Valorization of Vine Prunings by Slow Pyrolysis in a Fixed-Bed Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
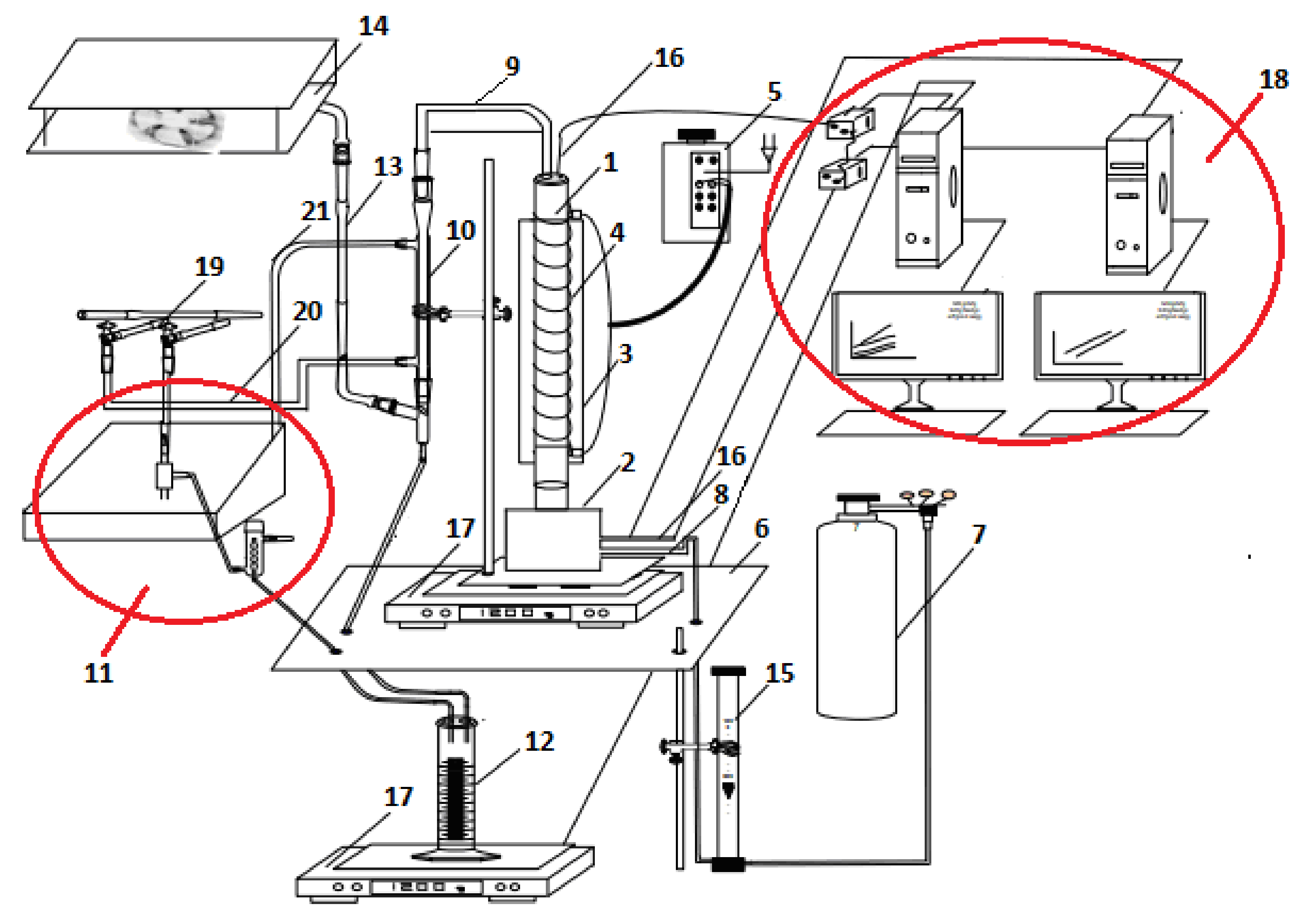
2.2. Equipment and Procedures
2.3. Independent and Dependent Process Variables
2.4. Biochar Characterization
2.5. Bio-Oil Characterization
3. Results and Discussion
3.1. Experimental Performances of Slow Pyrolysis
3.2. Predicted Performances of Slow Pyrolysis
3.3. Biochar and Bio-Oil Characterization
3.3.1. Biochar Characterization
3.3.2. Bio-Oil Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Che, Q.; Li, S.; Liu, Z.; Yang, H.; Chen, Y.; Wang, X.; Shao, J.; Chen, H. Recent developments in lignocellulosic biomass catalytic fast pyrolysis: Strategies for the optimization of bio-oil quality and yield. Fuel Process. Technol. 2019, 196, 106180. [Google Scholar] [CrossRef]
- El Hanandeh, A.; Albalasmeh, A.; Gharaibeh, M. Effect of pyrolysis temperature and biomass particle size on the heating value of biocoal and optimization using surface methodology. Biomass Bioenerg. 2021, 151, 106163. [Google Scholar] [CrossRef]
- Ghosh, P.; Sengupta, S.; Singh, L.; Sahay, A. Life cycle assessment of waste-to-bionergy processes: A review. In Bioreactors; Singh, L., Yousuf, A., Mahapatra, D.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 105–122. ISBN 9780128212646. [Google Scholar] [CrossRef]
- Kimura, L.M.; Santos, L.C.; Vieira, P.F.; Parreira, P.M.; Henrique, H.M. Biomass pyrolysis: Use of some agricultural wastes for alternative fuel production. In Proceedings of the 7th International Latin American Conference on Powder Technology, Atibaia, Brazil, 8–10 November 2009; pp. 274–279. [Google Scholar]
- Tian, B.; Wang, X.; Zhao, W.; Xu, L.; Bai, L. Pyrolysis behaviors, kinetics and gaseous product evolutions of two typical biomass wastes. Catal. Today 2021, 374, 77–85. [Google Scholar] [CrossRef]
- Wang, G.; Dai, G.; Ding, S.; Wu, J.; Wang, S. A new insight into pyrolysis mechanism of three typical actual biomass: The influence of structural differences on pyrolysis process. J. Anal. Appl. Pyrolysis 2021, 156, 105184. [Google Scholar] [CrossRef]
- Ansari, K.B.; Kamal, B.; Beg, S.; Khan, M.A.W.; Khan, M.S.; Al Mesfer, M.K.; Danish, M. Recent developments in investigating reactions chemistry and transport effects in biomass fast pyrolysis: A review. Renew. Sust. Energ. Rev. 2021, 150, 111454. [Google Scholar] [CrossRef]
- Cusenza, M.A.; Longo, S.; Cellura, M.; Guarino, F.; Messineo, A.; Mistretta, M.; Volpe, M. Environmental assessment of a waste-to-bioenergy practice: The pyrolysis of agro-industrial biomass residues. Sustain. Prod. Consum. 2021, 28, 866–876. [Google Scholar] [CrossRef]
- Li, Y.; Xing, B.; Ding, Y.; Han, X.; Wang, S. A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocellulosic biomass. Bioresour. Technol. 2020, 312, 123614. [Google Scholar] [CrossRef]
- Mlonka-Medrala, A.; Evangelopoulos, P.; Sieradzka, M.; Zajemska, M.; Magdziarz, A. Pyrolysis of agricultural waste biomass towards production of gas fuel and high-quality char: Experimental and numerical investigations. Fuel 2021, 296, 120611. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, Y.; Yang, H.; Liu, M.; Xiao, H.; Wang, S.; Chen, H.; Naqvi, S.R. Machine learning prediction if pyrolytic gas yield and compositions with feature reduction methods: Effect of pyrolysis conditions and biomass characteristics. Bioresour. Technol. 2021, 339, 125581. [Google Scholar] [CrossRef]
- Usino, D.O.; Ylitervo, P.; Moreno, A.; Sipponen, M.H. Primary interactions of biomass components during fast pyrolysis. J. Anal. Appl. Pyrolysis 2021, 159, 105297. [Google Scholar] [CrossRef]
- Pârvulescu, O.C.; Gavrilă, A.I.; Dobre, T.; Ceatră, L. Effects of process factors on slow pyrolysis of sorghum waste. Rev. Chim. 2016, 67, 2254–2257. [Google Scholar]
- Dobre, T.; Pârvulescu, O.C.; Iavorschi, G.; Stoica, A.; Stroescu, M. Catalytic effects at pyrolysis of wheat grains impregnated with nickel salts. Int. J. Chem. React. Eng. 2010, 8, 1968–1992. [Google Scholar] [CrossRef]
- Dobre, T.; Pârvulescu, O.C.; Rodriguez Ramos, I.; Ceatră, L.; Stroescu, M.; Stoica, A.; Mirea, R. Global reaction kinetics and enthalpy in slow pyrolysis of vegetal materials. Rev. Chim. 2012, 63, 54–59. [Google Scholar]
- Pârvulescu, O.C.; Dobre, T.; Ceatră, L.; Iavorschi, G.; Mirea, R. Characteristics of corn grains pyrolysis in a fixed bed reactor. Rev. Chim. 2011, 62, 89–94. [Google Scholar]
- Balat, M.; Balat, M.; Kirtay, E.; Balat, H. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems. Energ. Convers. Manag. 2009, 50, 3147–3157. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Di Blasi, C. Modelling chemical and physical processes of wood and biomass pyrolysis. Prog. Energy Combust. Sci. 2008, 34, 47–90. [Google Scholar] [CrossRef]
- Chen, W.; Meng, J.; Han, X.; Lan, Y.; Zhang, W. Past, present, and future of biochar. Biochar 2019, 1, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, J.F.; Encinar, J.M.; Canito, J.L.; Sabio, E.; Chacon, M. Pyrolysis of cherry stones: Energy uses of the different fractions and kinetic study. J. Anal. Appl. Pyrolysis 2003, 67, 165–190. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielska, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Khan, M.B.; Cui, X.; Jilani, G.; Tang, L.; Lu, M.; Cao, X.; Sahito, Z.A.; Hamid, Y.; Hussain, B.; Yang, X.; et al. New insight into the impact of biochar during vermi-stabilization of divergent biowastes: Literature synthesis and research pursuits. Chemosphere 2000, 238, 124679. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Bera, T.; Bhaduri, D.; Sarkar, B.; Mandal, S.; Wade, P.; Kumari, S.; Biswas, S.; Menon, M.; Pathak, H.; et al. A review on biochar modulated soil condition improvements and nutrient dynamics concerning crop yields: Pathways to climate change mitigation and global food security. Chemosphere 2019, 227, 345–365. [Google Scholar] [CrossRef]
- Ceatră, L.; Pârvulescu, O.C.; Rodriguez Ramos, I.; Dobre, T. Preparation, characterization, and testing of a carbon-supported catalyst obtained by slow pyrolysis of nickel salt impregnated vegetal material. Ind. Eng. Chem. Res. 2016, 55, 1491–1502. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Yorgun, S.; Şensöz, S.; Koçkar, Ö.M. Characterization of the pyrolysis oil produced in the slow pyrolysis of sunflower-extracted bagasse. Biomass Bioenerg. 2001, 20, 141–148. [Google Scholar] [CrossRef]
- Al Jamri, M.; Li, J.; Smith, R. Molecular characterisation of biomass pyrolysis oil and petroleum fraction blends. Comput. Chem. Eng. 2020, 140, 106906. [Google Scholar] [CrossRef]
- ASTM D5142. Standard Test Methods for Proximate Analysis of the Analysis Sample of Coal and Coke by Instrumental Procedures; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Bikbulatova, S.; Tahmasebi, A.; Zhang, Z.; Rish, S.K.; Yu, J. Understanding water retention behavior and mechanism in bio-char. Fuel Process. Technol. 2018, 169, 101–111. [Google Scholar] [CrossRef]
- Antal, M.J.J.; Varhegyi, G. Cellulose pyrolysis kinetics: The current state of knowledge? Ind. Eng. Chem. Res. 1995, 34, 703–717. [Google Scholar] [CrossRef]
- Babiker, M.E.; Aziz, A.R.A.; Heilkal, M.; Yusup, S.; Abakar, M. Pyrolysis characteristics of Phoenix dactylifera date palm seeds using thermo-gravimetric analysis (TGA). Int. J. Environ. Sci. Dev. 2013, 4, 521–524. [Google Scholar] [CrossRef]
- Damartzis, T.; Vamvuka, D.; Sfakiotakis, S.; Zabaniotou, A. Thermal degradation studies and kinetic modeling of cardoon (Cynara cardunculus) pyrolysis using thermogravimetric analysis (TGA). Bioresour. Technol. 2011, 102, 6230–6238. [Google Scholar] [CrossRef]
- El-Sayed, S.A.; Mostafa, M.E. Kinetic parameters determination of biomass pyrolysis fuels using TGA and DTA techniques. Waste Biomass Valor. 2015, 6, 401–415. [Google Scholar] [CrossRef]
- El-Sayed, S.A.; Mostafa, M.E. Pyrolysis characteristics and kinetic parameters determination of biomass fuel powders by differential thermal gravimetric analysis (TGA/DTG). Energy Convers. Manag. 2014, 85, 165–172. [Google Scholar] [CrossRef]
- Idris, S.S.; Rahman, N.A.; Ismail, K.; Alias, A.B.; Rashid, Z.A.; Aris, M.J. Investigation on thermochemical behaviour of low rank Malaysian coal, oil palm biomass and their blends during pyrolysis via thermogravimetric analysis (TGA). Bioresour. Technol. 2010, 101, 4584–4592. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Mohanty, K.; Wang, X. Pyrolysis kinetic behavior and Py-GC–MS analysis of waste dahlia flowers into renewable fuel and value-added chemicals. Fuel 2020, 260, 116338. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. In-depth investigation of biomass pyrolysis based on three major components: Hemicellulose, cellulose and lignin. Energy Fuels 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Nasser, R.A.; Salem, M.Z.M.; Al-Mefarrej, H.A.; Abdel-Aal, M.A.; Soliman, S.S. Fuel characteristics of vine prunings (Vitis vinifera L.) as a potential source for energy production. BioResources 2014, 9, 482–496. [Google Scholar] [CrossRef] [Green Version]
- Aboulkas, A.; Hammani, H.; El Achaby, M.; Bilal, E.; Barakat, A.; El Harf, K. Valorization of algal waste via pyrolysis in a fxed-bed reactor: Production and characterization of bio-oil and bio-char. Bioresour. Technol. 2017, 243, 400–408. [Google Scholar] [CrossRef]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; de Melo, I.C.N.A.; Melo, L.C.A.; Magriotis, Z.M.; Sánchez-Monedero, M.A. Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef] [Green Version]
- Melo, L.C.A.; Coscione, A.R.; Abreu, C.A.; Puga, A.P.; Camargo, O.A. Influence of pyrolysis temperature on cadmium and zinc sorption capacity of sugarcane straw-derived biochar. BioResources 2013, 8, 4992–5004. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Yang, M.; Feng, Q.; McGrouther, K.; Wang, H.L.; Lu, H.H.; Chen, Y.X. Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenerg. 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Ray, A.; Banerjee, A.; Dubey, A. Characterization of biochars from various agricultural by-products using FTIR spectroscopy, SEM focused with image processing. IJAEB 2020, 13, 423–430. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, L.; Zhang, S.; Fu, H.; Chen, J. Hydrothermal liquefaction of macroalgae Enteromorpha prolifera to bio-oil. Energy Fuels 2010, 24, 4054–4061. [Google Scholar] [CrossRef]
- Abnisa, F.; Arami-Niya, A.; Daud, W.M.A.W.; Sahu, J.N. Characterization of bio-oil and bio-char from pyrolysis of palm oil wastes. Bioenergy Res. 2013, 6, 830–840. [Google Scholar] [CrossRef]
- Aldobouni, I.A.; Fadhil, A.B.; Saied, I.K. Conversion of de-oiled castor seed cake into bio-oil and carbon adsorbents. Energy Source Part A 2015, 37, 2617–2624. [Google Scholar] [CrossRef]
- Fadhil, A.B. Evaluation of apricot (Prunus armeniaca L.) seed kernel as a potential feedstock for the production of liquid bio-fuels and activated carbons. Energy Convers. Manag. 2017, 133, 307–317. [Google Scholar] [CrossRef]
- Haarlemmer, G.; Guizani, C.; Anouti, S.; Deniel, M.; Roubaud, A.; Valin, S. Analysis and comparison of bio-oils obtained by hydrothermal liquefaction and fast pyrolysis of beech wood. Fuel 2016, 174, 180–188. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Abdulkhani, A.O.; Saha, B. Recent insights into lignocellulosic biomass pyrolysis: A critical review on pretreatment, characterization, and products upgrading. Processes 2020, 8, 799. [Google Scholar] [CrossRef]
- Naik, D.K.; Monika, K.; Prabhakar, S.; Parthasarathy, R.; Satyavathi, B. Pyrolysis of sorghum bagasse biomass into bio-char and bio-oil products. J. Thermal Anal. Calorim. 2017, 127, 1277–1289. [Google Scholar] [CrossRef]
- Jouad, E.M.; Allain, M.; Khan, M.A.; Bouet, G.M. Structural and spectral studies of thiosemicarbazones derived from 3-furaldehyde and 3-(2-furyl)prop-2-enal. J. Mol. Struct. 2002, 604, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Caputo, F.; Vignoli, T.; Maremmani, I.; Bernardi, M.; Zoli, G. Gamma hydroxybutyric acid (GHB) for the treatment of alcohol dependence: A Review. Int. J. Environ. Res. Public Health 2009, 6, 1917–1929. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.H.; Choi, S.Y.; Kim, J.Y.; Kim, D.H.; Lee, J.W.; Choi, J.S.; Chung, H.Y. 3-Methyl-1,2-cyclopentanedione down-regulates age-related NF-κB signaling cascade. J. Agric. Food Chem. 2007, 55, 6787–6792. [Google Scholar] [CrossRef]
- Samanta, A.K.; Pandey, P.; Bandyopadhyay, B.; Mukhopadhyay, A.; Chakraborty, T. Intra- and intermolecular H-bond mediated tautomerization and dimerization of 3-methyl-1,2-cyclopentanedione: Infrared spectroscopy in argon matrix and CCl4 solution. J. Mol. Struct. 2011, 994, 97–103. [Google Scholar] [CrossRef]


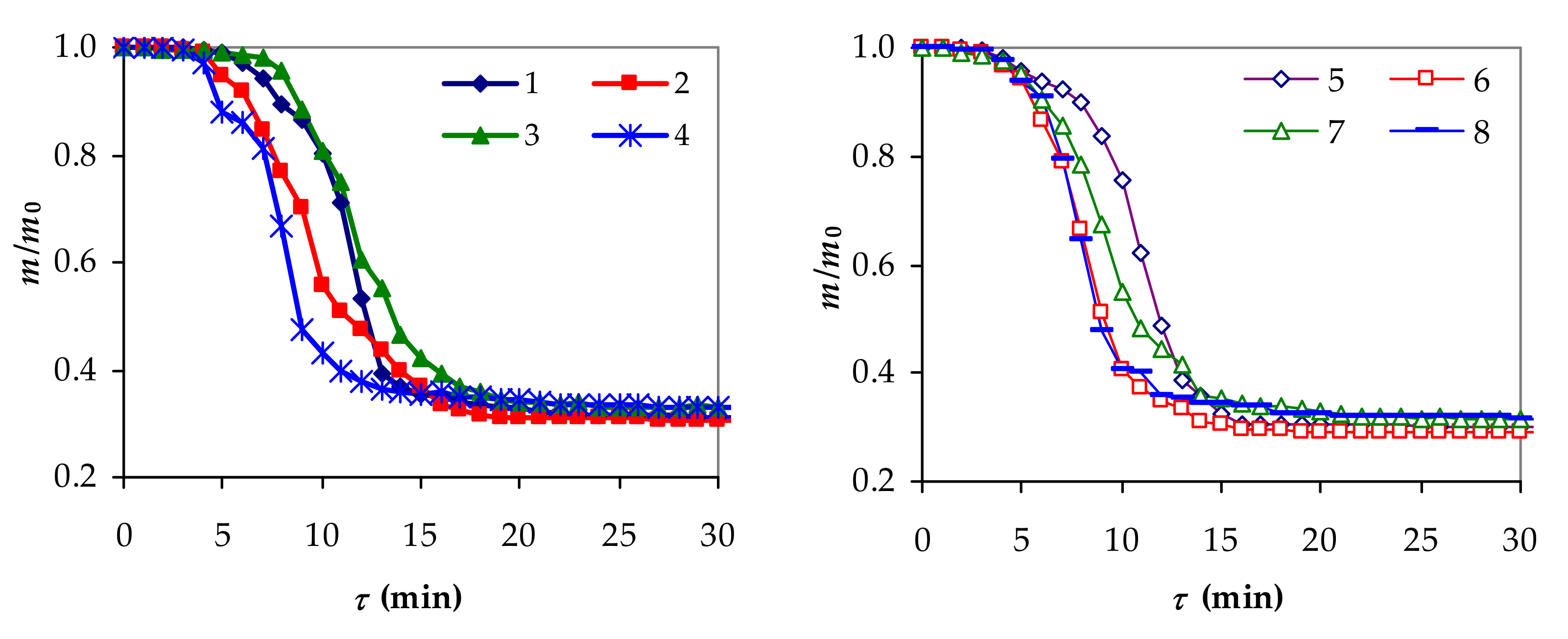

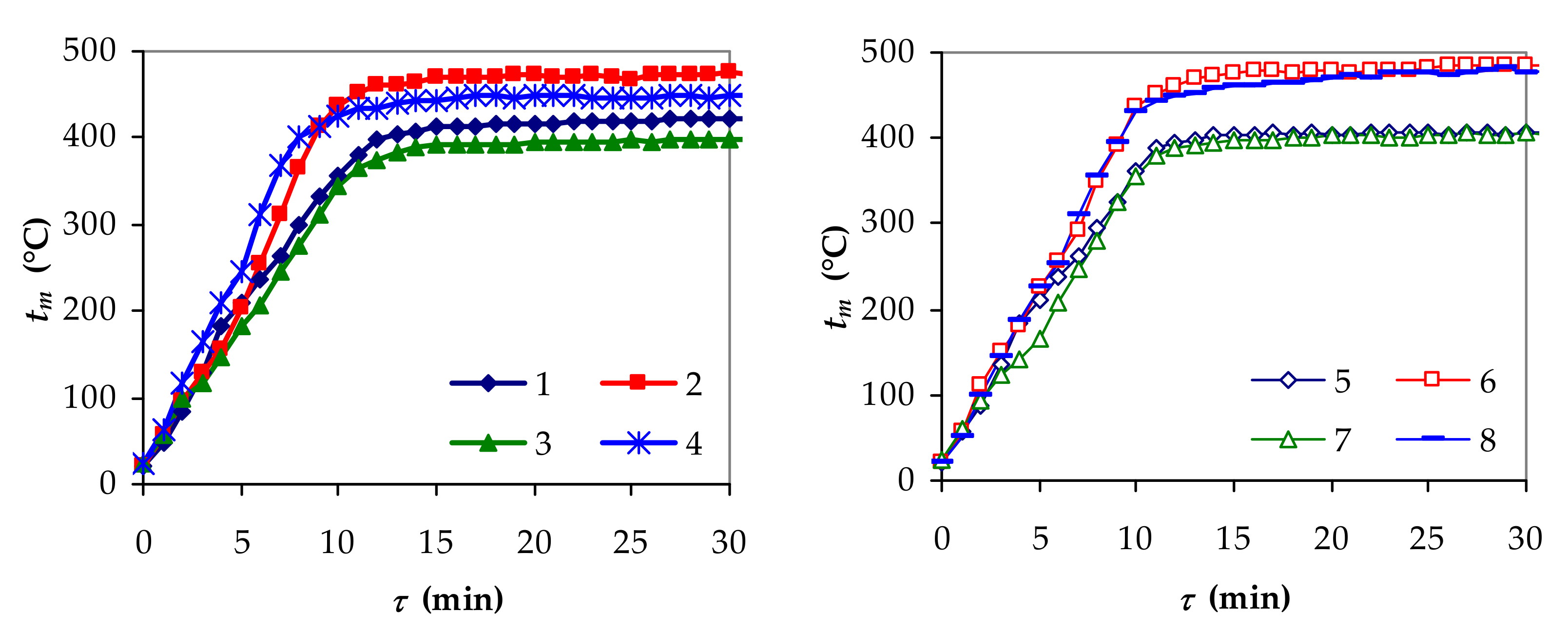
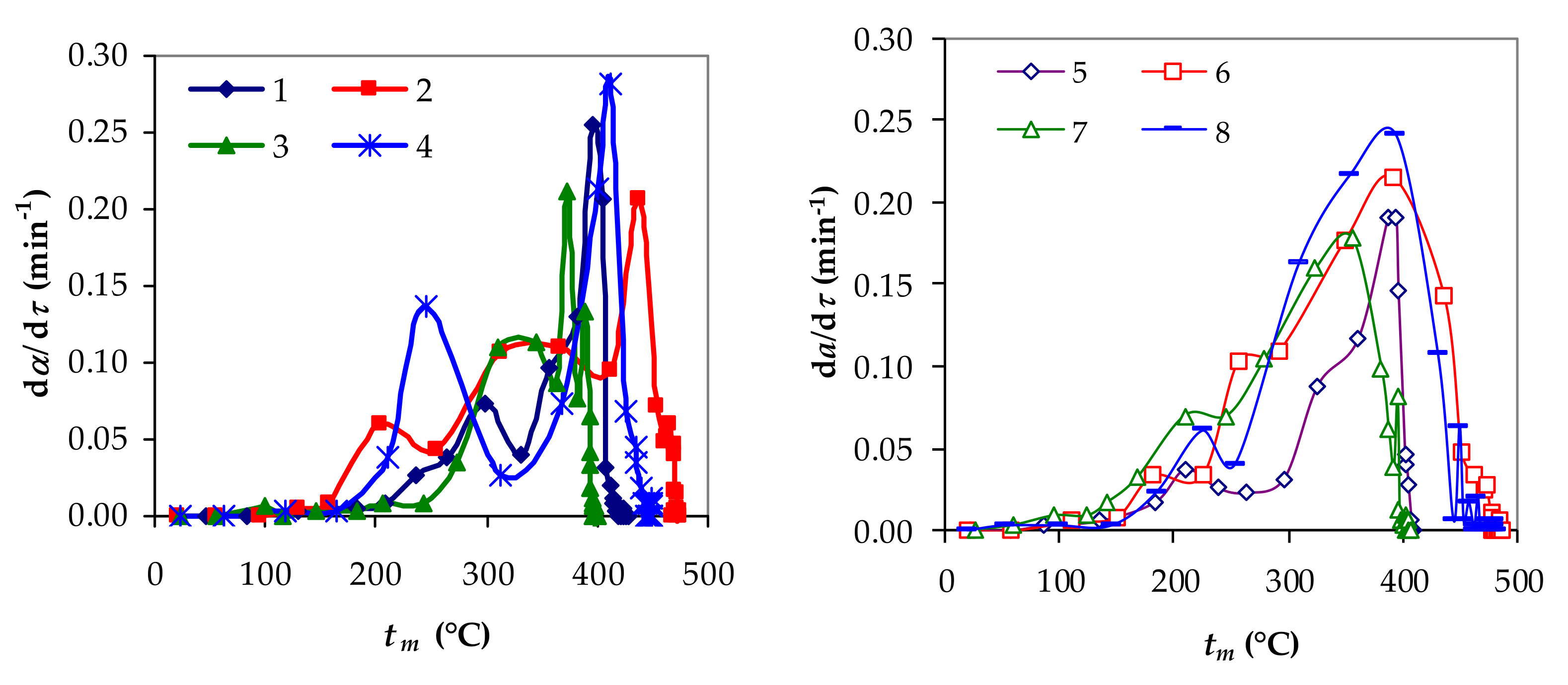





| Exp. | q (W/m2) | w (m/s) | d (m) | x1 | x2 | x3 | mf/m0 | mLf/m0 | tmf (°C) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4244 | 0.004 | 0.007 | −1 | −1 | −1 | 0.312 | 0.278 | 426.6 |
| 2 | 5777 | 0.004 | 0.007 | 1 | −1 | −1 | 0.306 | 0.328 | 473.6 |
| 3 | 4244 | 0.008 | 0.007 | −1 | 1 | −1 | 0.328 | 0.260 | 401.1 |
| 4 | 5777 | 0.008 | 0.007 | 1 | 1 | −1 | 0.324 | 0.346 | 449.2 |
| 5 | 4244 | 0.004 | 0.011 | −1 | −1 | 1 | 0.298 | 0.296 | 406.9 |
| 6 | 5777 | 0.004 | 0.011 | 1 | −1 | 1 | 0.286 | 0.350 | 486.5 |
| 7 | 4244 | 0.008 | 0.011 | −1 | 1 | 1 | 0.314 | 0.328 | 406.3 |
| 8 | 5777 | 0.008 | 0.011 | 1 | 1 | 1 | 0.310 | 0.304 | 479.1 |
| 9 | 5010 | 0.006 | 0.009 | 0 | 0 | 0 | 0.312 | 0.306 | 438.8 |
| 10 | 5010 | 0.006 | 0.009 | 0 | 0 | 0 | 0.309 | 0.312 | 442.7 |
| 11 | 5010 | 0.006 | 0.009 | 0 | 0 | 0 | 0.305 | 0.314 | 444.2 |
| Proximate Analysis (% wb) | |
|---|---|
| Dry Matter Content, DM | 98.14 ± 0.51 |
| Volatile Matter Content, VM | 32.29 ± 1.60 |
| Ash Content, Ash | 7.90 ± 0.59 |
| Fixed Carbon Content, FC | 57.96 ± 1.25 |
| Ultimate Analysis (% dafb) | |
| C | 69.37 ± 0.22 |
| H | 3.59 ± 0.05 |
| N | 2.33 ± 0.04 |
| S | 0 |
| O | 24.71 ± 1.43 |
| Bulk Density, BD (g/cm3) | 0.112 ± 0.001 |
| Electrical Conductivity, EC (dS/m) | 0.55 ± 0.03 |
| pH | 10.35 ± 0.06 |
| Water Holding Capacity, WHC (%) | 58.99 ± 14.51 |
| Parameter (Units) | Mean Value ± SD |
|---|---|
| Water Content, W (%) | 33.2 ± 1.27 |
| Density, ρ (g/cm3) | 1.027 ± 0.014 |
| pH | 3.34 ± 0.02 |
| Refractive Index, RI | 1.3553 ± 0.0027 |
| Iodine Value, IV (g I2/100 g Bio-Oil) | 87.98 ± 4.38 |
| No. | Chemical Compound | Molecular Formula | CAS Number | Retention Time τR (min) | Peak Area A (%) |
|---|---|---|---|---|---|
| 1 | Cyclopentanone | C5H8O | 120-92-3 | 3.07 | 1.19 |
| 2 | 2-Methylpyridine | C6H7N | 109-06-8 | 3.43 | 1.26 |
| 3 | 3-Furaldehyde | C5H4O2 | 498-60-2 | 3.6 | 10.71 |
| 4 | 2-Furanmethanol | C5H6O2 | 98-00-0 | 3.93 | 4.56 |
| 5 | 1-(Acetyloxy)-2-propanone | C5H8O3 | 592-20-1 | 4.08 | 4.14 |
| 6 | 2-Methyl-2-cyclopenten-1-one | C6H8O | 1120-73-6 | 4.72 | 2.91 |
| 7 | 4-Hydroxybutanoic acid | C4H8O3 | 591-81-1 | 4.82 | 7.90 |
| 8 | 1,2-Cyclopentanedione | C5H6O2 | 3008-40-0 | 5.04 | 2.30 |
| 9 | 3-Methyl-2-cyclopenten-1-one | C6H8O | 2758-18-1 | 5.74 | 4.05 |
| 10 | Phenol | C6H6O | 108-95-2 | 6.03 | 10.75 |
| 11 | Tetrahydrofuran-2-carbonyl chloride | C5H7ClO2 | 52449-98-6 | 6.48 | 2.56 |
| 12 | 3-Methyl-1,2-cyclopentanedione | C6H8O2 | 765-70-8 | 6.85 | 5.49 |
| 13 | 2,3-Dimethyl-2-cyclopenten-1-one | C7H10O | 1121-05-7 | 7.06 | 1.42 |
| 14 | 2-Methylphenol | C7H8O | 95-48-7 | 7.37 | 3.05 |
| 15 | 3-Methylphenol | C7H8O | 108-39-4 | 7.79 | 4.09 |
| 16 | 2-Methoxyphenol (guaiacol) | C7H8O2 | 90-05-1 | 8.01 | 6.74 |
| 17 | 3-Ethyl-2-hydroxy-2-cyclopenten-1-one | C7H10O2 | 21835-01-8 | 8.54 | 1.60 |
| 18 | Creosol | C8H10O2 | 93-51-6 | 9.89 | 1.95 |
| 19 | 4-Ethyl-2-methoxyphenol | C9H12O2 | 2785-89-9 | 11.38 | 1.55 |
| 20 | 2,6-Dimethoxyphenol (syringol) | C8H10O3 | 91-10-1 | 12.58 | 13.25 |
| 21 | 3,5-Dimethoxy-4-hydroxytoluene | C9H12O3 | 6638-05-7 | 14.1 | 2.85 |
| 22 | 1,2,3-Trimethoxy-5-methylbenzene | C10H14O3 | 6443-69-2 | 15.28 | 2.24 |
| 23 | 1-(4-Hydroxy-3,5-dimethoxyphenyl)ethanone | C10H12O4 | 2478-38-8 | 18.22 | 0.54 |
| 24 | Syringylacetone | C11H14O4 | 19037-58-2 | 18.68 | 2.09 |
| 25 | 1-(4-Hydroxy-3,5-dimethoxyphenyl)-1-propanone | C11H14O4 | 5650-43-1 | 19.44 | 0.18 |
| 26 | 5,10-Diethoxy-2,3,7,8-tetrahydro-1H,6H-dipyrrolo [1,2-a:1′,2′-d]pyrazine | C14H22N2O2 | - | 20.93 | 0.44 |
| 27 | Hexanedioic acid dioctyl ester | C22H42O4 | 123-79-5 | 25.9 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcan, S.I.; Pârvulescu, O.C.; Ion, V.A.; Răducanu, C.E.; Bădulescu, L.; Dobre, T.; Egri, D.; Moț, A.; Popa, V.; Crăciun, M.E. Valorization of Vine Prunings by Slow Pyrolysis in a Fixed-Bed Reactor. Processes 2022, 10, 37. https://doi.org/10.3390/pr10010037
Calcan SI, Pârvulescu OC, Ion VA, Răducanu CE, Bădulescu L, Dobre T, Egri D, Moț A, Popa V, Crăciun ME. Valorization of Vine Prunings by Slow Pyrolysis in a Fixed-Bed Reactor. Processes. 2022; 10(1):37. https://doi.org/10.3390/pr10010037
Chicago/Turabian StyleCalcan, Suzana Ioana, Oana Cristina Pârvulescu, Violeta Alexandra Ion, Cristian Eugen Răducanu, Liliana Bădulescu, Tănase Dobre, Diana Egri, Andrei Moț, Vlad Popa, and Mihaela Emanuela Crăciun. 2022. "Valorization of Vine Prunings by Slow Pyrolysis in a Fixed-Bed Reactor" Processes 10, no. 1: 37. https://doi.org/10.3390/pr10010037
APA StyleCalcan, S. I., Pârvulescu, O. C., Ion, V. A., Răducanu, C. E., Bădulescu, L., Dobre, T., Egri, D., Moț, A., Popa, V., & Crăciun, M. E. (2022). Valorization of Vine Prunings by Slow Pyrolysis in a Fixed-Bed Reactor. Processes, 10(1), 37. https://doi.org/10.3390/pr10010037






