S.; Truitt, M.J.; White, J.L.; Willson, C.G. Degradable Cross-Linkers and Strippable Imaging Materials for Step-and-Flash Imprint Lithography. Macromolecules 2008, 41, 719–726. [Google Scholar] [CrossRef]
- Sanchez, A.; Pedroso, E.; Grandas, A. Maleimide-dimethylfuran exo adducts: Effective maleimide protection in the synthesis of oligonucleotide conjugates. Org. Lett. 2011, 13, 4364–4367. [Google Scholar] [CrossRef] [PubMed]
- Budd, M.E.; Stephens, R.; Afsar, A.; Salimi, S.; Hayes, W. Exploiting thermally-reversible covalent bonds for the controlled release of microencapsulated isocyanate crosslinkers. React. Funct. Polym. 2019, 135, 23–31. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels-Alder Reaction in Total Synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Jarosz, S.; Mach, M.; Szewczyk, K.; Skóra, S.; Ciunik, Z. Synthesis of Sugar-Derived 2′- and 3′-Substituted Furans and Their Application in Diels−Alder Reactions. Eur. J. Org. Chem. 2001, 2001, 2955–2964. [Google Scholar] [CrossRef]
- Uemura, N.; Toyoda, S.; Ishikawa, H.; Yoshida, Y.; Mino, T.; Kasashima, Y.; Sakamoto, M. Asymmetric Diels-Alder Reaction Involving Dynamic Enantioselective Crystallization. J. Org. Chem. 2018, 83, 9300–9304. [Google Scholar] [CrossRef]
- Jegat, C.; Mignard, N. Effect of the polymer matrix on the thermal behaviour of a furan-maleimide type adduct in the molten state. Polym. Bull. 2008, 60, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Oparina, L.A.; Vysotskaya, O.V.; Stepanov, A.V.; Ushakov, I.A.; Apartsin, K.A.; Gusarova, N.K.; Trofimov, B.A. Furfuryl vinyl ethers in [4+2]-cycloaddition reactions. Russ. J. Org. Chem. 2017, 53, 203–209. [Google Scholar] [CrossRef]
- Ding, X.; Nguyen, S.T.; Williams, J.D.; Peet, N.P. Diels-Alder reactions of five-membered heterocycles containing one heteroatom. Tetrahedron Lett. 2014, 55, 7002–7006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastin, L.D.; Nigam, M.; Martinus, S.; Maloney, J.E.; Benyack, L.L.; Gainer, B. Synthesis of substituted N-phenylmaleimides and use in a Diels-Alder reaction: A green multi-step synthesis for an undergraduate organic chemistry laboratory. Green Chem. Lett. Rev. 2019, 12, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.; John, J.M.; Schrock, R.R. Formation of Alternating trans-A-alt-B Copolymers through Ring-Opening Metathesis Polymerization Initiated by Molybdenum Imido Alkylidene Complexes. Organometallics 2015, 34, 5136–5145. [Google Scholar] [CrossRef]
- Park, J.; Heo, J.M.; Seong, S.; Noh, J.; Kim, J.M. Self-assembly using a retro Diels-Alder reaction. Nat. Commun. 2021, 12, 4207. [Google Scholar] [CrossRef]
- Czifrak, K.; Lakatos, C.; Karger-Kocsis, J.; Daroczi, L.; Zsuga, M.; Keki, S. One-Pot Synthesis and Characterization of Novel Shape-Memory Poly(epsilon-Caprolactone) Based Polyurethane-Epoxy Co-networks with Diels(-)Alder Couplings. Polymers 2018, 10, 504. [Google Scholar] [CrossRef] [Green Version]
- Potts, K.T.; Walsh, E.B. Furfural dimethylhydrazone: A versatile diene for arene cycloaromatization. J. Org. Chem. 2002, 49, 4099–4101. [Google Scholar] [CrossRef]
- Higson, S.; Subrizi, F.; Sheppard, T.D.; Hailes, H.C. Chemical cascades in water for the synthesis of functionalized aromatics from furfurals. Green Chem. 2016, 18, 1855–1858. [Google Scholar] [CrossRef] [Green Version]
- Karaluka, V.; Murata, K.; Masuda, S.; Shiramatsu, Y.; Kawamoto, T.; Hailes, H.C.; Sheppard, T.D.; Kamimura, A. Development of a microwave-assisted sustainable conversion of furfural hydrazones to functionalised phthalimides in ionic liquids. RSC Adv. 2018, 8, 22617–22624. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Wang, Y.; Gong, J.; Zhang, X. Dynamic dye emission ON/OFF systems by a furan moiety exchange protocol. Dyes Pigm. 2021, 184, 108652. [Google Scholar] [CrossRef]
- Li, F.; Li, X.; Zhang, X. Dynamic Diels-Alder reactions of maleimide-furan amphiphiles and their fluorescence ON/OFF behaviours. Org. Biomol. Chem. 2018, 16, 7871–7877. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, A.B.; Hsiao, D.; Jin, G.; Gates, B.D.; Branda, N.R. An efficient method based on the photothermal effect for the release of molecules from metal nanoparticle surfaces. Angew. Chem. Int. Ed. 2009, 48, 4166–4169. [Google Scholar] [CrossRef]
- Gregoritza, M.; Brandl, F.P. The Diels-Alder reaction: A powerful tool for the design of drug delivery systems and biomaterials. Eur. J. Pharm. Biopharm. 2015, 97, 438–453. [Google Scholar] [CrossRef] [PubMed]
- Durand, H.; Baussanne, I.; Demeunynck, M.; Viger-Gravel, J.; Emsley, L.; Bardet, M.; Zeno, E.; Belgacem, N.; Bras, J. Two-step immobilization of metronidazole prodrug on TEMPO cellulose nanofibrils through thiol-yne click chemistry for in situ controlled release. Carbohydr. Polym. 2021, 262, 117952. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Durand, H.; Zeno, E.; Balsollier, C.; Watbled, B.; Sillard, C.; Fort, S.; Baussanne, I.; Belgacem, N.; Lee, D.; et al. The surface chemistry of a nanocellulose drug carrier unravelled by MAS-DNP. Chem. Sci. 2020, 11, 3868–3877. [Google Scholar] [CrossRef] [Green Version]
- Bliman, D.; Demeunynck, M.; Leblond, P.; Meignan, S.; Baussane, I.; Fort, S. Enzymatically Activated Glyco-Prodrugs of Doxorubicin Synthesized by a Catalysis-Free Diels-Alder Reaction. Bioconjug. Chem. 2018, 29, 2370–2381. [Google Scholar] [CrossRef]
- Mancuso, L.; Knobloch, T.; Buchholz, J.; Hartwig, J.; Moller, L.; Seidel, K.; Collisi, W.; Sasse, F.; Kirschning, A. Preparation of thermocleavable conjugates based on ansamitocin and superparamagnetic nanostructured particles by a chemobiosynthetic approach. Chem. A Eur. J. 2014, 20, 17541–17551. [Google Scholar] [CrossRef]
- Guldris, N.; Gallo, J.; Garcia-Hevia, L.; Rivas, J.; Banobre-Lopez, M.; Salonen, L.M. Orthogonal Clickable Iron Oxide Nanoparticle Platform for Targeting, Imaging, and On-Demand Release. Chem. -A Eur. J. 2018, 24, 8624–8631. [Google Scholar] [CrossRef]
- Dirlam, P.T.; Strange, G.A.; Orlicki, J.A.; Wetzel, E.D.; Costanzo, P.J. Controlling surface energy and wetability with Diels-Alder chemistry. Langmuir 2010, 26, 3942–3948. [Google Scholar] [CrossRef]
- Yasir, M.; Liu, P.; Tennie, I.K.; Kilbinger, A.F.M. Catalytic living ring-opening metathesis polymerization with Grubbs’ second- and third-generation catalysts. Nat. Chem. 2019, 11, 488–494. [Google Scholar] [CrossRef]
- Pal, S.; Alizadeh, M.; Kong, P.; Kilbinger, A.F.M. Oxanorbornenes: Promising new single addition monomers for the metathesis polymerization. Chem. Sci. 2021, 12, 6705–6711. [Google Scholar] [CrossRef]
- Sun, H.; Kabb, C.P.; Dai, Y.; Hill, M.R.; Ghiviriga, I.; Bapat, A.P.; Sumerlin, B.S. Macromolecular metamorphosis via stimulus-induced transformations of polymer architecture. Nat. Chem. 2017, 9, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Shi, Q.; Zhang, L.; Liu, B.; Zhang, Y.; Gao, Y.; Jia, R.; Zhang, Z.; Zhu, X. Stereoisomeric furan/maleimide adducts as latent monomers for one-shot sequence-controlled polymerization. Polym. Chem. 2020, 11, 1614–1620. [Google Scholar] [CrossRef]
- Ax, J.; Wenz, G. Thermoreversible Networks by Diels–Alder Reaction of Cellulose Furoates With Bismaleimides. Macromol. Chem. Phys. 2012, 213, 182–186. [Google Scholar] [CrossRef]
- Navarro, J.R.; Conzatti, G.; Yu, Y.; Fall, A.B.; Mathew, R.; Eden, M.; Bergstrom, L. Multicolor fluorescent labeling of cellulose nanofibrils by click chemistry. Biomacromolecules 2015, 16, 1293–1300. [Google Scholar] [CrossRef]
- Ma, K.; Chen, G.; Zhang, Y. Thermal cross-link between 2,5-furandicarboxylic acid-based polyimides and bismaleimide via Diels-Alder reaction. J. Polym. Sci. 2020, 58, 2951–2962. [Google Scholar] [CrossRef]
- Mukherjee, S.; Brooks, W.L.A.; Dai, Y.; Sumerlin, B.S. Doubly-dynamic-covalent polymers composed of oxime and oxanorbornene links. Polym. Chem. 2016, 7, 1971–1978. [Google Scholar] [CrossRef]
- Banella, M.B.; Giacobazzi, G.; Vannini, M.; Marchese, P.; Colonna, M.; Celli, A.; Gandini, A.; Gioia, C. A Novel Approach for the Synthesis of Thermo-Responsive Co-Polyesters Incorporating Reversible Diels–Alder Adducts. Macromol. Chem. Phys. 2019, 220, 1900247. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Polymer engineering based on reversible covalent chemistry: A promising innovative pathway towards new materials and new functionalities. Prog. Polym. Sci. 2018, 80, 39–93. [Google Scholar] [CrossRef]
- Briou, B.; Ameduri, B.; Boutevin, B. Trends in the Diels-Alder reaction in polymer chemistry. Chem. Soc. Rev. 2021, 50, 11055–11097. [Google Scholar] [CrossRef]
- Zheng, N.; Xu, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: A Molecular Platform for Designing Functions beyond Chemical Recycling and Self-Healing. Chem. Rev. 2021, 121, 1716–1745. [Google Scholar] [CrossRef] [PubMed]
- Chakma, P.; Konkolewicz, D. Dynamic Covalent Bonds in Polymeric Materials. Angew. Chem. Int. Ed. 2019, 58, 9682–9695. [Google Scholar] [CrossRef] [PubMed]
- Munkhbat, O.; Gok, O.; Sanyal, R.; Sanyal, A. Multiarm star polymers with a thermally cleavable core: A “grafting-from” approach paves the way. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 885–893. [Google Scholar] [CrossRef]
- Lorenzini, R.G.; Sotzing, G.A. Furan/imide Diels-Alder polymers as dielectric materials. J. Appl. Polym. Sci. 2014, 131, 40179. [Google Scholar] [CrossRef]
- Gaina, C.; Ursache, O.; Gaina, V. Thermal Behavior of New Polymaleamides. Polym.-Plast. Technol. Eng. 2014, 53, 353–364. [Google Scholar] [CrossRef]
- Gaina, C.; Ursache, O.; Gaina, V.; Varganici, C.D. Poly(urethane-benzoxazine)s. J. Polym. Res. 2014, 21, 586. [Google Scholar] [CrossRef]
- Satoh, H.; Mineshima, A.; Nakamura, T.; Teramoto, N.; Shibata, M. Thermo-reversible Diels–Alder polymerization of difurfurylidene diglycerol and bismaleimide. React. Funct. Polym. 2014, 76, 49–56. [Google Scholar] [CrossRef]
- Micheel, M.; Ahner, J.; Frey, M.; Neumann, C.; Hager, M.D.; Dietzek, B. Photophysics of a Bis-Furan-Functionalized 4,7-bis(Phenylethynyl)-2,1,3-benzothiadiazole: A Building Block for Dynamic Polymers. ChemPhotoChem 2019, 3, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Ahner, J.; Dahlke, J.; Pretzel, D.; Schubert, U.S.; Dietzek, B.; Hager, M.D. Thermally Switchable Fluorescence Resonance Energy Transfer via Reversible Diels-Alder Reaction of pi-Conjugated Oligo-(Phenylene Ethynylene)s. Macromol. Rapid Commun. 2018, 39, e1700789. [Google Scholar] [CrossRef]
- Wu, C.-S.; Kao, T.-H.; Li, H.-Y.; Liu, Y.-L. Preparation of polybenzoxazine-functionalized Fe3O4 nanoparticles through in situ Diels–Alder polymerization for high performance magnetic polybenzoxazine/Fe3O4 nanocomposites. Compos. Sci. Technol. 2012, 72, 1562–1567. [Google Scholar] [CrossRef]
- Liu, X.; Du, P.; Liu, L.; Zheng, Z.; Wang, X.; Joncheray, T.; Zhang, Y. Kinetic study of Diels–Alder reaction involving in maleimide–furan compounds and linear polyurethane. Polym. Bull. 2013, 70, 2319–2335. [Google Scholar] [CrossRef]
- Hsu, Y.-I.; Masutani, K.; Kimura, Y.; Yamaoka, T. A Novel Bioabsorbable Gel Formed from a Mixed Micelle Solution of Poly(oxyethylene)-block-poly(L-lactide) and Poly(oxyethylene)-block-poly(D-lactide) by Concomitant Stereocomplexation and Chain Extension. Macromol. Chem. Phys. 2013, 214, 1559–1568. [Google Scholar] [CrossRef]
- Aizpurua, J.; Martin, L.; Formoso, E.; González, A.; Irusta, L. One pot stimuli-responsive linear waterborne polyurethanes via Diels-Alder reaction. Prog. Org. Coat. 2019, 130, 31–43. [Google Scholar] [CrossRef]
- Motoki, S.; Nakano, T.; Tokiwa, Y.; Saruwatari, K.; Tomita, I.; Iwamura, T. Synthesis of recyclable molecular LEGO block polymers utilizing the Diels-Alder reaction. Polymer 2016, 101, 98–106. [Google Scholar] [CrossRef]
- Dolci, E.; Michaud, G.; Simon, F.; Boutevin, B.; Fouquay, S.; Caillol, S. Remendable thermosetting polymers for isocyanate-free adhesives: A preliminary study. Polym. Chem. 2015, 6, 7851–7861. [Google Scholar] [CrossRef]
- Lacerda, T.M.; Carvalho, A.J.F.; Gandini, A. A minimalist furan–maleimide AB-type monomer and its thermally reversible Diels–Alder polymerization. RSC Adv. 2016, 6, 45696–45700. [Google Scholar] [CrossRef]
- Gandini, A.; Silvestre, A.J.D.; Coelho, D. Reversible click chemistry at the service of macromolecular materials. Polym. Chem. 2011, 2, 1713. [Google Scholar] [CrossRef] [Green Version]
- Platonova, E.; Chechenov, I.; Pavlov, A.; Solodilov, V.; Afanasyev, E.; Shapagin, A.; Polezhaev, A. Thermally Remendable Polyurethane Network Cross-Linked via Reversible Diels-Alder Reaction. Polymers 2021, 13, 1935. [Google Scholar] [CrossRef] [PubMed]
- Strachota, B.; Morand, A.; Dybal, J.; Matejka, L. Control of Gelation and Properties of Reversible Diels-Alder Networks: Design of a Self-Healing Network. Polymers 2019, 11, 930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A thermally re-mendable cross-linked polymeric material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef]
- Mineo, P.; Barbera, V.; Romeo, G.; Ghezzo, F.; Scamporrino, E.; Spitaleri, F.; Chiacchio, U. Thermally reversible highly cross-linked polymeric materials based on furan/maleimide Diels-Alder adducts. J. Appl. Polym. Sci. 2015, 132, 42314. [Google Scholar] [CrossRef]
- Varganici, C.-D.; Ursache, O.; Gaina, C.; Gaina, V.; Rosu, D.; Simionescu, B.C. Synthesis and Characterization of a New Thermoreversible Polyurethane Network. Ind. Eng. Chem. Res. 2013, 52, 5287–5295. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Z.; Su, X.; Cao, J.; Chen, M.; Liu, R. Stiff UV-Curable self-healing coating based on double reversible networks containing diels-alder cross-linking and hydrogen bonds. Prog. Org. Coat. 2020, 146, 105699. [Google Scholar] [CrossRef]
- Shen, X.; Liu, X.; Dai, J.; Liu, Y.; Zhang, Y.; Zhu, J. How Does the Hydrogen Bonding Interaction Influence the Properties of Furan-Based Epoxy Resins. Ind. Eng. Chem. Res. 2017, 56, 10929–10938. [Google Scholar] [CrossRef]
- Nayab, S.; Trouillet, V.; Gliemann, H.; Weidler, P.G.; Azeem, I.; Tariq, S.R.; Goldmann, A.S.; Barner-Kowollik, C.; Yameen, B. Reversible Diels-Alder and Michael Addition Reactions Enable the Facile Postsynthetic Modification of Metal-Organic Frameworks. Inorg. Chem. 2021, 60, 4397–4409. [Google Scholar] [CrossRef]
- Jiang, Y.; Hadjichristidis, N. Diels-Alder Polymer Networks with Temperature-Reversible Cross-Linking-Induced Emission. Angew. Chem. Int. Ed. 2021, 60, 331–337. [Google Scholar] [CrossRef]
- Yu, F.; Cao, X.; Du, J.; Wang, G.; Chen, X. Multifunctional Hydrogel with Good Structure Integrity, Self-Healing, and Tissue-Adhesive Property Formed by Combining Diels-Alder Click Reaction and Acylhydrazone Bond. ACS Appl. Mater. Interfaces 2015, 7, 24023–24031. [Google Scholar] [CrossRef]
- Truong, T.T.; Nguyen, H.T.; Phan, M.N.; Nguyen, L.-T.T. Study of Diels-Alder reactions between furan and maleimide model compounds and the preparation of a healable thermo-reversible polyurethane. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1806–1814. [Google Scholar] [CrossRef]
- Zeng, C.; Seino, H.; Ren, J.; Hatanaka, K.; Yoshie, N. Self-healing bio-based furan polymers cross-linked with various bis-maleimides. Polymer 2013, 54, 5351–5357. [Google Scholar] [CrossRef]
- Zeng, C.; Seino, H.; Ren, J.; Hatanaka, K.; Yoshie, N. Bio-Based Furan Polymers with Self-Healing Ability. Macromolecules 2013, 46, 1794–1802. [Google Scholar] [CrossRef]
- Hayashi, S.; Narita, A.; Wasano, T.; Tachibana, Y.; Kasuya, K.-I. Synthesis and cross-linking behavior of biobased polyesters composed of bi(furfuryl alcohol). Eur. Polym. J. 2019, 121, 109333. [Google Scholar] [CrossRef]
- Tremblay-Parrado, K.-K.; Bordin, C.; Nicholls, S.; Heinrich, B.; Donnio, B.; Averous, L. Renewable and Responsive Cross-Linked Systems Based on Polyurethane Backbones from Clickable Biobased Bismaleimide Architecture. Macromolecules 2020, 53, 5869–5880. [Google Scholar] [CrossRef]
- Chang, H.; Kim, M.S.; Huber, G.W.; Dumesic, J.A. Design of closed-loop recycling production of a Diels–Alder polymer from a biomass-derived difuran as a functional additive for polyurethanes. Green Chem. 2021, 23, 9479–9488. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Pham, H.Q.; Thi Phung, D.T.; Truong, T.T.; Nguyen, H.T.; Chanh Duc Doan, T.; Dang, C.M.; Le Tran, H.; Mai, P.T.; Tran, D.T.; et al. Macromolecular design of a reversibly crosslinked shape-memory material with thermo-healability. Polymer 2020, 188, 122144. [Google Scholar] [CrossRef]
- Heo, Y.; Sodano, H.A. Self-Healing Polyurethanes with Shape Recovery. Adv. Funct. Mater. 2014, 24, 5261–5268. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, J.; Zhou, L.; Luo, Y. Promoting healing progress in polymer composites based on Diels-Alder reaction by constructing silver bridges. Polym. Adv. Technol. 2020, 32, 1239–1250. [Google Scholar] [CrossRef]
- Feng, L.; He, X.; Zhang, Y.; Qu, D.; Chai, C. Triple Roles of Thermoplastic Polyurethane in Toughening, Accelerating and Enhancing Self-healing Performance of Thermo-reversible Epoxy Resins. J. Polym. Environ. 2020, 29, 829–836. [Google Scholar] [CrossRef]
- Li, M.; Zhang, R.; Li, X.; Wu, Q.; Chen, T.; Sun, P. High-performance recyclable cross-linked polyurethane with orthogonal dynamic bonds: The molecular design, microstructures, and macroscopic properties. Polymer 2018, 148, 127–137. [Google Scholar] [CrossRef]
- Zhang, B.; Digby, Z.A.; Flum, J.A.; Foster, E.M.; Sparks, J.L.; Konkolewicz, D. Self-healing, malleable and creep limiting materials using both supramolecular and reversible covalent linkages. Polym. Chem. 2015, 6, 7368–7372. [Google Scholar] [CrossRef]
- Xu, J.; Ye, S.; Fu, J. Novel sea cucumber-inspired material based on stiff, strong yet tough elastomer with unique self-healing and recyclable functionalities. J. Mater. Chem. A 2018, 6, 24291–24297. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, J.; Liang, H.; Ye, S.; Zou, J.; Yang, H. A novel polyurethane elastomer with super mechanical strength and excellent self-healing performance of wide scratches. Prog. Org. Coat. 2020, 149, 105943. [Google Scholar] [CrossRef]
- Li, X.P.; Yu, R.; He, Y.Y.; Zhang, Y.; Yang, X.; Zhao, X.J.; Huang, W. Four-dimensional printing of shape memory polyurethanes with high strength and recyclability based on Diels-Alder chemistry. Polymer 2020, 200, 122532. [Google Scholar] [CrossRef]
- Wu, P.; Liu, L.; Wu, Z. Synthesis of Diels-Alder Reaction-Based Remendable Epoxy Matrix and Corresponding Self-healing Efficiency to Fibrous Composites. Macromol. Mater. Eng. 2020, 305, 2000359. [Google Scholar] [CrossRef]
- Bai, N.; Saito, K.; Simon, G.P. Synthesis of a diamine cross-linker containing Diels–Alder adducts to produce self-healing thermosetting epoxy polymer from a widely used epoxy monomer. Polym. Chem. 2013, 4, 724–730. [Google Scholar] [CrossRef]
- Min, Y.; Huang, S.; Wang, Y.; Zhang, Z.; Du, B.; Zhang, X.; Fan, Z. Sonochemical Transformation of Epoxy–Amine Thermoset into Soluble and Reusable Polymers. Macromolecules 2015, 48, 316–322. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, H.; Yang, H.; Xiong, L.; Zhou, J.; Huang, S.; Zhao, C.; Zhong, J.; Fan, X. UV-curable self-healing polyurethane coating based on thiol-ene and Diels-Alder double click reactions. Prog. Org. Coat. 2019, 137, 105282. [Google Scholar] [CrossRef]
- Ke, X.; Liang, H.; Xiong, L.; Huang, S.; Zhu, M. Synthesis, curing process and thermal reversible mechanism of UV curable polyurethane based on Diels-Alder structure. Prog. Org. Coat. 2016, 100, 63–69. [Google Scholar] [CrossRef]
- Heo, Y.; Sodano, H.A. Thermally responsive self-healing composites with continuous carbon fiber reinforcement. Compos. Sci. Technol. 2015, 118, 244–250. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, T.H.; Park, Y.I.; Nam, J.H.; Noh, S.M.; Cheong, I.W.; Kim, J.C. Influence of material properties on scratch-healing performance of polyacrylate-graft-polyurethane network that undergo thermally reversible crosslinking. Polymer 2017, 128, 135–146. [Google Scholar] [CrossRef]
- Salvati, M.E.; Balog, A.; Shan, W.; Rampulla, R.; Giese, S.; Mitt, T.; Furch, J.A.; Vite, G.D.; Attar, R.M.; Jure-Kunkel, M.; et al. Identification and optimization of a novel series of [2.2.1]-oxabicyclo imide-based androgen receptor antagonists. Bioorg. Med. Chem. Lett. 2008, 18, 1910–1915. [Google Scholar] [CrossRef]
- Kuang, X.; Liu, G.; Dong, X.; Liu, X.; Xu, J.; Wang, D. Facile fabrication of fast recyclable and multiple self-healing epoxy materials through diels-alder adduct cross-linker. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2094–2103. [Google Scholar] [CrossRef]
- Kuang, X.; Liu, G.; Dong, X.; Wang, D. Triple-shape memory epoxy based on Diels–Alder adduct molecular switch. Polymer 2016, 84, 1–9. [Google Scholar] [CrossRef]
- Elschner, T.; Obst, F.; Heinze, T. Furfuryl- and Maleimido Polysaccharides: Synthetic Strategies Toward Functional Biomaterials. Macromol. Biosci. 2018, 18, e1800258. [Google Scholar] [CrossRef]
- Maassen, E.E.L.; Anastasio, R.; Breemen, L.C.A.; Sijbesma, R.P.; Heuts, J.P.A. Thermally Reversible Diels–Alder Bond-Containing Acrylate Networks Showing Improved Lifetime. Macromol. Chem. Phys. 2020, 221, 2000208. [Google Scholar] [CrossRef]
- Durand-Silva, A.; Cortés-Guzmán, K.P.; Johnson, R.M.; Perera, S.D.; Diwakara, S.D.; Smaldone, R.A. Balancing Self-Healing and Shape Stability in Dynamic Covalent Photoresins for Stereolithography 3D Printing. ACS Macro Lett. 2021, 10, 486–491. [Google Scholar] [CrossRef]
- Dobbins, D.J.; Scheutz, G.M.; Sun, H.; Crouse, C.A.; Sumerlin, B.S. Glass-transition temperature governs the thermal decrosslinking behavior of Diels–Alder crosslinked polymethacrylate networks. J. Polym. Sci. 2019, 58, 193–203. [Google Scholar] [CrossRef]
- Stevenson, R.; Zhang, M.; De Bo, G. Mechanical activation of polymers containing two adjacent mechanophores. Polym. Chem. 2020, 11, 2864–2868. [Google Scholar] [CrossRef]
- N’Guyen, T.T.T.; Contrel, G.; Montembault, V.; Dujardin, G.; Fontaine, L. Phosphonated furan-functionalized poly(ethylene oxide)s using orthogonal click chemistries: Synthesis and Diels–Alder reactivity. Polym. Chem. 2015, 6, 3024–3030. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Hu, K.; Ma, X.; Zhang, W.; Yin, J.; Jiang, X. Hierarchical 3D Patterns with Dynamic Wrinkles Produced by a Photocontrolled Diels-Alder Reaction on the Surface. Adv. Mater. 2020, 32, e1906712. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, L.; Liao, X.; Huang, K. Controlled-Release System of Small Molecules Triggered by the Photothermal Effect of Polypyrrole. Macromol. Rapid Commun. 2016, 37, 149–154. [Google Scholar] [CrossRef]
- Wang, Z.; Craig, S.L. Stereochemical effects on the mechanochemical scission of furan-maleimide Diels-Alder adducts. Chem. Commun. 2019, 55, 12263–12266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; De Bo, G. A Catenane as a Mechanical Protecting Group. J. Am. Chem. Soc. 2020, 142, 5029–5033. [Google Scholar] [CrossRef]
- Lyu, B.; Cha, W.; Mao, T.; Wu, Y.; Qian, H.; Zhou, Y.; Chen, X.; Zhang, S.; Liu, L.; Yang, G.; et al. Surface confined retro Diels-Alder reaction driven by the swelling of weak polyelectrolytes. ACS Appl. Mater. Interfaces 2015, 7, 6254–6259. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.-Y.; Wang, Y.-X.; Wang, L.-J.; Min, Y.-Q.; Zhang, X.-H.; Du, B.-Y. An Investigation of the Selective Chain Scission at Centered Diels–Alder Mechanophore under Ultrasonication. Macromolecules 2017, 50, 1353–1361. [Google Scholar] [CrossRef]
- Hu, X.; Zeng, T.; Husic, C.C.; Robb, M.J. Mechanically Triggered Small Molecule Release from a Masked Furfuryl Carbonate. J. Am. Chem. Soc. 2019, 141, 15018–15023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, R.; De Bo, G. Controlling Reactivity by Geometry in Retro-Diels-Alder Reactions under Tension. J. Am. Chem. Soc. 2017, 139, 16768–16771. [Google Scholar] [CrossRef]







 | |||||
| № | R2 | Furan | Conditions | Endo/Exo Ratio | Yield of DA Adducts (%), Citation |
|---|---|---|---|---|---|
| 1 | H | 2-MF | Et2O, RT, 3 days | N.d. | 21 (endo), [32] |
| 2 | H | 2-MF | THF, reflux, 4 h | 0:100 | 94 1, [33] |
| 3 | H | DMF | CH3CN, 60 °C, overnight | 1:4 | N.d., [34] |
| 4 | H | BHMF | Ethyl acetate, 24 °C, 16 h | >99:1 | 83, [35] |
| 5 2 | H | BHMF | H2O, 24 °C, 16 h | >99:1 | 75, [35] |
| 6 2 | H | BHMF diethyl ester | Ethyl acetate, 24 °C, 32 h | >99:1 | 62, [35] |
| 7 | H | BAMF | Ethyl acetate, 24 °C, 24 h | >97:3 | 42, [35] |
| 8 2 | H | BAMF | Ethyl acetate, 24 °C, 32 h | >97:3 | 76, [35] |
| 9 2 | H |  | Ethyl acetate, 24 °C, 32 h | N.d. | 51, [35] |
| 10 2 | H |  | Ethyl acetate, 24 °C, 32 h | N.d. | 42, [35] |
| 11 | H | HMF dioxolane acetal | THF, 50 °C, 3 days | 4:1 | 64.1 3, [36] |
| 12 | H |  | THF, 50 °C, 3 days | 4:1 | 94.7 3, [36] |
| 13 | H |  | THF, 50 °C, 3 days | 5:1 | 95.2 3, [36] |
| 14 | H |  | Et2O, 24 °C | N.d. | 35 (endo), [30] |
| 15 2 | H |  | THF, RT | N.d. | 51 (endo), [30] |
| 16 | Me | 2-MF | Toluene, 90 °C | 0:100 | 92, [37] |
| 17 | Me | FA | Et2O, 90 °C | 21:79 | 43, [38] |
| 18 | Me | FA acetate | CH2Cl2, 23 °C | 77:23 | N.d., [39] |
| 19 | Me | FA allyl ester | Toluene, 50 °C, 24 h | N.d. | 65 (endo), [40] |
| 20 | Me | FA tert-butyl ester | CH2Cl2, 23 °C | 71:29 | N.d., [39] |
| 21 | Me | Furfural dioxolane acetal | CH2Cl2, 23 °C | 87:13 | N.d., [39] |
| 22 | Me | R1 = Me, R2 = CH2OAc | CH2Cl2, 23 °C | 73:27 | N.d., [39] |
| 23 | Et | 2-MF | H2O, 65 °C | 1.4: 1 | 100, [41] |
| 24 | Et | DMF | H2O, RT | 3:2 | 100, [41] |
| 25 2 | Pr |  | THF, RT | 4:1 | 66, [30] |
| 26 | Pr | FA iso-propyl ester | CHCl3, 55 °C | 60:40 | N.d., [42] |
| 27 | Pr |  | CHCl3, 55 °C | 100:0 | N.d., [42] |
| 28 | tBu | 2-MF | H2O, 65°C | 0:100 | 100, [41] |
| 29 | tBu | DMF | H2O, RT | 1:8 | 100, [41] |
| 30 | tBu | FA iso-propyl ester | CHCl3, 55 °C | 51:49 | N.d., [42] |
| 31 | Bn | FA | CH3CN, 35 °C | 70:30 | 75, [43] |
| 32 | Bn | FA iso-propyl ester | CHCl3, 55 °C | 44:56 | N.d., [42] |
| 33 | Bn |  | CH3CN, 70 °C | 3:1 | 31 4, [44] |
| 34 | Bn |  | CH3CN, 70 °C, 16 h | N.d. | 69 (endo), 21 (exo), [44] |
| 35 | 2-Hydroxyethyl | FA | Benzene, reflux | 0:100 | 86, [45] |
| 36 | 2-Hydroxyethyl | DMF | CH3CN, 65 °C | 1:4 | 100, [46] |
| 37 | 2-Carboxyethyl | 2-MF | CHCl3, 38 °C | 28:72 | 100, [46] |
| 38 | 2-Carboxyethyl | DMF | CH2Cl2, RT | 78:22 | 100, [46] |
| 39 | 2-Carboxyethyl | DMF | CH3CN, 60 °C | 22:78 | 100, [46] |
| 40 | 3-Hydroxypropyl | FA | Toluene, 80 °C | 30:70 5 | 77, [47] |
| 41 | Methoxy-2-propyl | FA acetate | CH2Cl2, 23 °C | 76:24 | N.d., [39] |
 | |||||
| № | Ar | Furan | Conditions | Endo/Exo Ratio | Yield of DA Adducts (%), Citation |
|---|---|---|---|---|---|
| 1 | Ph | 2-MF | H2O, 65 °C | 1.6:1 | 100, [41] |
| 2 | Ph | 2-MF | 4:1 toluene/benzene, RT, 1.1 GPa | 1.66:1 | 85, [49] |
| 3 | Ph | 2-MF | CDCl3, 60 °C | Exo with traces of endo | 90, [50] |
| 4 | Ph | 2-MF | Hexane or heptane, TFA, glass beads, 80 °C, 5–8 days 1 | (−)-Exo, 86–90 ee | 80, [50] |
| 5 | Ph | FA | Neat, 140 °C, 8 min | Exo | 82, [51] |
| 6 | Ph | FA | RT, 12 h | 71:29 | 66, [51] |
| 7 | Ph | FA allyl ester | Toluene, 50 °C, 24 h | N.d. | 26 (exo), [40] |
| 8 | Ph | FA acetate | CH2Cl2, 23 °C | 65:35 | N.d., [39] |
| 9 | Ph | FA vinyl ester | Et2O, 22–24 °C | 1:2.8 | 47, [52] |
| 10 | Ph | FA vinyl ester | Toluene, 80 °C | 4:1 | 66, [52] |
| 11 | Ph | DMF | H2O, RT | 1.3:1 | 100, [41] |
| 12 | p-Tolyl | DMF | toluene, 60 °C, 3 h | Exo | 50, [53] |
| 13 | p-Tolyl | DMF | Neat, 94 °C, 1 h | Exo | 60, [54] |
| 14 | m-Tolyl | FA iso-butyl ester | CHCl3, 55 °C | 67:33 | N.d., [42] |
| 15 | PhF5 | 2-MF | Neat, reflux | Exo | 50, [55] |
| 16 | 4-Hydroxyphenyl | FA | Acetone, 55 °C | Exo | 71, [56] |
| 17 | 4-Hydroxyphenyl | FA | CH3CN, 35 °C | 80:20 | N.d., [56] |
| 18 | p-Methoxyphenyl | FA | CH3CN, 35 °C, 18 h | N.d. | >85 (endo), [44] |
| 19 | p-Methoxyphenyl | FA acetate | CH2Cl2, 23 °C | 67:33 | N.d., [39] |
| 20 | p-Methoxyphenyl | DMF | Neat, 94 °C, 1 h | 17:83 | 25, [54] |
| 21 | p-Methoxyphenyl |  | CH3CN, 75 °C, | N.d. | 61 (endo), <5 (exo) [44] |
| 22 | p-Methoxyphenyl |  | CH3CN, 75 °C, 8 h | N.d. | <5 (endo), 63 (exo) [44] |
| 23 | p-Chlorophenyl | DMF | Neat, 94 °C, 1 h | 6:94 | 46, [54] |
| 24 | m-Nitrophenyl | DMF | Neat, 94 °C, 1 h | 5:95 | 14, [54] |
| 25 | p-Nitrophenyl | FA | CH3CN, 40 °C | 70:23 | 52, [44] |
| 26 | p-Nitrophenyl | FA acetate | CH2Cl2, 23 °C | 55:45 | N.d., [39] |
| 27 | p-Nitrophenyl |  | CH3CN, 50 °C, 72 h | N.d. | 26 (endo), <5 (exo), [44] |
| 28 | p-Nitrophenyl |  | CH3CN, 80 °C | N.d. | <5 (endo), 31 (exo) [44] |
| 29 | BMI as dienophile | FA | Toluene, 75–80 °C, two days | Mostly exo | 92, [57] |
| 30 | BMI as dienophile | FA iso-propyl ester | CHCl3, 55 °C | 19:81 | N.d., [42] |
 | |||||
| № | Furanic Substrate | R2 | Conditions | Conversion 1/ Isolated Yield | Selectivity 1 |
|---|---|---|---|---|---|
| 1 | R = R1 = H | H | H2O, 60 °C, 16 h | 38 2 | endo/exo 8:30, endo’/exo’ 0:0 |
| 2 | R = R1 = H | Me | H2O, 60 °C, 16 h | 63 2 | endo/exo 18:40, endo’/exo’ 1:3 |
| 3 | R = R1 = H | Et | H2O, 60 °C, 16 h | 43 2 | endo/exo 8:28, endo’/exo’ 1:6 |
| 4 | R = R1 = H | nPr | H2O, 60 °C, 16 h | 20 2 | endo/exo 1:7, endo’/exo’ 1:11 |
| 5 | R = R1 = H | Ph | H2O, 60 °C, 16 h | 7 2 | endo/exo 0:1, endo’/exo’ 1:5 |
| 6 | R = Me, R1 = H | Me | H2O, 60 °C, 16 h | 14 2 | endo/exo 3:8, endo’/exo’ 0:3 |
| 7 | R = CH2OH, R1 = H | Me | H2O, 60 °C, 16 h | 50 2 | endo/exo 37:13, endo’/exo’ 0:0 |
| 8 | R = CH2OMe, R1 = H | Me | H2O, 60 °C, 16 h | 18 2 | endo/exo 7:5, endo’/exo’ 3:3 |
| 9 | R = H, R1 = CH3 | Me | H2O, 60 °C, 16 h | 32/32 | endo/exo trace:32 |
| 10 | R = H, R1 = OH | H | NaOH, H2O, 50 °C, 16 h | 95/68 | endo/exo trace:95 |
| 11 | R = H, R1 = OH | Me | NaOH, H2O, 50 °C, 16 h | 98/92 | endo/exo 1:97 |
| 12 | R = H, R1 = OH | nPr | NaOH, H2O, 50 °C, 16 h | 96/72 | endo/exo 3:93 |
| 13 | R = H, R1 = OH | Ph | NaOH, H2O, 50 °C, 16 h | 51/21 | endo/exo trace:51 |
| 14 | R = H, R1 = OH | Cy | NaOH, H2O-MeOH, 50 °C, 16 h | 56/31 | endo/exo 3:53 |
| 15 | R = H, R1 = OMe | H | H2O, 50 °C, 16 h | 67/43 | endo/exo 2:65 |
| 16 | R = H, R1 = OMe | Me | H2O, 50 °C, 16 h | 70/52 | endo/exo 5:65 |
| 17 | R = H, R1 = OMe | Et | H2O, 50 °C, 16 h | 65/47 | endo/exo 4:61 |
| 18 | R = H, R1 = OEt | Me | H2O, 50 °C, 16 h | 63/29 | endo/exo 4:59 |
| 19 | R = H, R1 = OiPr | Me | H2O, 50 °C, 16 h | 54/26 | endo/exo 4:50 |
| 20 | R = H, R1 = OtBu | Me | H2O, 50 °C, 16 h | 54/25 | endo/exo 3:51 |
| 21 | R = H, R1 = NH2 | Me | H2O, 50 °C, 16 h | 94/77 | endo/exo 3:91 |
| 22 | R = H, R1 = NMe2 | Me | H2O, 50 °C, 16 h | 81/41 | endo/exo 4:77 |
| 23 | R = H, R1 = NHOH | Me | H2O, 50 °C, 16 h | 92/69 | endo/exo 16:76 |
| 24 | R = Me, R1 = OH | Me | NaOH, H2O, 50 °C, 16 h | 93/75 | endo/exo 5:88 |
| 25 | R = CH2OH, R1 = OH | Me | NaOH, H2O, 50 °C, 16 h | 91/51 3 | endo/exo 19:72 |
| 26 | R = CH2OH, R1 = OH | Ph | NaOH, H2O, 50 °C, 16 h | 28/11 | endo/exo trace:28 |
| 27 4 | R = CHO, R1 = OH | Me | NaOH, H2O, 50 °C, 16 h | <10/N.d. | endo/exo trace:~5 |
| 28 4 | R = COOH, R1 = OH | Me | NaOH, H2O, 50 °C, 16 h | 20/N.d. | endo/exo 0:20 |
| 29 4 | R = COOH, R1 = OH | Me | NaOH, H2O, 50 °C, 16 h | 56/N.d. | endo/exo 0:56 |
 | |||||||
| № | Furan | Maleimide | Conditions | Mn (g mol−1) | PDI | rDA (°C) 1 | Citation |
|---|---|---|---|---|---|---|---|
| 1 | R = CH2 | R1 = (CH2)3 | THF, reflux, 24 h | 3650 | 2.45 | 100–122 | [85] |
| 2 | R = CH2-O-CH2 | R1 = (CH2)3 | THF, reflux, 24 h | 4540 | 2.31 | 140–161 | [85] |
| 3 | R = CH2-S-CH2 | R1 = (CH2)3 | THF, reflux, 24 h | 5660 | 1.72 | 118–130 | [85] |
| 4 | R = CH2-NH-CH2 | R1 = (CH2)3 | THF, reflux, 24 h | 2920 | 2.76 | 123–140 | [85] |
| 5 | R = CH2-O-(CH2)10-O-CH2 | BMI | 1,2-dichloroethane, 60 °C | 2900–7800 | 1.66–2.86 | 110–150 | [95] |
| 6 | R = CH2-(O-(CH2-CH2)3-O-CH2 | BMI | 1,2-dichloroethane, 60 °C | 18,000–38,000 | 3.5–5.81 | 110 | [95] |
| 7 |  | BMI | CHCl3, 60 °C, 48 h | 2200 | 2.45 | 140–170 | [88] |
| 8 |  | (CH2)6 | CHCl3, 55 °C, 48 h | 5920 | 1.5 | ~124 | [42] |
| 9 |  | CHCl3, 55 °C, 48 h | 3700 | 1.43 | ~124 | [42] | |
| 10 | BMI | CHCl3, 55 °C, 48 h | 1900 | 1.37 | ~124 | [42] | |
| 11 |  | - | TCE, 110 °C, 5 h, then 60 °C, 72 h | ~1800 | N.d. | 150 | [97] |
| 12 |  | - | TCE, 110 °C, 24 h, then 65 °C, 72 h | 1900 | 2.2 | N.d. | [98] |
| № | Type of Bifunctional Adduct | R, R1 | Type of Prepared CAN, Citation |
|---|---|---|---|
| 1 |  | BMI as a precursor | Polyacrylates [121] |
| 2 |  | BMI as a precursor, R1 = OH | Polyurethanes [122,123] |
| 3 | BMI as a precursor, R1 = NH2 | Epoxy resins [124] | |
| 4 | R = (CH2)8, R1 = NH2 | Epoxy resin [125] | |
| 5 |  | R = (CH2)6 | Epoxy thermosets [126] |
| 6 | BMI as a precursor | Polysiloxanes [66] | |
| 7 |  | R = OH | Polyurethanes [116,127,128,129,130], dendrimers [131] |
| 8 | R = NH2 | Epoxy resins [132,133] | |
| 9 |  | - | Polyurethanes [24,25] |
| 10 |  | R = H | Polyacrylates [45,134,135,136] |
| 11 | R = Me | Polyacrylates [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galkin, K.I.; Sandulenko, I.V.; Polezhaev, A.V. Diels–Alder Cycloadditions of Bio-Derived Furans with Maleimides as a Sustainable «Click» Approach towards Molecular, Macromolecular and Hybrid Systems. Processes 2022, 10, 30. https://doi.org/10.3390/pr10010030
Galkin KI, Sandulenko IV, Polezhaev AV. Diels–Alder Cycloadditions of Bio-Derived Furans with Maleimides as a Sustainable «Click» Approach towards Molecular, Macromolecular and Hybrid Systems. Processes. 2022; 10(1):30. https://doi.org/10.3390/pr10010030
Chicago/Turabian StyleGalkin, Konstantin I., Irina V. Sandulenko, and Alexander V. Polezhaev. 2022. "Diels–Alder Cycloadditions of Bio-Derived Furans with Maleimides as a Sustainable «Click» Approach towards Molecular, Macromolecular and Hybrid Systems" Processes 10, no. 1: 30. https://doi.org/10.3390/pr10010030
APA StyleGalkin, K. I., Sandulenko, I. V., & Polezhaev, A. V. (2022). Diels–Alder Cycloadditions of Bio-Derived Furans with Maleimides as a Sustainable «Click» Approach towards Molecular, Macromolecular and Hybrid Systems. Processes, 10(1), 30. https://doi.org/10.3390/pr10010030







