Electrical Discharge Coating a Potential Surface Engineering Technique: A State of the Art
Abstract
:1. Introduction
1.1. Need for Coatings on Engineered Surfaces
- (1)
- An improvement in wear resistance properties of cutting tool to enhance productivity [2].
- (2)
- Fabrication of hard and wear-resistant coating on a component made of low weight and strength for a higher power to weight ratio [2].
- (3)
- Fabrication of solid lubricant coatings to reduce frictional force, which allows for lower consumption of fuel and is also beneficial for applications in brakes, bolted joints, and safety connectors [2].
- (4)
- Application of coatings in many sliding components to reduce the tendency of sticking [2].
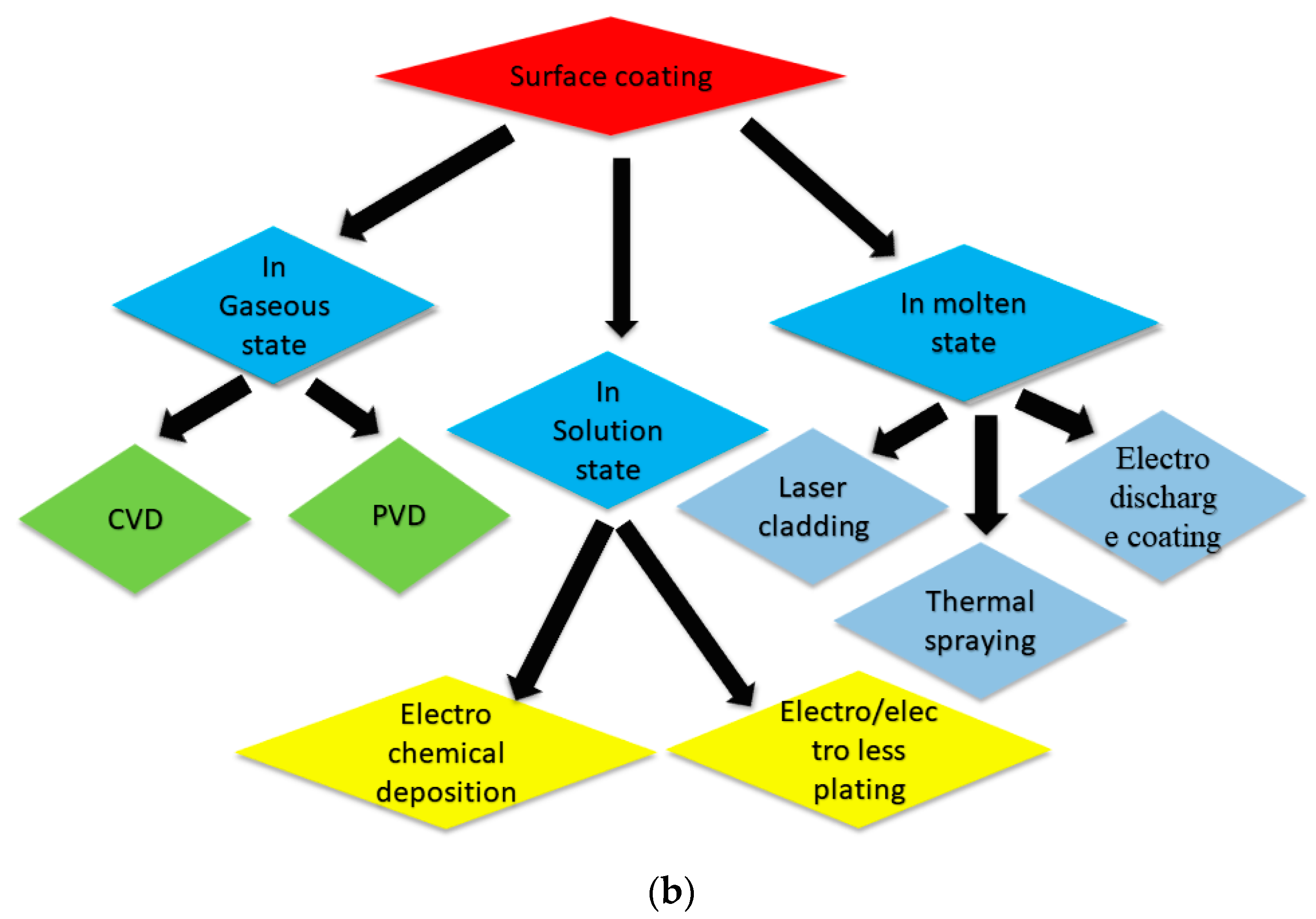
1.2. Methods of Coating, Advantages, and Limitations
1.2.1. Electroless Plating
1.2.2. Electrochemical Deposition
1.2.3. Chemical Vapor Deposition (CVD)
1.2.4. Physical Vapor Deposition (PVD)
1.2.5. Plasma Arc Coating
1.2.6. Laser Cladding
1.2.7. Electrical Discharge Coating (EDC)
1.3. Comparison between EDC and Other Processes
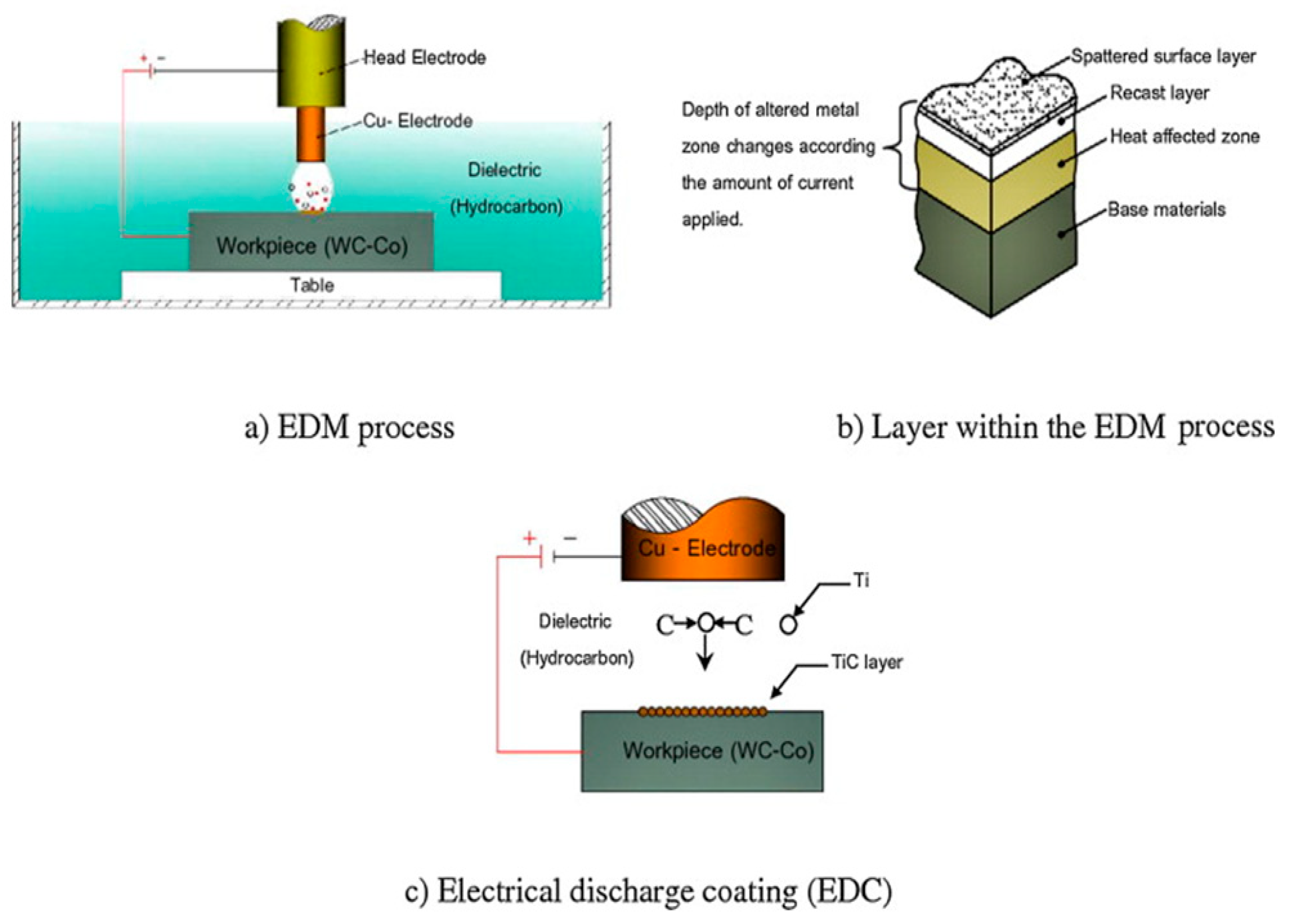
2. Background and Description of EDC
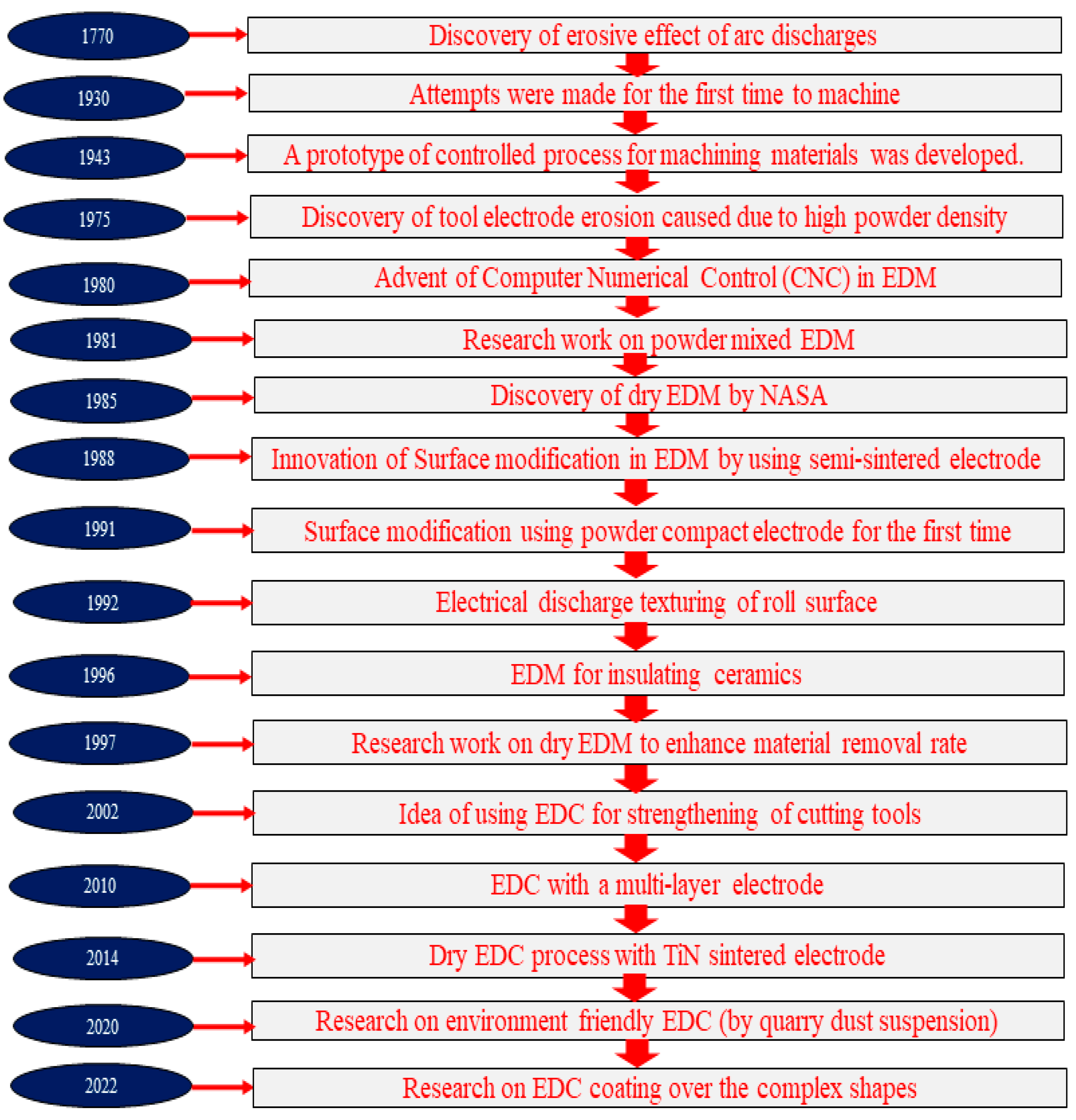
2.1. Origin of Electrical Discharge Coating (EDC)
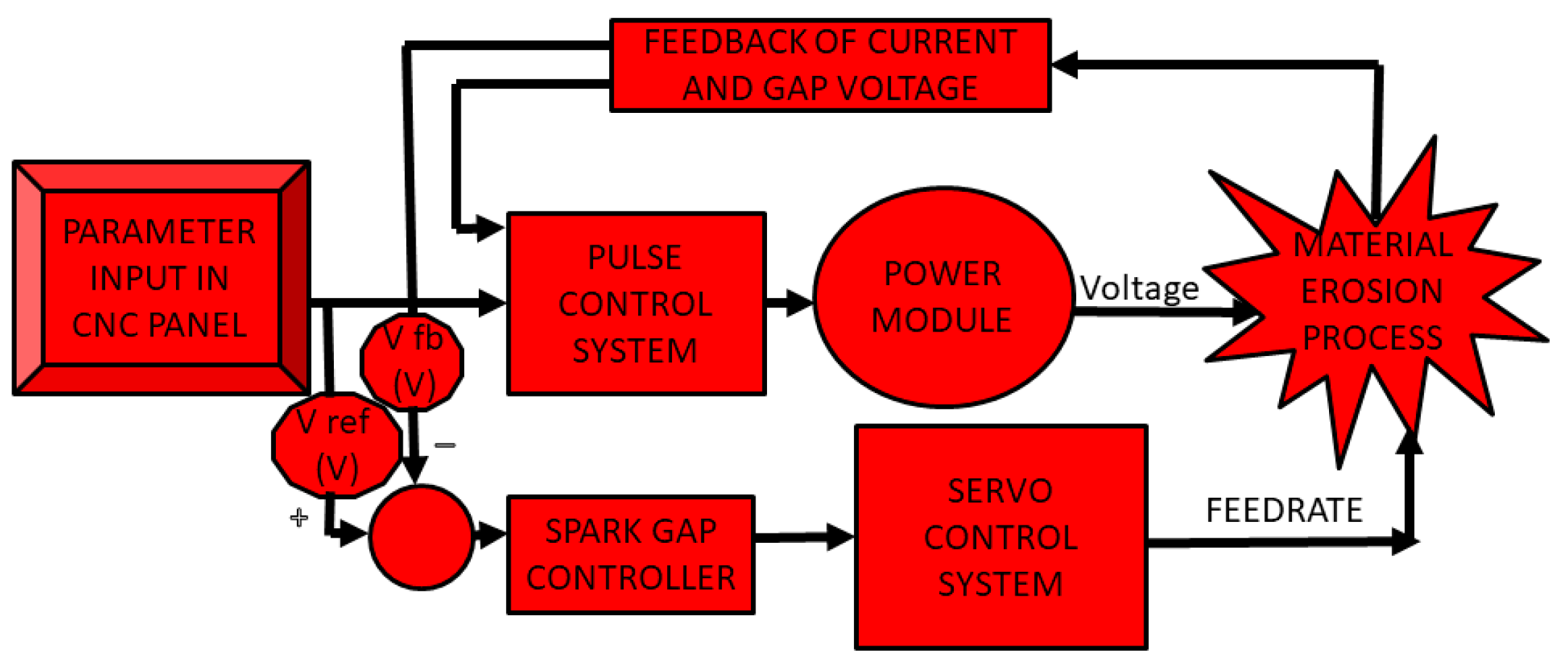
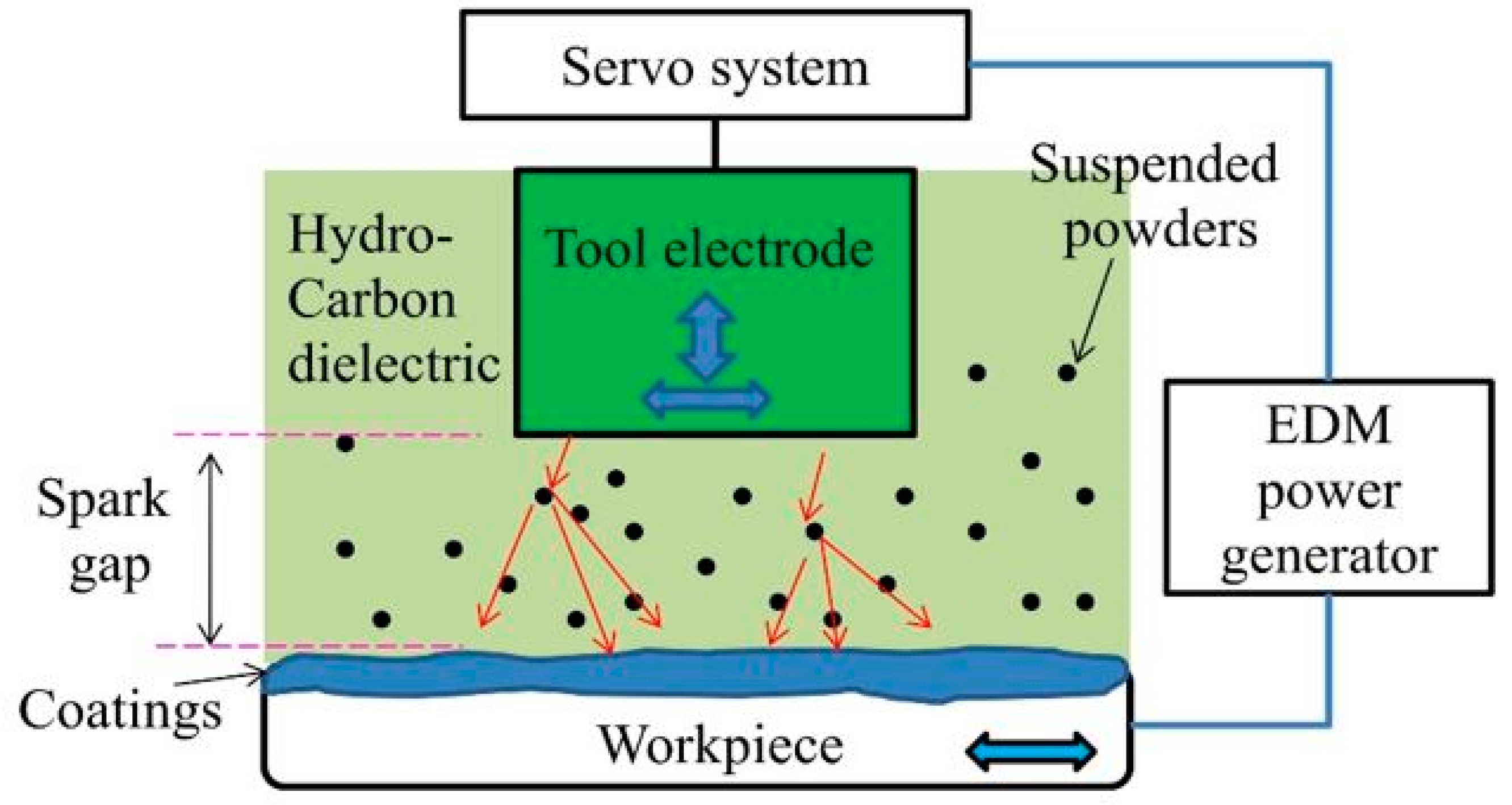
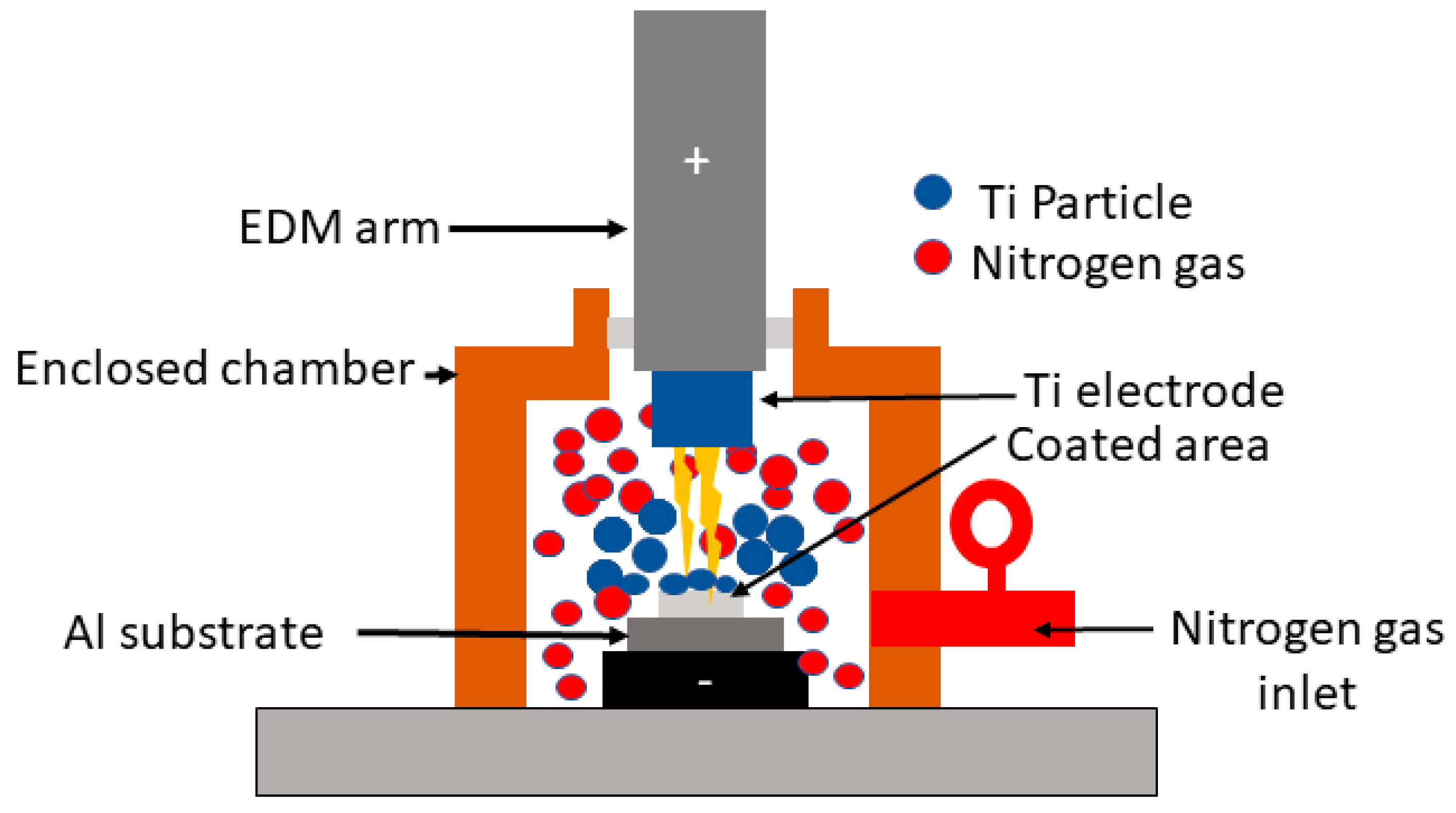
2.2. Working Principle of EDC
2.2.1. Advantages of EDC
- (1)
- The hard recast layer in EDM generally contains cracks, caused by the presence of residual stresses, which diminish the corrosion and wear resistance of components manufactured through EDM [104]. To remove and to restore the surface properties in the damaged layer, the EDC process is needed.
- (2)
- The recast layer thickness obtained in EDC is higher than EDM as a result of reverse polarity and loosely bonded particles in the green compact tool electrode. This reverse polarity with an optimal combination of process parameters assists in the maximum blending of the compact electrode material with parent material. Hence, EDC is adapted as an application of EDM by using the powder compact tool electrode and powder mixed EDM of the desired powder material.
- (3)
- (4)
- The process has the capability of machining and coating the substrate using the same tool electrode simultaneously. Hence, it is possible to use different shapes of an electrode for making complex components.
- (5)
- High-temperature plasma forms an electrical discharge process that can be effectively applied to prepare the coating of metals with a high melting point (hard-to-process ceramics materials) on a base material. Therefore, the wear-resistant ceramic coating layer on complex-shaped parts used in various machine components can easily be fabricated to enhance the lifespan [7].
- (6)
- EDC is an advanced and simple coating process that is applied for conductive materials due to certain advantages, i.e., good adhesion among the parent material and coating, high efficiency to achieve thick coating, and ability to balance the composition of coated layer by using the proper tool electrode material and dielectric fluid.
- (7)
- (8)
- As we know that EDC has the advantage of not needing a complicated set-up and high temperature and vacuum surrounding, it is thus classified as an economically viable coating process. This method of modifying the surface attracted researchers directly towards surface modification, especially for electrical discharge texturing with tools made of WC/Co and TiC/WC/Co, etc. [62,63,64,65,66,67,68].
2.3. Process Parameters and Their Influences
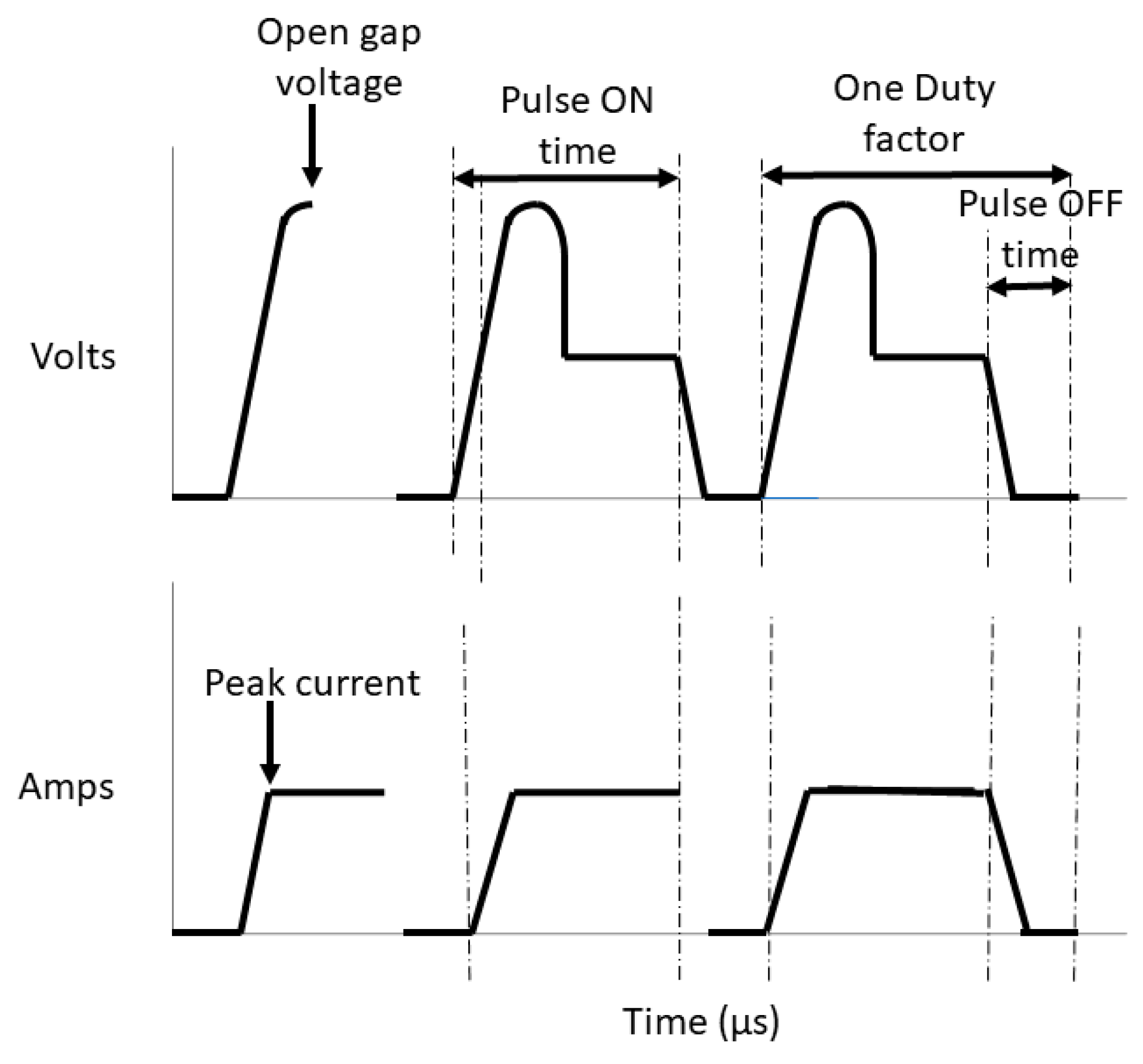
2.3.1. Gap Voltage and Discharge Voltage
2.3.2. Peak Current and Average Current
2.3.3. Pulse on and Pulse off Time
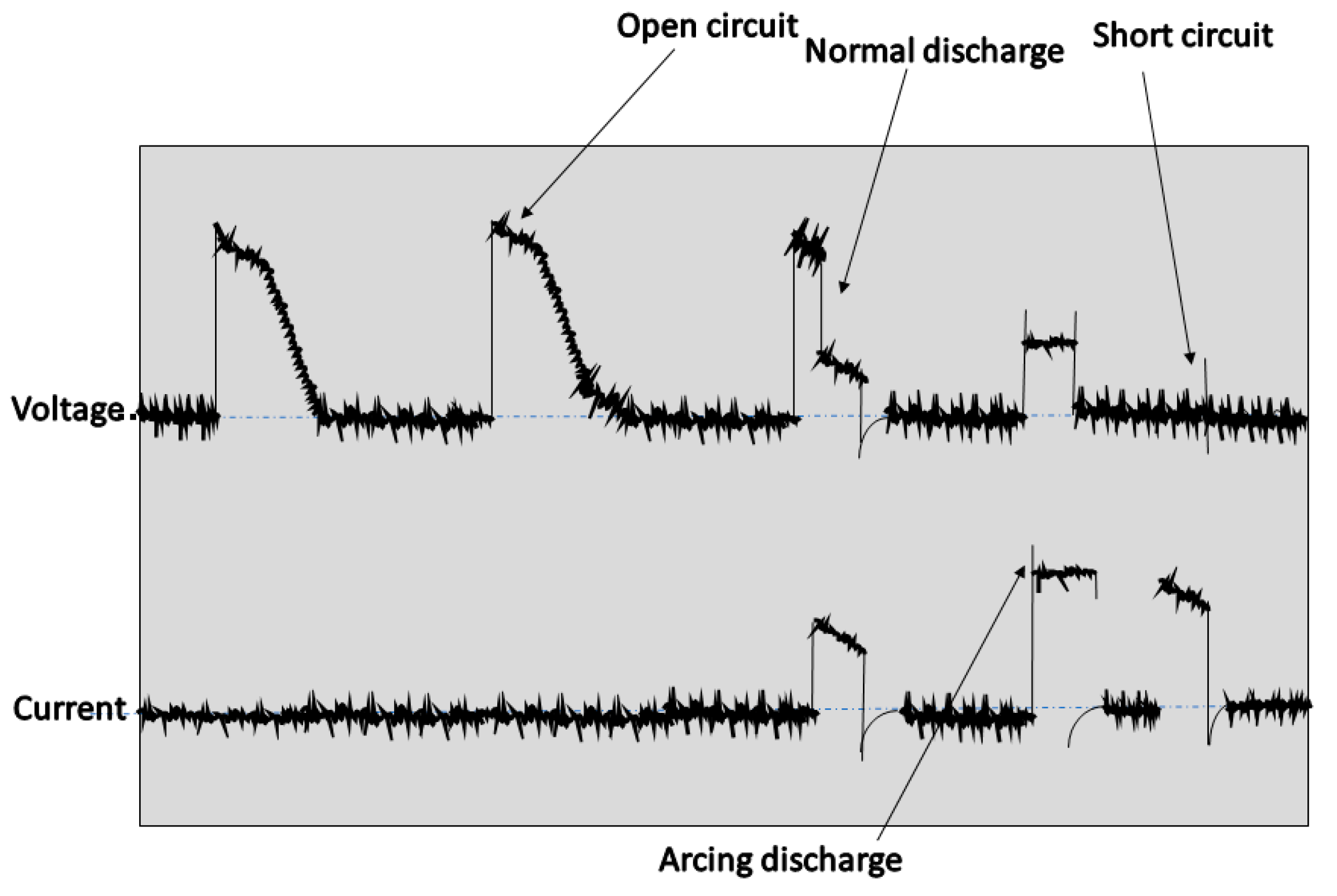
2.4. Analysis of Sparking, Arcing, and Short Circuit
3. Classification of the EDC Process
3.1. Green Compact Electrode Coating
3.1.1. Parameters for the Preparation of Green Compact Electrode
Powder Composition
Compaction Pressure
Sintering Temperature
3.1.2. Surface Coating Using Green Compact Electrodes
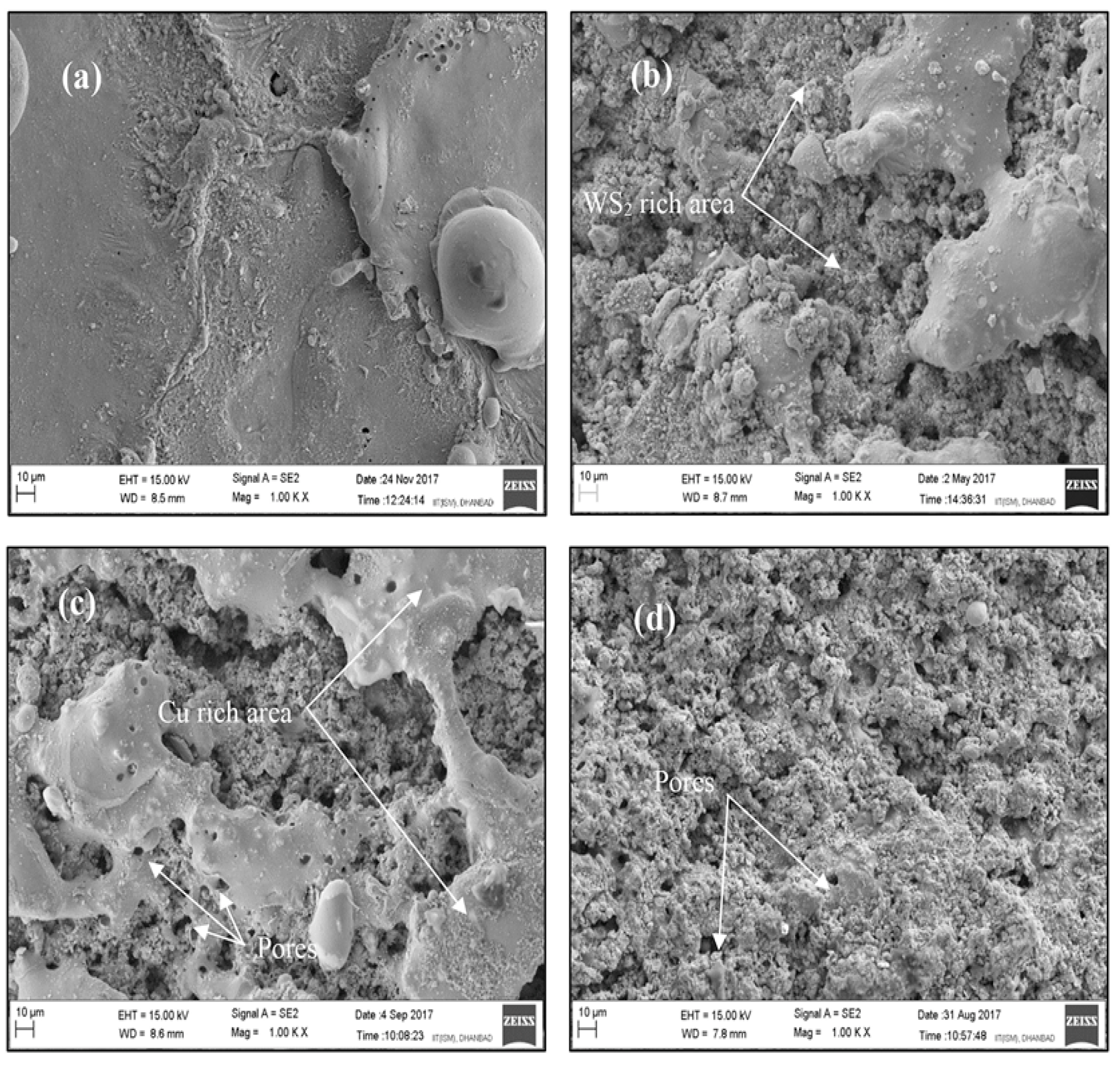
Using Copper and Its Alloys in a Powder Compact Tool Electrode
Using Copper/Silicon Carbide Powder Compact Tool Electrode
Using Tungsten/Tungsten Carbide Powder Compact Tool Electrode
Using Titanium/Titanium Carbide Powder Compact Tool Electrode
Using a Solid Lubricant Powder Compact Tool Electrode
Using Multi-Layer Electrode
3.2. Powder Mixed Dielectric Coating
3.2.1. Surface Coating Using a Suspension of Powder/Gas in Dielectric
The Suspension of Solid Lubricant
The Suspension of Titanium Powder
Using Hydroxy-Apatite (HA) Mixed Dielectric
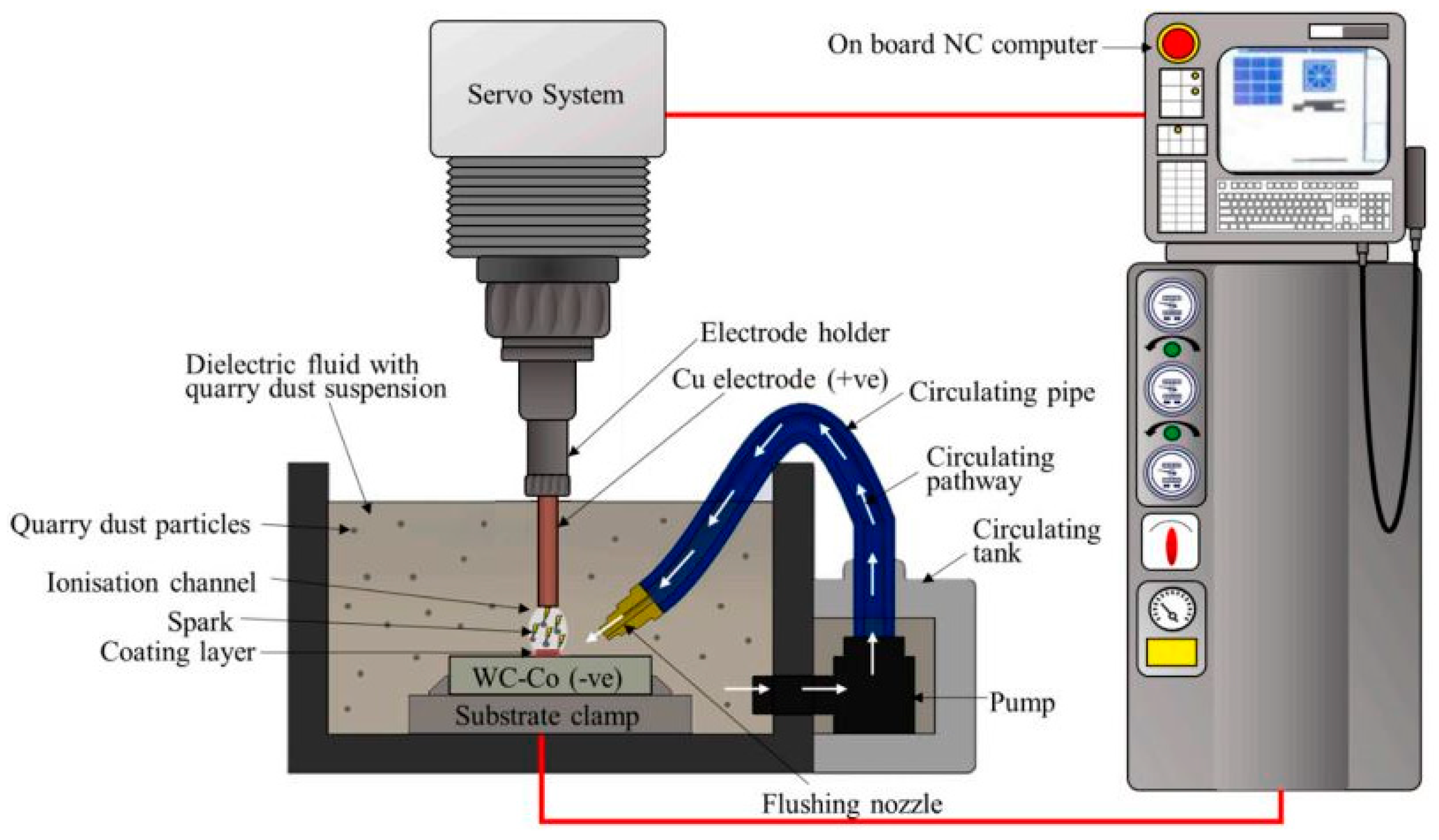
Quarry Dust Suspension
Using Dry EDC Process
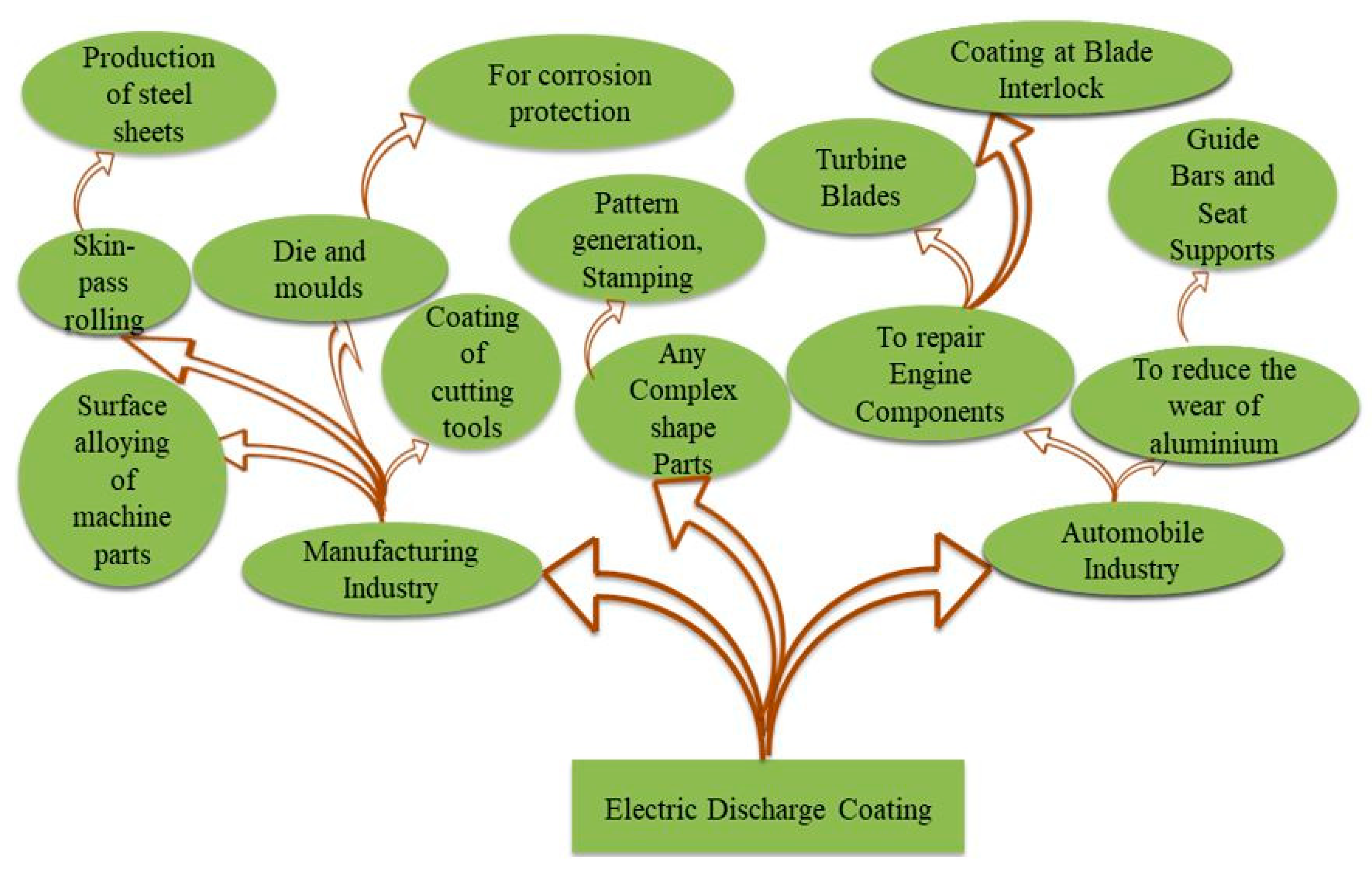
4. Applications of Surface Modified by EDC
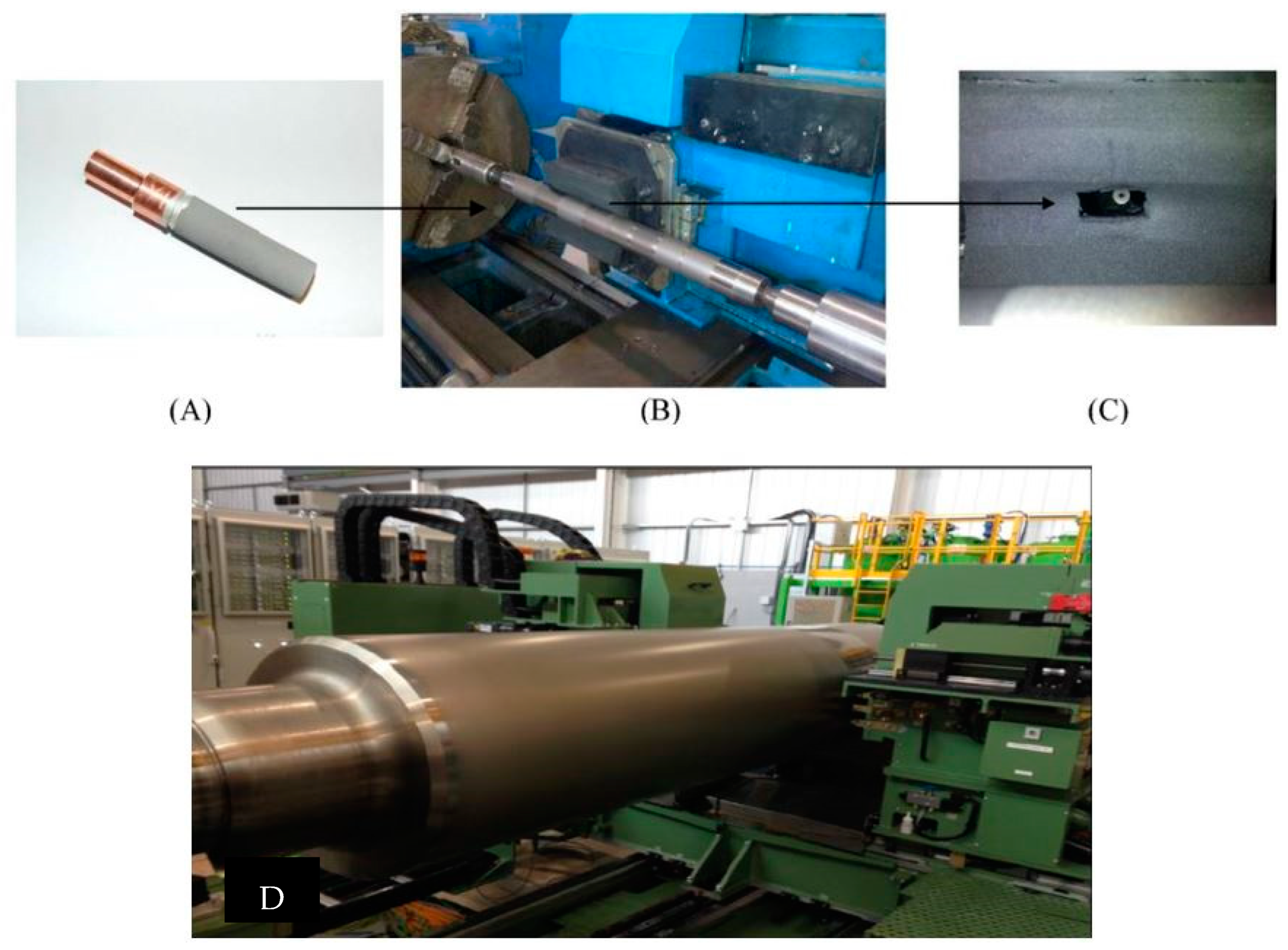
4.1. In Roll Surface Texturing
4.2. To Repair the Turbine Blade
4.3. Pattern Generation and Stamping
4.4. In Guide Bars, Seat Supports/Bearing Plates
4.5. In Die and Mould Coating
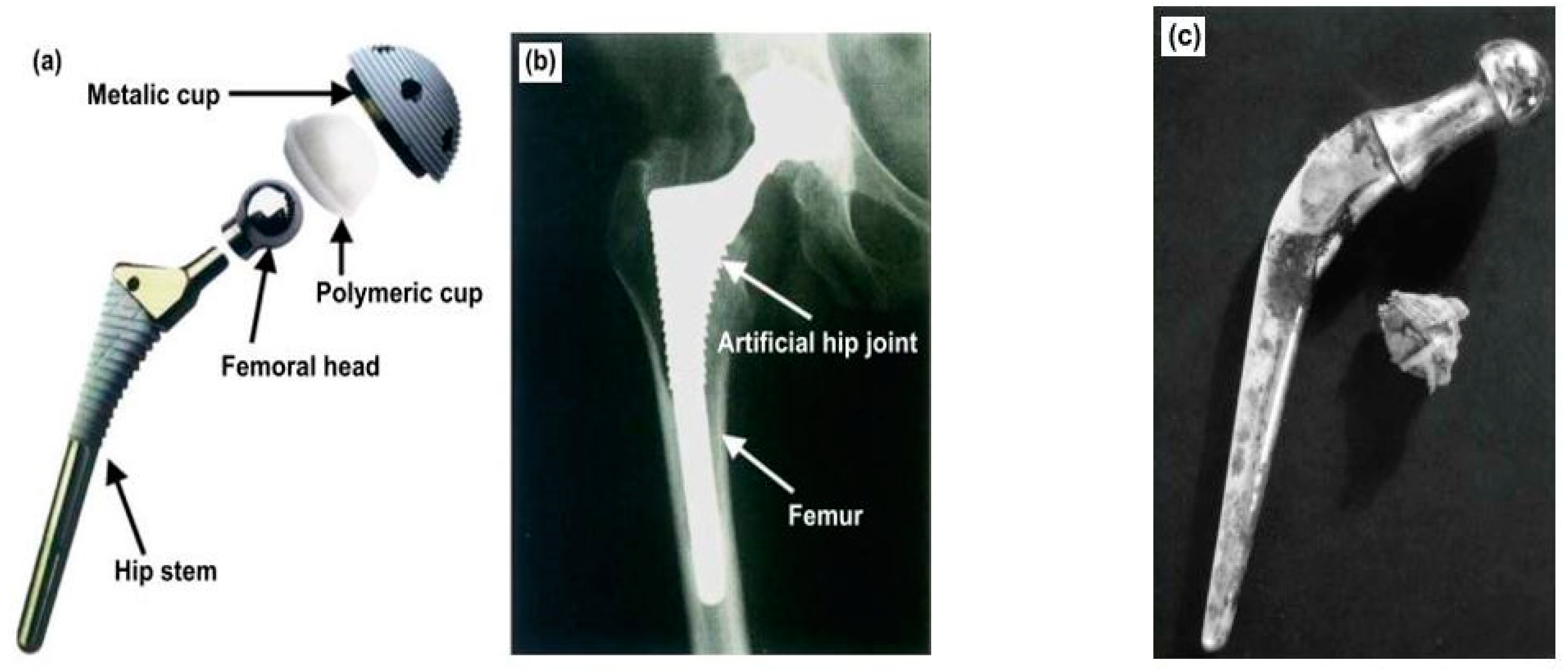
4.6. In the Biomedical Field
5. Challenges in the EDC and Future Scope
- A major drawback in EDC process is that the position of tool electrode and workpiece should be parallel, which influences the uniformity in the coating.
- The phenomenon results in accretion in which a new layer formation and material removal occur together, which needs to be identifiable. Hence, the tool wear should be increased as compared to the cutting speed of the base material.
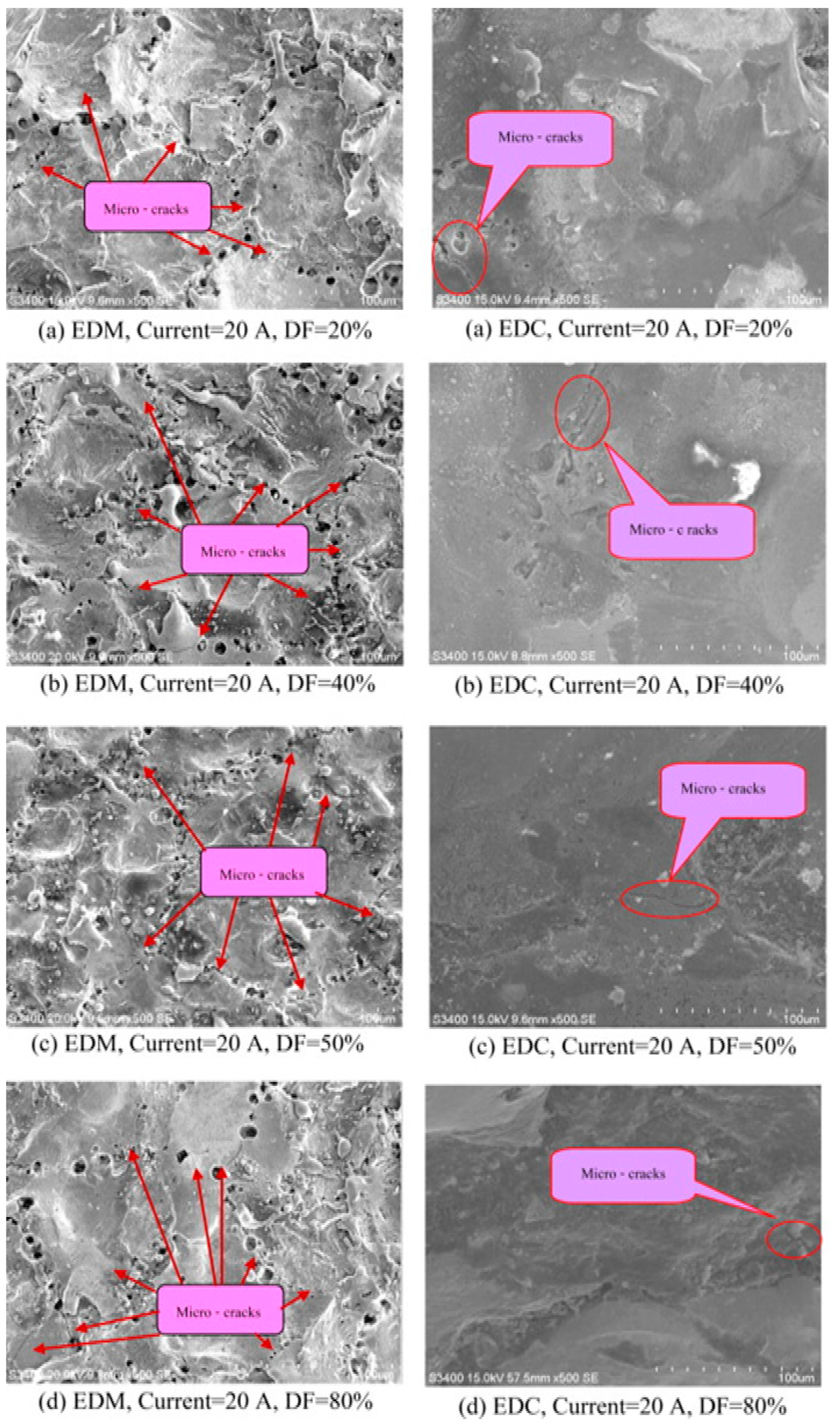
- Despite the minimization of defects in EDC as compared to EDM, the surface roughness of the coating gets compromised, which needs to be improved.
- The circulation of liquid dielectric and debris removal are general problems in coating, which restrict the efficiency of the coating process.
- There is no/light flushing used in ED process; hence, arcing is the major issue in EDC that deteriorates the surface finish and restricts the proper deposition, which leads to an unstable coating process.
- Degradation of surface roughness in EDC is observed for an improper composite powder mixing duration in mortar and for using dielectric oil with a random selection of process parameters.
6. Conclusions
- The process of surface coating by means of green compact electrodes and the powder suspension EDC method proposed is a good option in place of other costlier techniques of surface coating like laser cladding, thermal spraying, electroless plating, etc. In EDC, the current and pulse on-time in EDC is identified as a major effective parameter in the EDC process.
- The green compact tool prepared with hard powders (TiC and WC) showed high hardness and more defects, and the coating with soft powders (WS2, MoS2 and hbn) showed low hardness with less defects. Therefore, researchers have suggested both hard and soft powder in the green compact tool to impart desirable properties.
- By mixing the powder in the working dielectric, the green compact electrode preparation process can be excluded in EDC. By preparing a hydroxy-apatite (HA) coating by powder mixed EDC, hydrophilic properties can be achieved. Further, the TiC powder mixed coating also showed high hardness, but the presence of defects needs to be reduced.
- The multi-layer electrode was also used in EDC, and researchers have tried to use multi-layer green compact electrodes to reduce the defects in TiC coatings. However, a low recast layer thickness was obtained by using this type of electrode, which cannot be used for complex-shaped parts.
- Among all these processes, the dry EDC process proved to be the best alternative to reduce the environmental pollution. In this study, the tribological behavior of the coating was missing, and the coating also contained a few voids in the film due to incomplete coverage and microcracks.
- From an application point of view, EDC has a large range of applications in both the automotive and manufacturing industries. The application has also been extended to orthopedic implants for enhancement of wear and corrosion resistance, mechanical properties, and fatigue life. Further, the process can also be applied in biomedical applications.
Author Contributions
Funding
Conflicts of Interest
References
- Khadem, M.; Penkov, O.V.; Yang, H.K.; Kim, D.E. Tribology of Multilayer Coatings for Wear Reduction: A Review. Friction 2017, 5, 248–262. [Google Scholar] [CrossRef]
- Hogmark, S.; Jacobson, S.; Larsson, M. Design and Evaluation of Tribological Coatings. Wear 2000, 246, 20–33. [Google Scholar] [CrossRef]
- Arun, I.; Yuvaraj, C.; Selvarani, P.; Senthilkumaar, J.S.; Thamizhmanii, S.; Muruganandam, P. Synthesis of Electrical Discharge Metal Matrix Composite Coating through Compacted Semi-Sintered Electrode and Its Tribological Studies. J. Braz. Soc. Mech. Sci. Eng. 2019, 41, 213. [Google Scholar] [CrossRef]
- Sheng, C.; He, G.; Hu, Z.; Chou, C.; Shi, J.; Li, J.; Meng, Q.; Ning, X.; Wang, L.; Ning, F. Yarn on Yarn Abrasion Failure Mechanism of Ultrahigh Molecular Weight Polyethylene Fiber. J. Eng. Fibers Fabr. 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Liew, P.J.; Yap, C.Y.; Nurlishafiqa, Z.; Othman, I.S.; Chang, S.Y.; Toibah, A.R.; Wang, J. Material Deposition on Aluminium by Electrical Discharge Coating (Edc) with a Tungsten Powder Suspension. J. Adv. Manuf. Technol. 2018, 12, 133–145. [Google Scholar]
- Tyagi, R.; Das, A.K.; Mandal, A. Electrical Discharge Coating Using WS2 and Cu Powder Mixture for Solid Lubrication and Enhanced Tribological Performance. Tribol. Int. 2018, 120, 80–92. [Google Scholar] [CrossRef]
- Algodi, S.J.; Murray, J.W.; Brown, P.D.; Clare, A.T. Wear Performance of TiC/Fe Cermet Electrical Discharge Coatings. Wear 2018, 402–403, 109–123. [Google Scholar] [CrossRef]
- Elkoca, O. A Study on the Characteristics of Electrical Discharge Textured Skin Pass Mill Work Roll. Surf. Coat. Technol. 2008, 202, 2765–2774. [Google Scholar] [CrossRef]
- Prakash, C.; Kansal, H.K.; Pabla, B.S.; Puri, S. Effect of surface nano-porosities fabricated by powder mixed electric discharge machining on bone-implant interface: An experimental and finite element study. Nanosci. Nanotechnol. Lett. 2016, 8, 815–826. [Google Scholar] [CrossRef]
- Ning, F.; He, G.; Sheng, C.; He, H.; Wang, J.; Zhou, R.; Ning, X. Yarn on Yarn Abrasion Performance of High Modulus Polyethylene Fiber Improved by Graphene/Polyurethane Composites Coating. J. Eng. Fibers Fabr. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Huang, H.; Huang, M.; Zhang, W.; Pospisil, S.; Wu, A.T. Experimental Investigation on Rehabilitation of Corroded RC Columns with BSP and HPFL under Combined Loadings. J. Struct. Eng. 2020, 146, 8. [Google Scholar] [CrossRef]
- Pramanik, A.; Basak, A.; Prakash, C. Understanding the wire electrical discharge machining of Ti6Al4V alloy. Heliyon 2019, 5, e01473. [Google Scholar] [CrossRef] [PubMed]
- Mughal, M.P.; Farooq, M.U.; Mumtaz, J.; Mia, M.; Shareef, M.; Javed, M.; Jamil, M.; Pruncu, C.I. Surface Modification for Osseointegration of Ti6Al4V ELI Using Powder Mixed Sinking EDM. J. Mech. Behav. Biomed. Mater. 2021, 113, 104145. [Google Scholar] [CrossRef] [PubMed]
- Balanou, M.; Karmiris-Obratański, P.; Leszczyńska-Madej, B.; Papazoglou, E.L.; Markopoulos, A.P. Investigation of surface modification of 60CrMoV18-5 steel by EDM with Cu-ZrO2 powder metallurgy green compact electrode. Machines 2021, 9, 268. [Google Scholar] [CrossRef]
- Murray, J.W.; Algodi, S.J.; Fay, M.W.; Brown, P.D.; Clare, A.T. Formation Mechanism of Electrical Discharge TiC-Fe Composite Coatings. J. Mater. Process. Technol. 2017, 243, 143–151. [Google Scholar] [CrossRef]
- Machkale, P.D.; Dabade, B.M. Experimental Investigation of Tungsten and Copper Carbide Coating on AISI 1020 Steel Using Electro Discharge Coating Process. Mater. Today Proc. 2020, 26, 2915–2920. [Google Scholar] [CrossRef]
- Patowari, P.K.; Saha, P.; Mishra, P.K. Artificial Neural Network Model in Surface Modification by EDM Using Tungsten-Copper Powder Metallurgy Sintered Electrodes. Int. J. Adv. Manuf. Technol. 2010, 51, 627–638. [Google Scholar] [CrossRef]
- Ahmed, A. Deposition and Analysis of Composite Coating on Aluminum Using Ti-B 4C Powder Metallurgy Tools in EDM. Mater. Manuf. Process. 2016, 31, 467–474. [Google Scholar] [CrossRef]
- Matsukawa, K.; Satoh, K.; Goto, A.; Saito, N.; Mohri, N. Wear Properties of Surface Modified Hard Layers Using Electrical Discharge Machine. J. Adv. Mech. Des. Syst. Manuf. 2008, 2, 629–639. [Google Scholar] [CrossRef]
- Spadło, S.; Młynarczyk, P. Influence of the of Electrical Discharge Alloying Methods on the Surface Quality of Carbon Steel. Int. J. Adv. Manuf. Technol. 2017, 89, 1529–1534. [Google Scholar] [CrossRef]
- Ahmed, N.; Murray, J.W.; Yuzawa, T.; Nakagawa, T.; Sarugaku, S.; Saito, D.; Brown, P.D.; Clare, A.T. Formation of Thick Electrical Discharge Coatings. J. Mater. Process. Technol. 2020, 285, 116801. [Google Scholar] [CrossRef]
- Ahmed, N.; Murray, J.W.; Yuzawa, T.; Kurokawa, T.; Nakagawa, T.; Sarugaku, S.; Saito, D.; Clare, A.T. Residual Stress in Electrical Discharge Coatings. Surf. Coat. Technol. 2021, 416, 127156. [Google Scholar] [CrossRef]
- Goto, A.; Akiyoshi, M.; Ochiai, H.; Watanabe, M. Development of Micro Spark Coating. In Proceedings of the 24th International Congress of the Aeronautical Sciences, Yokohama, Japan, 29 August–3 September 2004; pp. 1–7. Available online: https://www.semanticscholar.org/paper/DEVELOPMENT-OF-MICRO-SPARK-COATING-Goto-Akiyoshi/0e4e5f31907babf4a7517bb160ba70b725736377 (accessed on 31 July 2022).
- Nakano, Y.; Araki, T.; Yamada, A.; Teramoto, H.; Okane, M.; Goto, A. Development of Innovative Coating Technology, MSCoating, Using Electrical Discharge. In Proceedings of the Turbo Expo: Power for Land, Sea, and Air, Glasgow, UK, 14–18 June 2010; Volume 43963, pp. 929–937. [Google Scholar]
- Dong, H.; Bell, T. State-of-the-Art Overview: Ion Beam Surface Modification of Polymers towards Improving Tribological Properties. Surf. Coat. Technol. 1999, 111, 29–40. [Google Scholar] [CrossRef]
- Lee, Y.; Han, S.; Lim, H.; Kim, Y.; Kim, H. Surface Analysis of Polymers Electrically Improved by Plasma-Source Ion-Implantation. Anal. Bioanal. Chem. 2002, 373, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Jagatheeshwaran, M.S.; Elayaperumal, A.; Arulvel, S. Wear Characteristics of Electroless NiP/Bio-Composite Coatings on En8 Steel. J. Manuf. Process. 2015, 20, 206–214. [Google Scholar] [CrossRef]
- Rajput, M.S.; Pandey, P.M.; Jha, S. Modelling of High Speed Selective Jet Electrodeposition Process. J. Manuf. Process. 2015, 17, 98–107. [Google Scholar] [CrossRef]
- Wu, Z. Empirical Modeling for Processing Parameters’ Effects on Coating Properties in Plasma Spraying Process. J. Manuf. Process. 2015, 19, 1–13. [Google Scholar] [CrossRef]
- Wang, G.; Huang, Z.; Xiao, P.; Zhu, X. Spraying of Fe-Based Amorphous Coating with High Corrosion Resistance by HVAF. J. Manuf. Process. 2016, 22, 34–38. [Google Scholar] [CrossRef]
- Ando, T.; Nishitani-Gamo, M.; Rawles, R.E.; Yamamoto, K.; Kamo, M.; Sato, Y. Chemical Modification of Diamond Surfaces Using a Chlorinated Surface as an Intermediate State. Diam. Relat. Mater. 1996, 5, 1136–1142. [Google Scholar] [CrossRef]
- Fuke, I.; Prabhu, V.; Baek, S. Computational Model for Predicting Coating Thickness in Electron Beam Physical Vapor Deposition. J. Manuf. Process. 2005, 7, 140–152. [Google Scholar] [CrossRef]
- De Damborenea, J. Surface Modification of Metals by High Power Lasers. Surf. Coat. Technol. 1998, 100–101, 377–382. [Google Scholar] [CrossRef]
- Zhang, P.R.; Liu, Z.Q.; Guo, Y.B. Machinability for Dry Turning of Laser Cladded Parts with Conventional vs. Wiper Insert. J. Manuf. Process. 2017, 28, 494–499. [Google Scholar] [CrossRef]
- Dini, J.W. Properties of Coatings: Comparisons of Electroplated, Physical Vapor Deposited, Chemical Vapor Deposited, and Plasma Sprayed Coatings. Mater. Manuf. Process. 1997, 12, 437–472. [Google Scholar] [CrossRef]
- Legg, K.O.; Graham, M.; Chang, P.; Rastagar, F.; Gonzales, A.; Sartwell, B. The Replacement of Electroplating. Surf. Coat. Technol. 1996, 81, 99–105. [Google Scholar] [CrossRef]
- Pawlowski, L. The Science and Engineering of Thermal Spray Coatings; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Madhaw, S. Surface Modification by Electro-Discharge Coating with WC-Cu P/M Electrode Tool. Bachelor’s Thesis, National Institute of Technology, Rourkela, India, 2013. [Google Scholar]
- Krishna, M.E.; Patowari, P.K. Parametric Optimisation of Electric Discharge Coating Process with Powder Metallurgy Tools Using Taguchi Analysis. Surf. Eng. 2013, 29, 703–711. [Google Scholar] [CrossRef]
- Bhushan, B. Principles and Applications of Tribology; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- ASM. ASM Handbook, Volume 18: Friction, Lubrication, and Wear Technology; ASM International: Almere, The Netherlands, 1992. [Google Scholar]
- Holmberg, K.; Erdemir, A. The Impact of Tribology on Energy Use and CO2 Emission Globally and in Combustion Engine and Electric Cars. Tribol. Int. 2019, 135, 389–396. [Google Scholar] [CrossRef]
- Sudagar, J.; Tamilarasan, R.; Sanjith, U.; Rajendran, R.; Kumar, R. Electroless Deposition of Nanolayered Metallic Coatings. In Nanoscaled Film and Layers; InTechOpen: London, UK, 2017; p. 27. [Google Scholar]
- Mallory, G.O.; Hajdu, J.B. Electroless Plating: Fundamentals and Applications; William Andrew: Norwich, NY, USA, 1990. [Google Scholar]
- Simko, M.; Silva, E.A.; Wolf, R.H.; Davis, S.P. Sheet Steel: Coated; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Bhanvase, B.A.; Pawade, V.B.; Dhoble, S.J.; Sonawane, S.H.; Ashokkumar, M. Nanomaterials for Green Energy; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Gunputh, U.; Le, H. Composite Coatings for Implants and Tissue Engineering Scaffolds. In Biomedical Composites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 111–138. [Google Scholar]
- Shang, S.M.; Zeng, W. Conductive Nanofibres and Nanocoatings for Smart Textiles. In Multidisciplinary Know-How for Smart-Textiles Developers; Elsevier: Amsterdam, The Netherlands, 2013; pp. 92–128. [Google Scholar]
- Park, J.-H.; Sudarshan, T.S. Chemical Vapor Deposition; ASM International: Almere, The Netherlands, 2001; Volume 2. [Google Scholar]
- Beaux, M.F.; Vodnik, D.R.; Peterson, R.J.; Bennett, B.L.; Salazar, J.J.; Holesinger, T.G.; King, G.; Maloy, S.A.; Devlin, D.J.; Usov, I.O. Chemical Vapor Deposition of Mo Tubes for Fuel Cladding Applications. Surf. Coat. Technol. 2018, 337, 510–515. [Google Scholar] [CrossRef]
- Mattox, D.M. Handbook of Physical Vapor Deposition (PVD) Processing; William Andrew: Norwich, NY, USA, 2010. [Google Scholar]
- Mattox, D.M. Ion Plating. In ASM Handbook Volume 5: Surface Engineering; ASM International: Almere, The Netherlands, 1994. [Google Scholar]
- Fadavi, M.; Baboukani, A.R.; Edris, H.; Salehi, M. Study on High-Temperature Oxidation Behaviors of Plasma-Sprayed TiB 2-Co Composite Coatings. J. Korean Ceram. Soc. 2018, 55, 178–184. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Surmeneva, M.A.; Shugurov, V.V.; Koval, N.N.; Shulepov, I.A.; Surmenev, R.A. Physico-Mechanical Properties of Ti-Zr Coatings Fabricated via Ion-Assisted Arc-Plasma Deposition. Vacuum 2018, 149, 129–133. [Google Scholar] [CrossRef]
- Toyserkani, E.; Khajepour, A.; Corbin, S.F. Laser Cladding; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Kumar, V.; Rakshit, R.; Das, A.K. Mechanical and Tribological Performance of Fiber Laser Cladded H-BN + SS316 Composite on SS316 Surface. J. Mater. Process. Technol. 2020, 278, 116509. [Google Scholar] [CrossRef]
- Baldridge, T.; Poling, G.; Foroozmehr, E.; Kovacevic, R.; Metz, T.; Kadekar, V.; Gupta, M.C. Laser Cladding of Inconel 690 on Inconel 600 Superalloy for Corrosion Protection in Nuclear Applications. Opt. Lasers Eng. 2013, 51, 180–184. [Google Scholar] [CrossRef]
- Kumar, S.; Mandal, A.; Das, A.K.; Dixit, A.R. Parametric Study and Characterization of AlN-Ni-Ti6Al4V Composite Cladding on Titanium Alloy. Surf. Coat. Technol. 2018, 349, 37–49. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, G.; Wang, Y.; Li, H.; Qin, Z.; Lu, X. Crack-Free Fe-Based Amorphous Coating Synthesized by Laser Cladding. Mater. Lett. 2018, 210, 46–50. [Google Scholar] [CrossRef]
- Goto, A. Development of Electrical Discharge Coating Method. Proc. Int. Symp. Electro-Mach. 2001, 2, 581–588. [Google Scholar]
- Beri, N.; Maheshwari, S.; Sharma, C.; Kumar, A. Technological Advancement in Electrical Discharge Machining with Powder Metallurgy Processed Electrodes: A Review. Mater. Manuf. Process. 2010, 25, 1186–1197. [Google Scholar] [CrossRef]
- Samuel, M.P.; Philip, P.K. Power Metallurgy Tool Electrodes for Electrical Discharge Machining. Int. J. Mach. Tools Manuf. 1997, 37, 1625–1633. [Google Scholar] [CrossRef]
- Mohri, N.; Saito, N.; Tsunekawa, Y.; Kinoshita, N. Metal Surface Modification by Electrical Discharge Machining with Composite Electrode. CIRP Ann.-Manuf. Technol. 1993, 42, 219–222. [Google Scholar] [CrossRef]
- Mohri, N.; Fukusima, Y.; Fukuzawa, Y.; Tani, T.; Saito, N. Layer Generation Process on Work-Piece in Electrical Discharge Machining. CIRP Ann.-Manuf. Technol. 2003, 52, 157–160. [Google Scholar] [CrossRef]
- Hao, R.; Lu, Z.; Ding, H.; Chen, L. A Nonlinear Vibration Isolator Supported on a Flexible Plate: Analysis and Experiment. Nonlinear Dyn. 2022, 108, 941–958. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Yi, D.; Liu, B.; Zhu, J.; Li, J.; Gao, C.; Wang, L. Effects of NbC Content on Microstructural Evolution and Mechanical Properties of Laser Cladded Fe50Mn30Co10Cr10-xNbC Composite Coatings. Intermetallics 2021, 138, 107309. [Google Scholar] [CrossRef]
- Li, X.; Yan, J.; Yu, T.; Zhang, B. Versatile Nonfluorinated Superhydrophobic Coating with Self-Cleaning, Anti-Fouling, Anti-Corrosion and Mechanical Stability. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128701. [Google Scholar] [CrossRef]
- Aspinwall, D.K.; Dewes, R.C.; Lee, H.G.; Simao, J. Electrical Discharge Surface Alloying of Ti and Fe Workpiece Materials Using Refractory Powder Compact Electrodes and Cu Wire. CIRP Ann.-Manuf. Technol. 2003, 52, 151–156. [Google Scholar] [CrossRef]
- Guan, X.; Wang, Y.; Zhang, G.; Jiang, X.; Wang, L.; Xue, Q. Microstructures and Properties of Zr/CrN Multilayer Coatings Fabricated by Multi-Arc Ion Plating. Tribol. Int. 2017, 106, 78–87. [Google Scholar] [CrossRef]
- Cui, G.; Han, B.; Zhao, J.; Li, M. Comparative Study on Tribological Properties of the Sulfurizing Layers on Fe, Ni and Co Based Laser Cladding Coatings. Tribol. Int. 2019, 134, 36–49. [Google Scholar] [CrossRef]
- Vuchkov, T.; Evaristo, M.; Yaqub, T.B.; Polcar, T.; Cavaleiro, A. Synthesis, Microstructure and Mechanical Properties of W–S–C Self-Lubricant Thin Films Deposited by Magnetron Sputtering. Tribol. Int. 2020, 150, 106363. [Google Scholar] [CrossRef]
- Thakur, A.; Gangopadhyay, S. Influence of Tribological Properties on the Performance of Uncoated, CVD and PVD Coated Tools in Machining of Incoloy 825. Tribol. Int. 2016, 102, 198–212. [Google Scholar] [CrossRef]
- Kumari, S.; Masanta, M. Ceramic-Metal Composite Coating on Steel Using a Powder Compact Tool Electrode by the Electro-Discharge Coating Process. Silicon 2018, 10, 1625–1637. [Google Scholar]
- Wang, Z.L.; Fang, Y.; Wu, P.N.; Zhao, W.S.; Cheng, K. Surface Modification Process by Electrical Discharge Machining with a Ti Powder Green Compact Electrode. J. Mater. Process. Technol. 2002, 129, 139–142. [Google Scholar] [CrossRef]
- Hatipoglu, G.; Kartal, M.; Uysal, M.; Cetinkaya, T.; Akbulut, H. The Effect of Sliding Speed on the Wear Behavior of Pulse Electro Co-Deposited Ni/MWCNT Nanocomposite Coatings. Tribol. Int. 2016, 98, 59–73. [Google Scholar] [CrossRef]
- Du, S.; Li, Z.; He, Z.; Ding, H.; Wang, X.; Zhang, Y. Effect of Temperature on the Friction and Wear Behavior of Electroless Ni–P–MoS2–CaF2 Self-Lubricating Composite Coatings. Tribol. Int. 2018, 128, 197–203. [Google Scholar] [CrossRef]
- Murray, J.W.; Ahmed, N.; Yuzawa, T.; Nakagawa, T.; Sarugaku, S.; Saito, D.; Clare, A.T. Dry-Sliding Wear and Hardness of Thick Electrical Discharge Coatings and Laser Clads. Tribol. Int. 2020, 150, 106392. [Google Scholar] [CrossRef]
- Xie, Z.J.; Mai, Y.J.; Lian, W.Q.; He, S.L.; Jie, X.H. Titanium Carbide Coating with Enhanced Tribological Properties Obtained by EDC Using Partially Sintered Titanium Electrodes and Graphite Powder Mixed Dielectric. Surf. Coat. Technol. 2016, 300, 50–57. [Google Scholar] [CrossRef]
- Liew, P.J.; Yap, C.Y.; Wang, J.; Zhou, T.; Yan, J. Surface Modification and Functionalization by Electrical Discharge Coating: A Comprehensive Review. Int. J. Extrem. Manuf. 2020, 2, 012004. [Google Scholar] [CrossRef]
- Shepeleva, L.; Medres, B.; Kaplan, W.D.; Bamberger, M.; Weisheit, A. Laser Cladding of Turbine Blades. Surf. Coat. Technol. 2000, 125, 45–48. [Google Scholar] [CrossRef]
- Sexton, L.; Lavin, S.; Byrne, G.; Kennedy, A. Laser Cladding of Aerospace Materials. J. Mater. Process. Technol. 2002, 122, 63–68. [Google Scholar]
- Algodi, S.J.; Murray, J.W.; Fay, M.W.; Clare, A.T.; Brown, P.D. Electrical Discharge Coating of Nanostructured TiC-Fe Cermets on 304 Stainless Steel. Surf. Coat. Technol. 2016, 307, 639–649. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Chow, H.-M.; Lin, Y.-C.; Lin, C.-T. Surface Modification Using Semi-Sintered Electrodes on Electrical Discharge Machining. Int. J. Adv. Manuf. Technol. 2008, 36, 490–500. [Google Scholar] [CrossRef]
- Ho, K.H.; Newman, S.T. State of the Art Electrical Discharge Machining (EDM). Int. J. Mach. Tools Manuf. 2003, 43, 1287–1300. [Google Scholar] [CrossRef]
- Lazarenko, B.R. To Invert the Effect of Wear on Electric Power Contacts. Master’s Dissertation, All-Union Institute for Electro Technique, Moscow, Russian, 1943. [Google Scholar]
- Toren, M.; Zvirin, Y.; Winograd, Y. Melting and Evaporation Phenomena during Electrical Erosion. J. Heat Transfer. 1975, 97, 576–581. [Google Scholar] [CrossRef]
- Jameson, E.C. Electrical Discharge Machining: Tooling, Methods, and Applications; Society of Manufacturing Engineers: Southfield, MI, USA, 1983. [Google Scholar]
- Pey Tee, K.T.; Hosseinnezhad, R.; Brandt, M.; Mo, J. Pulse Discrimination for Electrical Discharge Machining with Rotating Electrode. Mach. Sci. Technol. 2013, 17, 292–311. [Google Scholar] [CrossRef]
- Jeswani, M.L. Effect of the Addition of Graphite Powder to Kerosene Used as the Dielectric Fluid in Electrical Discharge Machining. Wear 1981, 70, 133–139. [Google Scholar] [CrossRef]
- Tao, J.; Shih, A.J.; Ni, J. Experimental Study of the Dry and Near-Dry Electrical Discharge Milling Processes. J. Manuf. Sci. Eng. 2008, 130, 011002. [Google Scholar] [CrossRef]
- Mohri, N. Surface Modification by EDM-an Innovation in EDM with Semi-Conductive Electrodes. In Proceedings of the Winter annual meeting of the ASME, Chicago, IL, USA, 27 November 1988; Volume 34, pp. 21–31. [Google Scholar]
- Ueno, M.; Fujita, N.; Kimura, Y.; Nakata, N. Evaluation of Coating and Wear Characteristics of Roll Surface Coated with TiC by Electrical Discharge Coating. J. Mater. Process. Technol. 2016, 236, 9–15. [Google Scholar] [CrossRef]
- Mohri, N.; Fukuzawa, Y.; Tani, T.; Saito, N.; Furutani, K. Assisting Electrode Method for Machining Insulating Ceramics. CIRP Ann. 1996, 45, 201–204. [Google Scholar] [CrossRef]
- Kunieda, M.; Yoshida, M.; Taniguchi, N. Electrical Discharge Machining in Gas. CIRP Ann. 1997, 46, 143–146. [Google Scholar] [CrossRef]
- Hwang, Y.L.; Kuo, C.L.; Hwang, S.F. The Coating of TiC Layer on the Surface of Nickel by Electric Discharge Coating (EDC) with a Multi-Layer Electrode. J. Mater. Process. Technol. 2010, 210, 642–652. [Google Scholar] [CrossRef]
- Chen, H.J.; Wu, K.L.; Yan, B.H. Characteristics of Al Alloy Surface after EDC with Sintered Ti Electrode and TiN Powder Additive. Int. J. Adv. Manuf. Technol. 2014, 72, 319–332. [Google Scholar] [CrossRef]
- Kunieda, M.; Lauwers, B.; Rajurkar, K.P.; Schumacher, B.M. Advancing EDM through Fundamental Insight into the Process. CIRP Ann.-Manuf. Technol. 2005, 54, 64–87. [Google Scholar] [CrossRef]
- El-Hofy, H.A.-G. Fundamentals of Machining Processes: Conventional and Nonconventional Processes; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Zhang, Y.; Liu, Y.; Ji, R.; Cai, B. Study of the Recast Layer of a Surface Machined by Sinking Electrical Discharge Machining Using Water-in-Oil Emulsion as Dielectric. Appl. Surf. Sci. 2011, 257, 5989–5997. [Google Scholar] [CrossRef]
- Moro, T.; Mohri, N.; Otsubo, H.; Goto, A.; Saito, N. Study on the Surface Modification System with Electrical Discharge Machine in the Practical Usage. J. Mater. Process. Technol. 2004, 149, 65–70. [Google Scholar] [CrossRef]
- Yadav, A.; Mohanty, S.; Nag, A.; Dixit, A.R.; Das, A.K. A Concise Review on Improvement of Tribological Properties by Electrical Discharge Coating Process. AIP Conf. Proc. 2020, 2273, 50009. [Google Scholar]
- Zhong, Y.; Xie, J.; Chen, Y.; Yin, L.; He, P.; Lu, W. Microstructure and Mechanical Properties of Micro Laser Welding Nitinb/Ti6Al4V Dissimilar Alloys Lap Joints with Nickel Interlayer. Mater. Lett. 2022, 306, 130896. [Google Scholar] [CrossRef]
- Bernd, M. Schumacher. Efectividad de Biopreparados Cubanos de Producción Comercial En La Embriogénesis Somática de Papa. J. Mater. Process. Technol. 2004, 149, 376–381. [Google Scholar] [CrossRef]
- Yih-Fong, T.; Fu-Chen, C. Investigation into Some Surface Characteristics of Electrical Discharge Machined SKD-11 Using Powder-Suspension Dielectric Oil. J. Mater. Process. Technol. 2005, 170, 385–391. [Google Scholar] [CrossRef]
- Forster, E.O.; Yamashita, H.; Mazzetti, C.; Pompili, M.; Caroli, L.; Patrissi, S. The Effect of the Electrode Gap on the Breakdown Process in Liquid Dielectrics. In Proceedings of the 1993 IEEE 11th International Conference on Conduction and Breakdown in Dielectric Liquids (ICDL’93), Baden-Dattwil, Switzerland, 19–23 July 1993; pp. 383–389. [Google Scholar]
- Tyagi, R.; Pandey, K.; Mohanty, S.; Kumar, S.; Das, A.K.; Mandal, A. Optimization of Electrical Discharge Coating of WS 2 and Cu Powder Mixture Deposited Through Green Compact Electrode. In Advances in Industrial and Production Engineering; Springer: Berlin/Heidelberg, Germany, 2019; pp. 273–283. [Google Scholar]
- Kumar, S.; Singh, R.; Singh, T.P.; Sethi, B.L. Surface Modification by Electrical Discharge Machining: A Review. J. Mater. Process. Technol. 2009, 209, 3675–3687. [Google Scholar] [CrossRef]
- Prakash, V.; Shubham; Kumar, P.; Singh, P.K.; Das, A.K.; Chattopadhyaya, S.; Mandal, A.; Dixit, A.R. Surface Alloying of Miniature Components by Micro-Electrical Discharge Process. Mater. Manuf. Process. 2018, 33, 1051–1061. [Google Scholar] [CrossRef]
- Liew, P.J.; Yan, J.; Kuriyagawa, T. Experimental Investigation on Material Migration Phenomena in Micro-EDM of Reaction-Bonded Silicon Carbide. Appl. Surf. Sci. 2013, 276, 731–743. [Google Scholar] [CrossRef]
- Mansor, A.F.; Jamaluddin, R.; Azmi, A.I.; Lih, T.C.; Zain, M.Z.M. Surface Modification of Nitinol by Using Electrical Discharge Coatings in Deionized Water. IOP Conf. Ser. Mater. Sci. Eng. 2019, 670, 012010. [Google Scholar] [CrossRef]
- Davim, J.P. Microfabrication and Precision Engineering: Research and Development; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Khan, M.Y.; Rao, P.S.; Pabla, B.S. A Framework for Surface Modification by Electrical Discharge Coating Using Variable Density Electrodes. E3S Web Conf. 2021, 309, 01093. [Google Scholar] [CrossRef]
- Maddu, J.; Karrolla, B.; Shaik, R.U.; Burdhuhos-Nergis, D.-P. SWOT Analysis of Electrical Discharge Coatings: A Case Study of Copper Coating on Titanium Alloy. Surfaces 2022, 5, 290–307. [Google Scholar] [CrossRef]
- Janmanee, P.; Muttamara, A. Surface Modification of Tungsten Carbide by Electrical Discharge Coating (EDC) Using a Titanium Powder Suspension. Appl. Surf. Sci. 2012, 258, 7255–7265. [Google Scholar] [CrossRef]
- Zou, R.; Yu, Z.; Li, W.; Guo, M.; Li, J. Influence of Porous Structure on the Machining Performance of Micro EDM. J. Mater. Process. Technol. 2016, 232, 43–51. [Google Scholar] [CrossRef]
- Prakash, C.; Uddin, M.S. Surface Modification of β-Phase Ti Implant by Hydroaxyapatite Mixed Electric Discharge Machining to Enhance the Corrosion Resistance and in-Vitro Bioactivity. Surf. Coat. Technol. 2017, 326, 134–145. [Google Scholar] [CrossRef]
- Tyagi, R.; Pandey, K.; Das, A.K.; Mandal, A. Deposition of HBN + Cu Layer through Electrical Discharge Process Using Green Compact Electrode. Mater. Manuf. Process. 2019, 34, 1035–1048. [Google Scholar] [CrossRef]
- Yap, C.Y.; Liew, P.J.; Wang, J. Surface Modification of Tungsten Carbide Cobalt by Electrical Discharge Coating with Quarry Dust Powder: An Optimisation Study. Mater. Res. Express 2020, 7, 106407. [Google Scholar] [CrossRef]
- Taylor, P.; Krishna, M.E.; Patowari, P.K. Materials and Manufacturing Processes Parametric Study of Electric Discharge Coating Using Powder Metallurgical Green Compact Electrodes Parametric Study of Electric Discharge Coating Using Powder Metallurgical Green Compact Electrodes. Mater. Manuf. Processes 2014, 29, 1131–1138. [Google Scholar] [CrossRef]
- Mussada, E.K.; Patowari, P.K. Post Processing of the Layer Deposited by Electric Discharge Coating. Mater. Manuf. Process. 2017, 32, 442–449. [Google Scholar] [CrossRef]
- Yap, C.Y.; Liew, P.J. Surface Modification of Tungsten Carbide Cobalt by Electrical Discharge Coating with Quarry Dust Suspension. Int. J. Adv. Manuf. Technol. 2020, 111, 2105–2116. [Google Scholar] [CrossRef]
- Tyagi, R.; Patel, V.S.; Das, A.K.; Mandal, A. Investigation on Electrical Discharge Coating of Brass and Copper Powder. Trans. Indian Inst. Met. 2022, 77, 1–10. [Google Scholar] [CrossRef]
- Tyagi, R.; Das, A.K.; Mandal, A. Wettability and Performance of Cu-MoS2/SiC Coating Prepared by Electro-Discharge Coating Process. Trans. Indian Inst. Met. 2022, 75, 1563–1572. [Google Scholar] [CrossRef]
- Patowari, P.K.; Mishra, U.K.; Saha, P.; Mishra, P.K. Surface Modification of C40 Steel Using WC-Cu P/M Green Compact Electrodes in EDM. Int. J. Manuf. Technol. Manag. 2010, 21, 83–98. [Google Scholar] [CrossRef]
- Liang, L.; Xu, M.; Chen, Y.; Zhang, T.; Tong, W.; Liu, H.; Li, H. Effect of Welding Thermal Treatment on the Microstructure and Mechanical Properties of Nickel-Based Superalloy Fabricated by Selective Laser Melting. Mater. Sci. Eng. A 2021, 819, 141507. [Google Scholar] [CrossRef]
- Patowari, P.K.; Saha, P.; Mishra, P.K. Taguchi Analysis of Surface Modification Technique Using W-Cu Powder Metallurgy Sintered Tools in EDM and Characterization of the Deposited Layer. Int. J. Adv. Manuf. Technol. 2011, 54, 593–604. [Google Scholar] [CrossRef]
- Tyagi, R.; Das, A.K.; Mandal, A. Tribology International Formation of Superhydrophobic Surface with Enhanced Hardness and Wear Resistance by Electrical Discharge Coating Process. Tribol. Int. 2021, 157, 106897. [Google Scholar] [CrossRef]
- Arun, I.; Duraiselvam, M.; Senthilkumar, V.; Narayanasamy, R.; Anandakrishnan, V. Synthesis of Electric Discharge Alloyed Nickel-Tungsten Coating on Tool Steel and Its Tribological Studies. Mater. Des. 2014, 63, 257–262. [Google Scholar] [CrossRef]
- Tsai, H.C.; Yan, B.H.; Huang, F.Y. EDM Performance of Cr/Cu-Based Composite Electrodes. Int. J. Mach. Tools Manuf. 2003, 43, 245–252. [Google Scholar] [CrossRef]
- Khanra, A.K.; Sarkar, B.R.; Bhattacharya, B.; Pathak, L.C.; Godkhindi, M.M. Performance of ZrB2-Cu Composite as an EDM Electrode. J. Mater. Process. Technol. 2007, 183, 122–126. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Z.; Wu, Y.; Wang, Y.; Ma, G.; Shi, J.; Zhong, J.; Hong, Z.; Jin, J.; Zhao, Y. Synergic Realization of Electrical Insulation and Mechanical Strength in Liquid Nitrogen for High-Temperature Superconducting Tapes with Ultra-Thin Acrylic Resin Coating. Supercond. Sci. Technol. 2022, 35, 075014. [Google Scholar] [CrossRef]
- Singh, S.; Maheshwari, S.; Pandey, P.C. Some Investigations into the Electric Discharge Machining of Hardened Tool Steel Using Different Electrode Materials. J. Mater. Process. Technol. 2004, 149, 272–277. [Google Scholar] [CrossRef]
- Singh, S.; Maheshwari, S.; Pandey, P.C. Experimental Investigations into Die-Sinking Electric Discharge Machining of Hardened AISI 6150 Tool Steel Using Different Electrode Materials. Stroj. Cas.-J. Mech. Eng. 2005, 56, 197–210. [Google Scholar]
- Beri, N.; Kumar, A.; Maheshwari, S.; Sharma, C. Optimisation of Electrical Discharge Machining Process with CuW Powder Metallurgy Electrode Using Grey Relation Theory. Int. J. Mach. Mach. Mater. 2011, 9, 103–115. [Google Scholar] [CrossRef]
- Föhl, J.; Weissenberg, T.; Wiedemeyer, J. General Aspects for Tribological Applications of Hard Particle Coatings. Wear 1989, 130, 275–288. [Google Scholar] [CrossRef]
- Shunmugam, M.S.; Philip, P.K.; Gangadhar, A. Tribological Behaviour of an Electrodischarge Machined Surface with a Powder Metallurgy Bronze Electrode. Tribol. Int. 1993, 26, 109–113. [Google Scholar] [CrossRef]
- Ho, S.K.; Aspinwall, D.K.; Voice, W. Use of Powder Metallurgy (PM) Compacted Electrodes for Electrical Discharge Surface Alloying/Modification of Ti-6Al-4V Alloy. J. Mater. Process. Technol. 2007, 191, 123–126. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kar, S.; Dey, V.; Ghosh, S.K. Optimization and surface modification of al-6351 alloy using sic-cu green compact electrode by electro discharge coating process. Surf. Rev. Lett. 2017, 24, 1750007. [Google Scholar] [CrossRef]
- Pantelis, D.I.; Vaxevanidis, N.M.; Houndri, A.E.; Dumas, P.; Jeandin, M. Investigation into Application of Electrodischarge Machining as Steel Surface Modification Technique. Surf. Eng. 1998, 14, 55–61. [Google Scholar] [CrossRef]
- Das, S. Surface Alloying of Aluminium by W-Cu-Cr Powder Metallurgy Tool Electrode in EDM. Int. J. Latest Res. Eng. Technol. 2016, 2, 1–10. [Google Scholar]
- Moro, T.; Goto, A.; Mohri, N.; Saito, N.; Matsukawa, K.; Miyake, H. Surface Modification Process by Electrical Discharge Machining with TiC Semi-Sintered Electrode. J.-Jpn. Soc. Precis. Eng. 2001, 67, 114–119. [Google Scholar] [CrossRef]
- Miyake, H. Improvement of Tool Life through Surface Modification by Electrical Discharge Machining. Proc. ISEM 12 1998, 1405, 261–270. [Google Scholar]
- Tsunekawa, Y.; Okumiya, M.; Mohri, N.; Takahashi, I. Surface Modification of Aluminum by Electrical Discharge Alloying. Mater. Sci. Eng. A 1994, 174, 193–198. [Google Scholar] [CrossRef]
- Kruth, J.-P.; Stevens, L.; Froyen, L.; Lauwers, B. Study of the White Layer of a Surface Machined by Die-Sinking Electro-Discharge Machining. CIRP Ann. 1995, 44, 169–172. [Google Scholar] [CrossRef]
- Das, A.; Misra, J.P. Experimental Investigation on Surface Modification of Aluminum by Electric Discharge Coating Process Using TiC/Cu Green Compact Tool-Electrode. Mach. Sci. Technol. 2012, 16, 601–623. [Google Scholar] [CrossRef]
- Zeng, Z.Y.; Xiao, H.Q.; Jie, X.H.; Zhang, Y.M. Friction and Wear Behaviors of TiCN Coating Based on Electrical Discharge Coating. Trans. Nonferrous Met. Soc. China 2015, 25, 3716–3722. [Google Scholar] [CrossRef]
- Tyagi, R.; Das, A.K.; Mandal, A.; Saxena, K.K.; Tripathi, A. Hydrophobic Properties and Chemical State Analysis of Wear Resistant Coating Prepared by Electrical Discharge Process. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2022, 0954408922109. [Google Scholar] [CrossRef]
- Murray, J.W.; Clare, A.T. Morphology and Wear Behaviour of Single and Multi-Layer Electrical Discharge Coatings. Procedia CIRP 2016, 42, 236–239. [Google Scholar] [CrossRef]
- Murray, J.W.; Cook, R.B.; Senin, N.; Algodi, S.J.; Clare, A.T. Defect-Free TiC/Si Multi-Layer Electrical Discharge Coatings. Mater. Des. 2018, 155, 352–365. [Google Scholar] [CrossRef]
- Priadi, D.; Siradj, E.S.; Winarto, W. Surface Modification of SKD 61 by Electrical Discharge Coating (EDM/EDC) with Multilayer Cylindrical Electrode and Jatropha Curcas as Dielectric Fluid. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Zurich, Switzerland, 2013; Volume 319, pp. 96–101. [Google Scholar]
- Kansal, H.K.; Singh, S.; Kumar, P. Parametric Optimization of Powder Mixed Electrical Discharge Machining by Response Surface Methodology. J. Mater. Process. Technol. 2005, 169, 427–436. [Google Scholar] [CrossRef]
- Mohanty, S.; Das, A.K.; Dixit, A.R. Surface Integrity of Tribo-Adaptive Layer Prepared on Ti6Al4V through μEDC Process. Surf. Coat. Technol. 2021, 429, 127922. [Google Scholar] [CrossRef]
- OGATA, I. Carburizing and Decarburazing Phenomena in EDM’d Surface. Int. J. Jpn. Soc. Prec. Eng. 1993, 27, 197–202. [Google Scholar]
- Kiran, P.; Mohanty, S.; Das, A.K. Surface Modification through Sustainable Micro-EDM Process Using Powder Mixed Bio-Dielectrics. Mater. Manuf. Process. 2021, 37, 640–651. [Google Scholar] [CrossRef]
- Okada, A. Formation of Hard Layer by EDM with Carbon Powder Mixed Fluid. In Proceedings of the 5th International Conference on Progress of Machining Technology, New Orleans, LA, USA, 9–12 January 2000; Volume 464, pp. 464–469. [Google Scholar]
- Furutani, K.; Shimizu, Y. Experimental Analysis of Deposition Process of Lubricant Surface by Electrical Discharge Machining with Molybdenum Disulfide Powder Suspended in Working Oil. In Proceedings of the 18th Annual Meeting of American Society for Precision Engineering, Portland, OR, USA, 26–31 October 2003; pp. 547–550. [Google Scholar]
- Wu, K.L.; Yan, B.H.; Huang, F.Y.; Chen, S.C. Improvement of Surface Finish on SKD Steel Using Electro-Discharge Machining with Aluminum and Surfactant Added Dielectric. Int. J. Mach. Tools Manuf. 2005, 45, 1195–1201. [Google Scholar] [CrossRef]
- Amorim, F.L.; Dalcin, V.A.; Soares, P.; Mendes, L.A. Surface Modification of Tool Steel by Electrical Discharge Machining with Molybdenum Powder Mixed in Dielectric Fluid. Int. J. Adv. Manuf. Technol. 2017, 91, 341–350. [Google Scholar] [CrossRef]
- Mohanty, S.; Kumar, V.; Das, A.K.; Dixit, A.R. Surface Modification of Ti-Alloy by Micro-Electrical Discharge Process Using Tungsten Disulphide Powder Suspension. J. Manuf. Process. 2019, 37, 28–41. [Google Scholar] [CrossRef]
- Wong, Y.S.; Lim, L.C.; Rahuman, I.; Tee, W.M. Near-Mirror-Finish Phenomenon in EDM Using Powder-Mixed Dielectric. J. Mater. Process. Technol. 1998, 79, 30–40. [Google Scholar] [CrossRef]
- Furutania, K.; Saneto, A.; Takezawa, H.; Mohri, N.; Miyake, H. Accretion of Titanium Carbide by Electrical Discharge Machining with Powder Suspended in Working Fluid. Precis. Eng. 2001, 25, 138–144. [Google Scholar] [CrossRef]
- Ou, S.F.; Wang, C.Y. Fabrication of a Hydroxyapatite-Containing Coating on Ti-Ta Alloy by Electrical Discharge Coating and Hydrothermal Treatment. Surf. Coat. Technol. 2016, 302, 238–243. [Google Scholar] [CrossRef]
- Yee, C.; Jun, P.; Sharhida, I.; Othman, B.; Fadzli, M.; Abdollah, B. Surface & Coatings Technology Tribological Characteristics of Electrical Discharge Coated Layers Using Quarry Dust Suspension. Surf. Coat. Technol. 2021, 428, 127895. [Google Scholar] [CrossRef]
- Li, S.L.; Mai, Y.J.; Huang, M.Y.; Jie, X.H. Anti-Wear Hierarchical TiC Enhanced Cermet Coating Obtained via Electrical Discharge Coating Using a Reduced Graphene Oxide Nanosheets Mixed Dielectric. Ceram. Int. 2020, 46, 11933–11942. [Google Scholar] [CrossRef]
- Mohanty, S.; Das, A.K.; Dixit, A.R. Surface Integrity and Residual Stress Analysis of μEDM Coated Ti-Alloy Miniature Components. Mater. Manuf. Process. 2021, 36, 48–58. [Google Scholar] [CrossRef]
- Chen, H.-J.; Wu, K.-L.; Yan, B.-H. Dry Electrical Discharge Coating Process on Aluminum by Using Titanium Powder Compact Electrode. Mater. Manuf. Process. 2013, 28, 1286–1293. [Google Scholar] [CrossRef]
- Lee, H.-T.; Yur, J.-P. Characteristic Analysis of EDMed Surfaces Using the Taguchi Approach. Mater. Manuf. Process. 2000, 15, 781–806. [Google Scholar] [CrossRef]
- Okada, M.; Yoshida, A.; Furumoto, T.; Watanabe, H.; Asakawa, N.; Otsu, M. Mechanisms and Characteristics of Direct Cutting of Tungsten Carbide Using a Diamond-Coated Carbide End Mill. Int. J. Adv. Manuf. Technol. 2016, 86, 1827–1839. [Google Scholar] [CrossRef]
- Bröcking, R.; Meghwal, A.; Melzer, S.; Verdier, S.; Evans, G.; Lowbridge, T.; Vanhumbeeck, J.F.; Debrabandere, D.; Crahay, J. Development of Electrical Discharge Coating (EDC) as Chrome-Free Alternative for Increasing Campaign Length of Temper Mill Work Rolls. Iron Steel Technol. 2015, 12, 68–76. [Google Scholar]
- Mazarbhuiya, R.M.; Dutta, H.; Debnath, K.; Rahang, M. Surface Modification of CFRP Composite Using Reverse-EDM Method. Surf. Interfaces 2020, 18, 100457. [Google Scholar] [CrossRef]
- Mazarbhuiya, R.M.; Rahang, M. PCM Assisted Reverse Electro Discharge Machining Process for Pattern Generation. Mater. Manuf. Process. 2021, 37, 995–1002. [Google Scholar] [CrossRef]
- Mazarbhuiya, R.M.; Rahang, M. Reverse EDM Process for Pattern Generation Using Powder Metallurgical Green Compact Tool. Mater. Manuf. Process. 2020, 35, 1741–1748. [Google Scholar] [CrossRef]
- Chen, W.C.; Lin, H.M.; Uan, J.Y. Formation and Characterization of Self-Lubricated Carbide Layer on AA6082 Al–Mg–Si Aluminum Alloy by Electrical Discharge Alloying Process. Trans. Nonferrous Met. Soc. China 2016, 26, 3205–3218. [Google Scholar] [CrossRef]
- Jhavar, S.; Paul, C.P.; Jain, N.K. Causes of Failure and Repairing Options for Dies and Molds: A Review. Eng. Fail. Anal. 2013, 34, 519–535. [Google Scholar] [CrossRef]
- Aliyu, A.A.; Abdul-Rani, A.M.; Ginta, T.L.; Prakash, C.; Axinte, E.; Razak, M.A.; Ali, S. A Review of Additive Mixed-Electric Discharge Machining: Current Status and Future Perspectives for Surface Modification of Biomedical Implants. Adv. Mater. Sci. Eng. 2017, 2017, 8723239. [Google Scholar] [CrossRef]
- Devarani, N.; Joshi, S.N. Electric Discharge Alloying of Titanium and Aluminium on AISI P20 Mold Steel. Surf. Coat. Technol. 2021, 405, 126515. [Google Scholar] [CrossRef]
- Mohammed, M.T.; Khan, Z.A.; Siddiquee, A.N. Surface Modifications of Titanium Materials for Developing Corrosion Behavior in Human Body Environment: A Review. Procedia Mater. Sci. 2014, 6, 1610–1618. [Google Scholar] [CrossRef]
- Kiran, P.; Mohanty, S.; Das, A.K. Sustainable Surface Modification of Ti-Alloy Using Powder Mixed in Bio-Dielectrics through Micro-Electrical Discharge Coating Process. J. Clean. Prod. 2022, 362, 132375. [Google Scholar]
- Peng, P.W.; Ou, K.L.; Lin, H.C.; Pan, Y.N.; Wang, C.H. Effect of Electrical-Discharging on Formation of Nanoporous Biocompatible Layer on Titanium. J. Alloys Compd. 2010, 492, 625–630. [Google Scholar] [CrossRef]
- Walczak, J.; Shahgaldi, F.; Heatley, F. In Vivo Corrosion of 316L Stainless-Steel Hip Implants: Morphology and Elemental Compositions of Corrosion Products. Biomaterials 1998, 19, 229–237. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface Modification of Titanium, Titanium Alloys, and Related Materials for Biomedical Applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Wandra, R.; Prakash, C.; Singh, S. Investigation on Surface Roughness and Hardness of β-Ti Alloy by Ball Burnishing Assisted Electrical Discharge Cladding for Bio-Medical Applications. Mater. Today Proc. 2021, 50, 848–854. [Google Scholar] [CrossRef]
- Chen, S.-L.; Lin, M.-H.; Huang, G.-X.; Wang, C.-C. Research of the Recast Layer on Implant Surface Modified by Micro-Current Electrical Discharge Machining Using Deionized Water Mixed with Titanium Powder as Dielectric Solvent. Appl. Surf. Sci. 2014, 311, 47–53. [Google Scholar] [CrossRef]
- Sales, W.F.; Oliveira, A.R.F.; Raslan, A.A. Titanium Perovskite (CaTiO3) Formation in Ti6Al4V Alloy Using the Electrical Discharge Machining Process for Biomedical Applications. Surf. Coat. Technol. 2016, 307, 1011–1015. [Google Scholar] [CrossRef]
- Prakash, C.; Kansal, H.K.; Pabla, B.S.; Puri, S. Processing and Characterization of Novel Biomimetic Nanoporous Bio Surface on β-Ti Implant by Powder Mixed Electric Discharge Machining. J. Mater. Eng. Perform. 2015, 24, 3622–3633. [Google Scholar] [CrossRef]
- Yang, T.-S.; Huang, M.-S.; Wang, M.-S.; Lin, M.-H.; Tsai, M.-Y.; Wang, P.-Y.W. Effect of Electrical Discharging on Formation of Nanoporous Biocompatible Layer on Ti-6Al-4V Alloys. Implant Dent. 2013, 22, 374–379. [Google Scholar] [CrossRef] [PubMed]









| EDC | Laser Cladding | Thermal Spraying | PVD | CVD | Electroplating | |
|---|---|---|---|---|---|---|
| Working condition | Room temperature | Room temperature, sometimes need oxidation environment | Room temperature | High vacuum and High temperature | High temperature | Room temperature |
| Parent Material | Conductive metals | All materials | All materials | All materials | All materials | Conductive metals |
| Material of Coating | Any materials including metal matrix composite and hard to process ceramics | Any material except reflective materials | Metals, alloys, carbides, ceramics and polymers | Metals or ceramics | Metals or ceramics | Metal ions |
| Post coating process | Cleaning the sample to remove loose particles and dielectric | Cleaning the samples in acetone | Cleaning the oil and dirt then roughening the prepared samples to enhanced bond strength | Cleaning the surface | Cleaning the toxic exhaust gases by scrubber and need 6–8 h for cool down to room temperature | Cleaning and let the sample dry to prevent oxidation |
| Efficiency | High | High | High | Low | Low | Intermediate |
| Function | Improves the properties (roughness, microhardness, corrosion, and wear resistance) of original surface or surface with complicated shape | Improves the microhardness, oxidation, corrosion, and wear resistance of original surface and to repair components | Improves the wear and corrosion resistant | Enhance the microhardness, resistance to wear and oxidation | Ability to form uniform coating with less pores, even on workpiece of complex shape | Resistance to wear and corrosion, high electrical conductivity and reflectivity |
| Applications | Roll surface texturing, repair turbine blades, Bio-compatible implantation | Aerospace and automobile applications, repair applications for gas turbine engines | Automobile and Aircraft engine components, storage tank, rocket motor | Aerospace, automotive, surgical/medical, dies, and mold | Cutting tool | Electronic industry (semi conductor and printed circuit board) |
| EDM | EDC | |
|---|---|---|
| Process | Material removal from the workpiece takes place to acquire a desired shape | A material deposition process that is used to prepare a coating on the parent material surface. |
| Tool electrode | Conventional solid tool | Sacrificial/green compact |
| Thickness of recast layer | Low | High |
| Polarity used | Tool can be connected to either a positive or negative terminal. | Tool is connected to negative terminal and workpiece is connected to positive terminal. |
| Roughness | Low | High |
| Quality of surface after process | Low (high cracks and pores) | High (reduces cracks and pores) |
| Workpiece weight | Decreases due to removal of the material | Increases due to the deposition of the material |
| Application | Machining of conductive materials | Coating of conductive materials, even complex-shaped materials can be coated. |
| Author | Workpiece | Tool/Powder Mixed | Voltage (V) | Findings |
|---|---|---|---|---|
| Prakash, et al. [108] | Ti6Al4 | Nickel tool | 20, 30, 40, 50, 60 | Voltage affects the recast layer thickness. |
| Liew, et al. [109] | SiC | Tungsten | 60–110 | W particles were deposited in a high amount at 60 V (low voltage). However, this amount decreases as the voltage increases up to 110 V. |
| Liew, et al. [5] | Aluminum | Tungsten powder suspension | 20, 25, 30, 35, 40 V | When V increased to 40 V, the amount of tungsten and carbon increased because V influences the spark gap. |
| Mansor, et al. [110] | Nitinol | Nickel–titanium shape memory alloy | 70–160 | A low gap voltage was preferred in the EDC process in order to provide good surface roughness and a higher uniformity of the material deposition. |
| Author | Workpiece | Green Compact/Powder Mixed | Current (A) | Findings |
|---|---|---|---|---|
| Janmanee and Muttamara [114] | WC | Ti | 10, 15, 20, and 25 A | A low current faciliates the Ti powder to fill the microcracks over the WC surface. Although, the bonding strength drops as the current exceeds 20 A. |
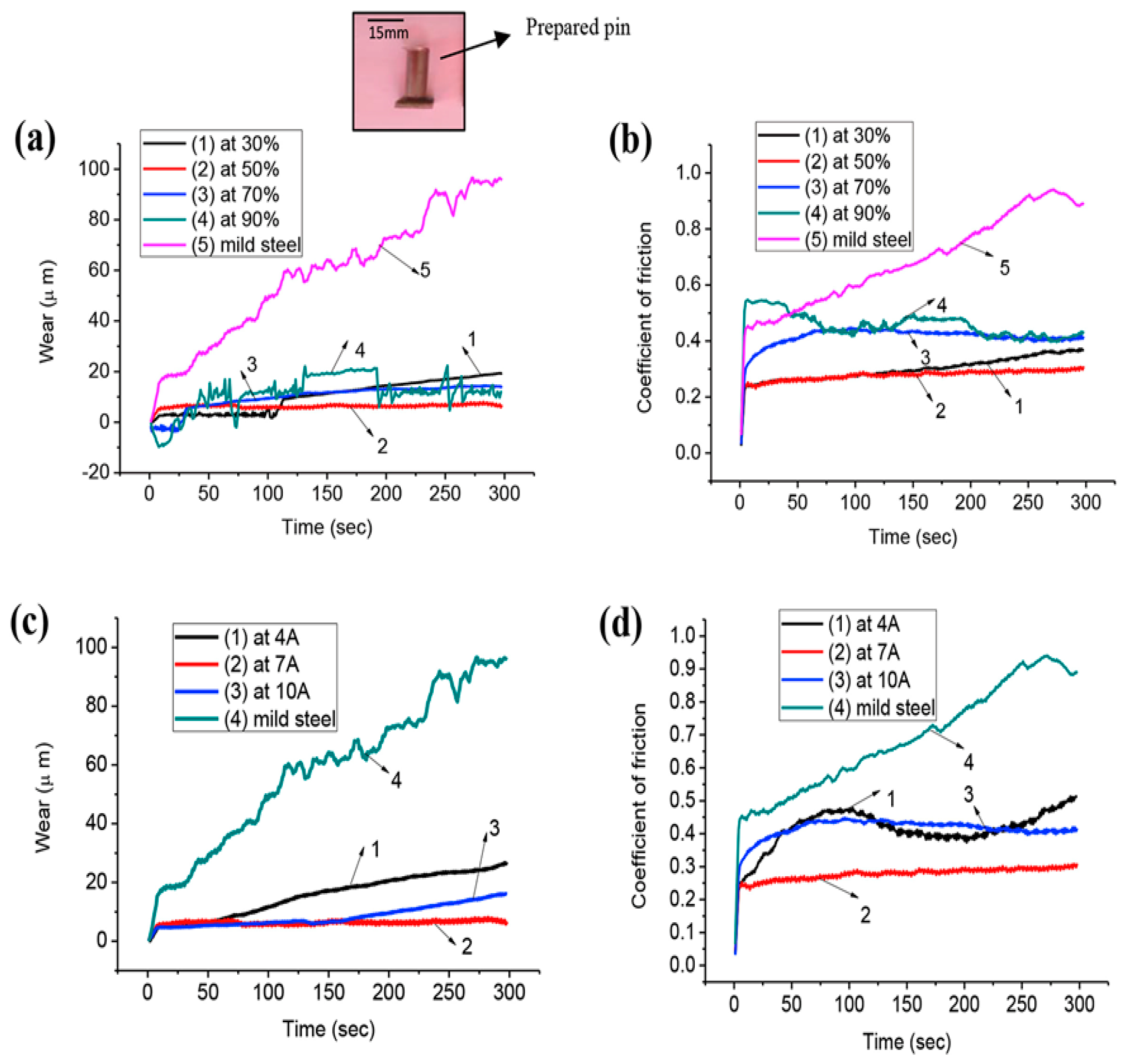
| Kumar, et al. [115] | WC-Co | SiC/Cu | 4, 7, 10 | Higher settings of currents lead to a thick coating layer but highly rough surface. A lower current leads to a smooth surface with less coating thickness. |
| Prakash and Uddin [116] | Titanium | HA powder | 15 | High peak current (15 A) and low pulse duration in deionised water resulted into uniform HA layer without any crack on the β-phase Ti implant surface. |
| Algodi, et al. [82] | SS 304 | TiC-Fe | 2, 6, 10, 14, 19 | A current of 2–10 A promotes a good value of hardness and surface finish with less presence of cracks and voids. |
| Tyagi, et al. [102] | Mild steel | MoS2 + Cu | 4, 7, 10 | A low thickness 0.446 mm was observed at a low peak current 4 A, and a maximum thickness of 0.647 mm was observed at 10 A. |
| Author | Workpiece | Tool/Powder Mix | Pulse on Time | Findings |
|---|---|---|---|---|
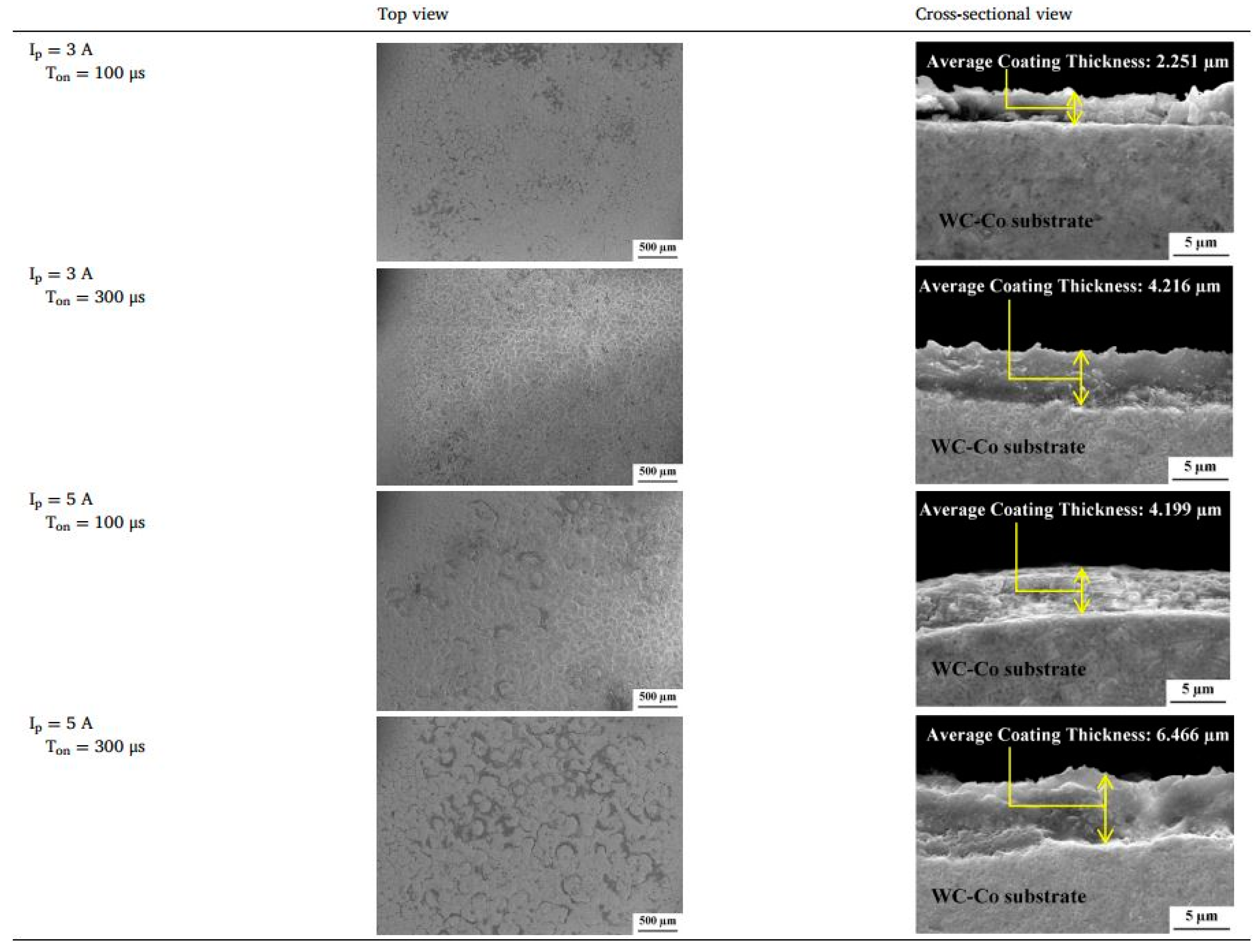
| Yap, et al. [118] | WC-Co | Quarry dust | 100–300 μs | Results showed that, with an increase in Ton, the hardness and layer thickness increased with a decreased surface finish. Ton = 341 μs is the optimum value for achieving a hard surface, thick coating layer, and low surface roughness. |
| Algodi, et al. [82] | SS 304 | TiC-Fe | 2, 4, 8, 16, 32, 64 | Presence of craters and surrounding microcracks with increasing pulse on-time was observed. With a 2 μs pulse duration, the surfaces showed less void and crack formation. |
| Taylor, et al. [119] | Mild steel | W-Cu | 25, 106, 463, 1010 | Vigorous sparking was observed at larhe on-time, which leads to thicker deposition. |
| Balanou, et al. [14] | 60CrMoV18-5 Steel | Cu-Zr2 | 12.8, 25. 50 | Higher MTR of 46.5 mgr/min is achieved at Ton = 25 µs, and the Ra varies from 3.72 µm to 7.12 µm |
| Mussada, et al. [120] | Aluminum 6351 | W-Cu | 25, 106, 463, 1010 | With increasing pulse duration, the microhardness decreases gradually as there are less carbon particles on the workpiece because particles flush away from the work surface at a longer pulse duration. |
| Author (Year) | Green Compact Tool | Findings | Deficiency in Research |
|---|---|---|---|
| Wang, Z.L.; Fang, Y.; Wu, P.N.; et al. (2002) [74] | Ti/Cu | Hardness of the w/p was increased due to formation of TiC layer over it whose hardness was 3 times that of w/p. | Tribological behavior of coating was not explored. Coating layer showed a large amount of pore formation. |
| Aspinwall, D.K.; Dewes, R.C.; Lee, H.G.; Simao, J. (2003) [68] | 60% Fe and 40% WC | Enhancement in abrasive wear resistance property was observed. | WC formation resulted in a larger number of cracks. The hard reinforcing phases such as carbides induced brittleness in the coating. |
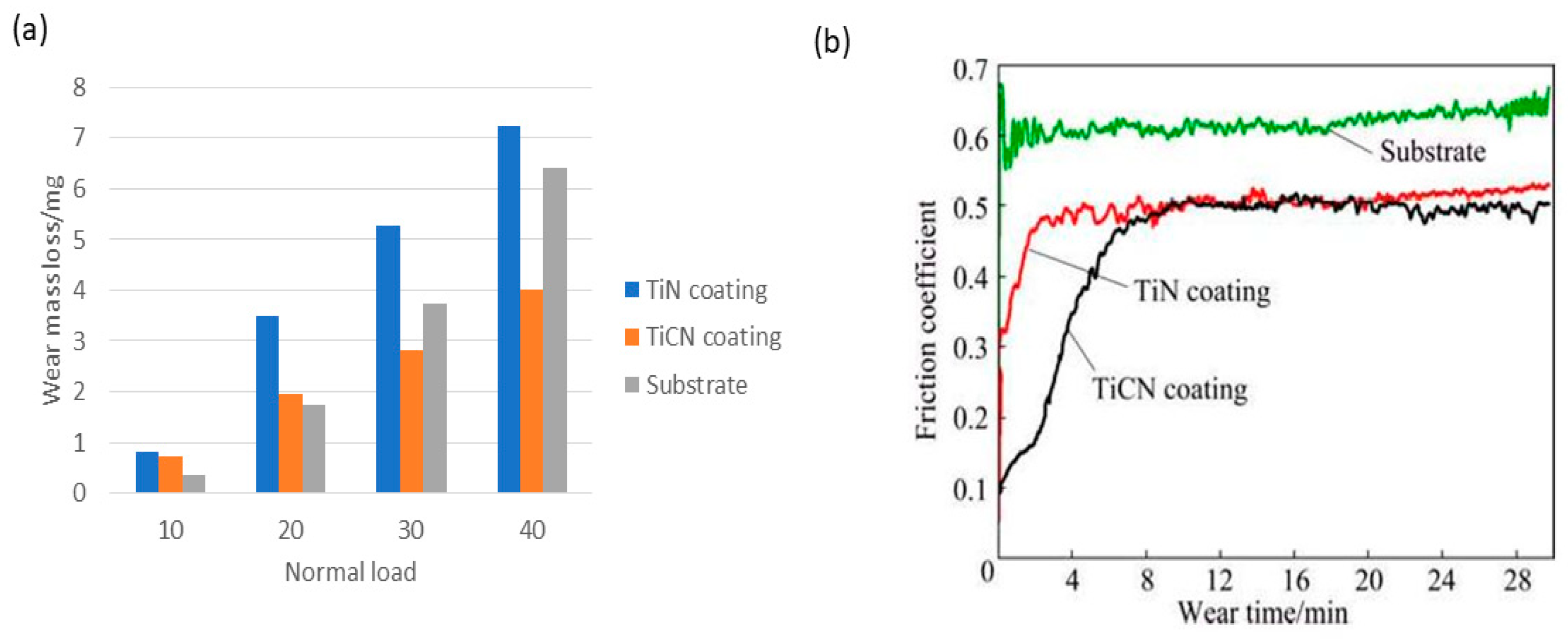
| Zeng, Z.Y.; Xiao, H.Q.; Jie, X.H.; Zhang, Y.M. (2015) [146] | TiC | The coefficient of friction and wear resistance to abrasion of the coated surface was found superior to the PVD TiN coating. | The maximum microhardness of PVD TiN coating (HV0.2 1980) was slightly higher as compared to TiCN EDC (HV0.2 1780). |
| Chakraborty, S.; Kar, S.; Dey, V.; Ghosh, S.K. (2017) [138] | SiC/Cu | Microhardness value of 1.5–3 times higher than Al-6351 alloy substrate and coating layer thickness of maximum 83.644 μm were achieved successfully. | SiC gets decomposed into Si and C, so improvement in hardness is less. Coating layer also showed a large amount of crater formation with an increment in the proportion of Cu compact pellets. |
| Ahmed, N.; Murray, J.W.; Yuzawa, T.; Nakagawa, T.; Sarugaku, S.; Saito, D.; Brown, P.D.; Clare, A.T. (2020) [112] | Stellite | Thick electrical discharge coatings, also known by the commercial name “MSCoating”, can be applied on complex shapes and cavities to repair components or act as protective coatings. | Wear and friction behavior of coating was not explored. |
| Tyagi, R.; Das, A.K.; Mandal, A. [123] | MoS2 + SiC | Hard and solid lubricant coating was deposited on the steel, which showed hydrophobic properties. | Hardness was distrubuted non uniformly over the substrate, which led to less improvement in hardness. |
| Tyagi, R.; Patel, V.S.; Das, A.K.; Mandal, A. [122] | Brass + Cu | Low friction coeffcient and corrosion was reported along with defect reduction. | Hardness is not elaborated. Reason of using brass with copper still need to be identified. |
| Tyagi, R.; Swaraj, S.; Mandal, A.; Das, A.K. [114] | MoS2 + hBN | Reduction in pores was observed by using hBN powder in MoS2 tool. | The resaon of using two solid lubricants together is not explained well. Additionally, the effect of process parameters on hardness is not explained. |
| Author (Year) | Workpiece | Green Compact Tool | Findings | Deficiency in Research |
|---|---|---|---|---|
| Murray, J.W.; Cook, R.B.; Senin, N.; Algodi, S.J.; Clare, A.T. 2020 [149] | 304 stainless steel | TiC, Si, Cu, WC, and Zr | A composite coating layer was formed. showing fewer cracks and pores with low surface roughness. | A small level of Si (~5–6% at maximum) present in the TiC coating served to reduce the mean hardness of the coating. |
| Priadi, D.; Siradj 2013 [150] | KD 61 steel | Graphite and copper | This study shows that jatropha curcas dielectric fluid has potential to be used in the EDM process since it produces a smoother surface and higher white layer hardness value. | This coating cannot be applied as an MS coating to repair components due to low recast layer thickness. |
| Hwang, Y.L.; Kuo, C.L.; Hwang, S.F. (2010) [95]. | Nickel | Ti and Gr | TiC due to a large amount of carbon from the graphite layer enhances the carbon concentration, which increases in the hardness of the coating. | Coated layer inhomogeneity was due to different electrode wear for each material. A few micron thickness was obtained; therefore, the coating cannot be used for complex shapes. |
| Author (Year) | Workpiece | Powder Additive | Findings | Deficiency in Research |
|---|---|---|---|---|
| Ou, S.-F.; Wang, C.-Y. (2016) [162] | 70Ti–30Ta alloy | hydroxyapatite (HA) with distilled water | A composite coating layer was formed showing fewer cracks and pores with low surface roughness. | A small level of Si (~5–6% at maximum) present in the TiC coating served to reduce the mean hardness of the coating notably. |
| Priadi, D.; Siradj 2013 [150] | Aluminium alloy | TiN powder additive | This study shows that jatropha curcas dielectric fluid has potential to be used in the EDM process since it produces a smoother surface and higher white layer hardness value. | Cannot be applied as MS coating to repair components due to low recast layer thickness. |
| Li, S.L.; Mai, Y.J., Huang, M.Y.; Jie, X.H. [164] | H13 steel | EDM oil with graphene oxide | Hierarchical TiC phase-enhanced cermet coatings with superior tribological performance have been successfully prepared by EDC process using a RGONS mixed dielectric. | Coated layer inhomogeneity was due to different electrode wear for each material. A few micron thickness was obtained and cannot be used for complex shapes. |
| Chen, H.J.; Wu, K.L.; Yan, B.H. [96] | Aluminium | TiN powder (0.5 μm) additive | TiN powder additive in kerosene not only can enhance the EDC surface quality, but also decrease COF and enhance the wear resistance of the Al alloy. | Only few micrometers recast layer thickness can be formed. |
| Mohanty, S.; Das, A.K.; Dixit, A.R. [165] | Titanium alloy | WS2, MoS2 | Residual stress has been reduced by using solid lubricant as a powder mixed additive. | Powder mixed EDC results in few micrometres recast layer thickness. |
| Mohanty, S.; Kumar, V.; Das, A.K.; Dixit, A.R. [159] | Titanium alloy | hBN powder mixed additive | This study shows that WS2 powder mixed coating results in in high wear resistance. | Cannot be applied as MS coating to repair components due to low recast layer thickness. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyagi, R.; Mandal, A.; Das, A.K.; Tripathi, A.; Prakash, C.; Campilho, R.; Saxena, K.K. Electrical Discharge Coating a Potential Surface Engineering Technique: A State of the Art. Processes 2022, 10, 1971. https://doi.org/10.3390/pr10101971
Tyagi R, Mandal A, Das AK, Tripathi A, Prakash C, Campilho R, Saxena KK. Electrical Discharge Coating a Potential Surface Engineering Technique: A State of the Art. Processes. 2022; 10(10):1971. https://doi.org/10.3390/pr10101971
Chicago/Turabian StyleTyagi, Rashi, Amitava Mandal, Alok Kumar Das, Ashutosh Tripathi, Chander Prakash, Raul Campilho, and Kuldeep K. Saxena. 2022. "Electrical Discharge Coating a Potential Surface Engineering Technique: A State of the Art" Processes 10, no. 10: 1971. https://doi.org/10.3390/pr10101971
APA StyleTyagi, R., Mandal, A., Das, A. K., Tripathi, A., Prakash, C., Campilho, R., & Saxena, K. K. (2022). Electrical Discharge Coating a Potential Surface Engineering Technique: A State of the Art. Processes, 10(10), 1971. https://doi.org/10.3390/pr10101971











