Preparation of Rice Bran Protein (RBP) Powder Using Spray Drying Method at the Optimal Condition and Its Protein Quality
Abstract
:1. Introduction
2. Methodology
2.1. Raw Material
2.2. Extraction Process
2.3. Spray-Drying Process
- Wfinal (g) = Weight of the cylone after the spray-drying process;
- Winitial (g)= Weight of the cylone before the spray-drying process; and
- WRRB (g)= Weight of raw rice bran.
2.4. Other Drying Methods for Rice Bran Protein (RBP) Powder
2.5. Optimization of Spray-Drying Process Using Response Surface Methodology (RSM)
- A0 = Regression coefficients of variables for intercept terms;
- Ai = Regression coefficients of variables for linear terms;
- Aii = Regression coefficients of variables for quadratic terms;
- Aij = Regression coefficients of variables for interaction terms; and
- Xi, Xj = Independent variables.
2.6. Statistical Analysis
2.7. Model Validation
2.8. High-Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS) for Protein Quality Analysis
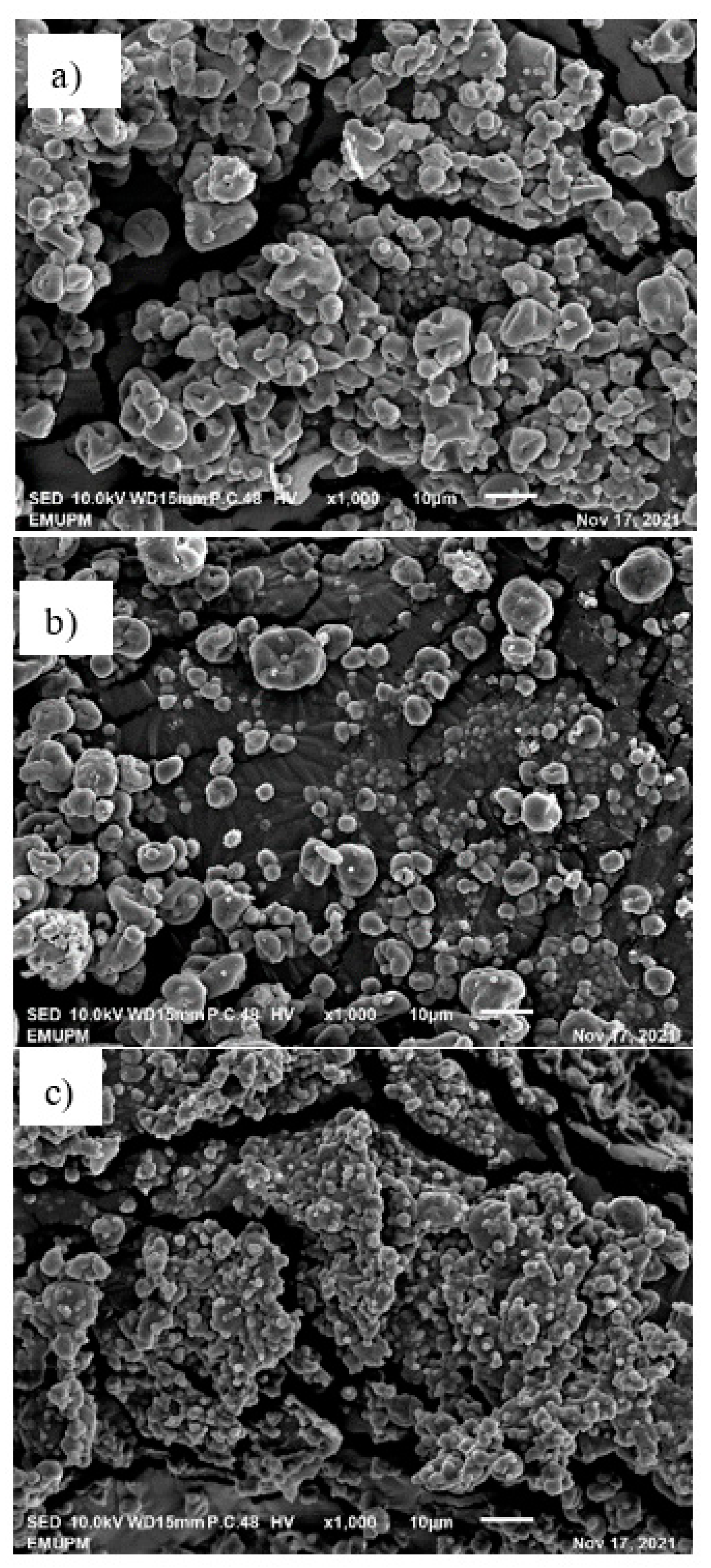
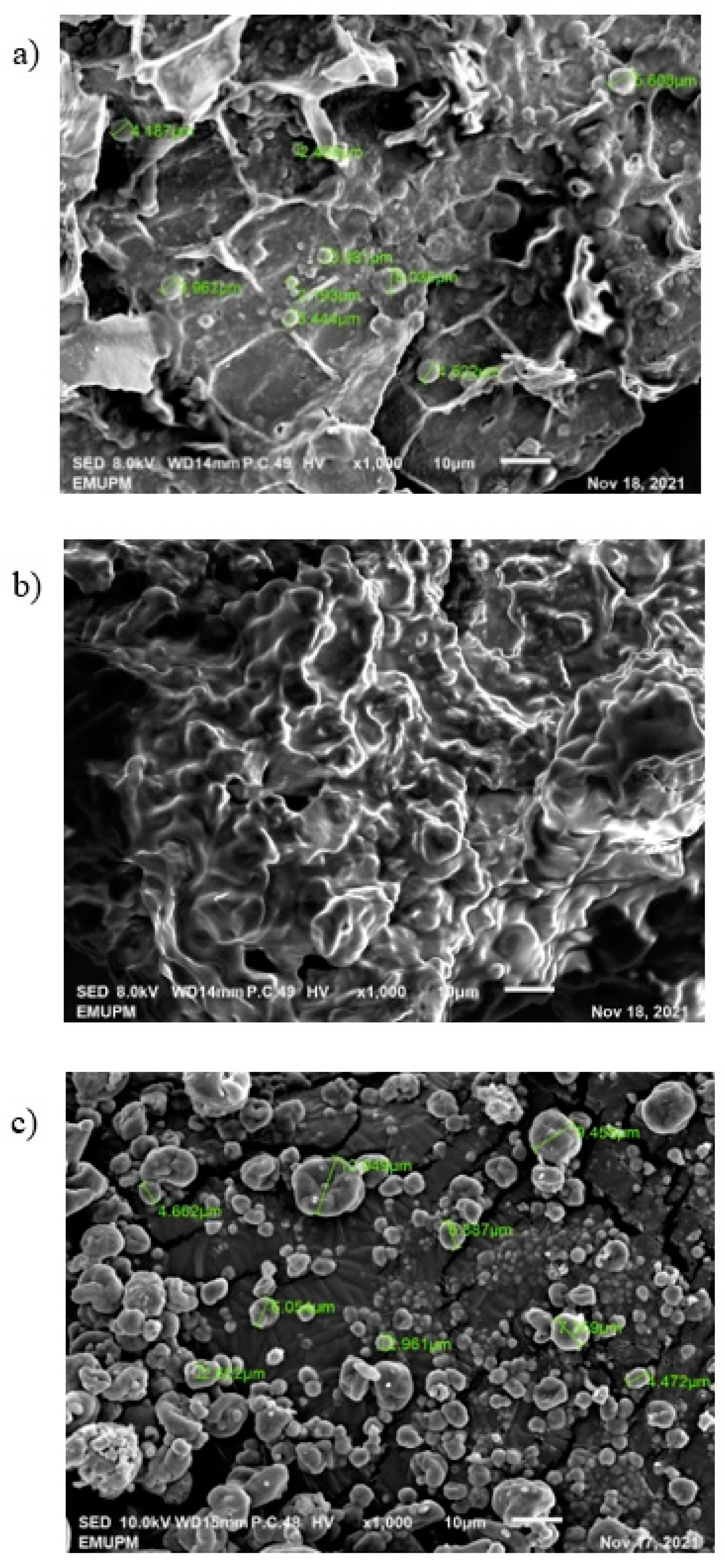
2.9. Scanning Electron Microscope (SEM)
3. Result and Discussion
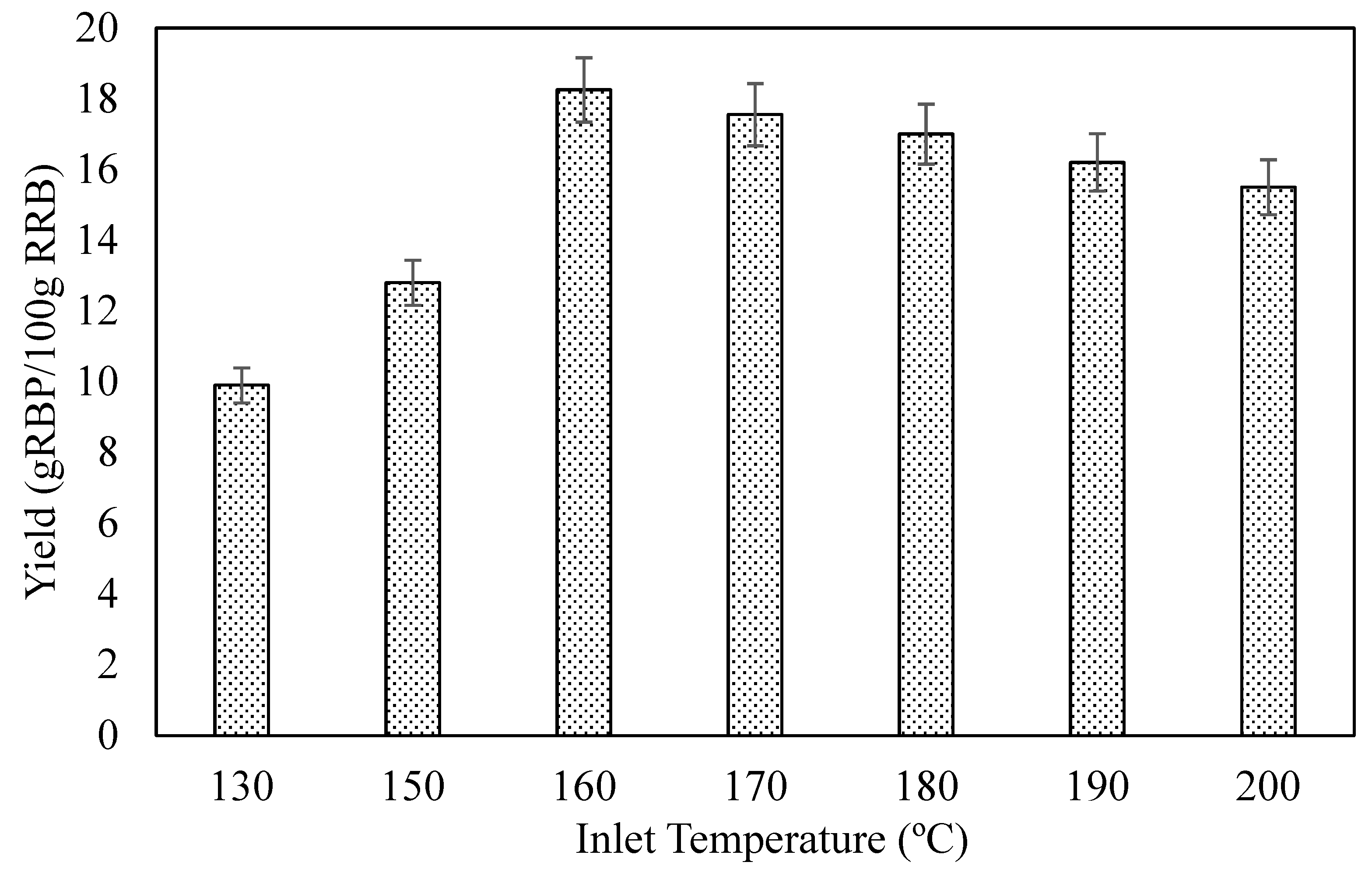
3.1. Effect of Inlet Temperature on Spray-Drying Process on RBP Powder Yield
3.2. Effect of Feed Flowrate on Spray-Drying Process on the RBP Powder Yield
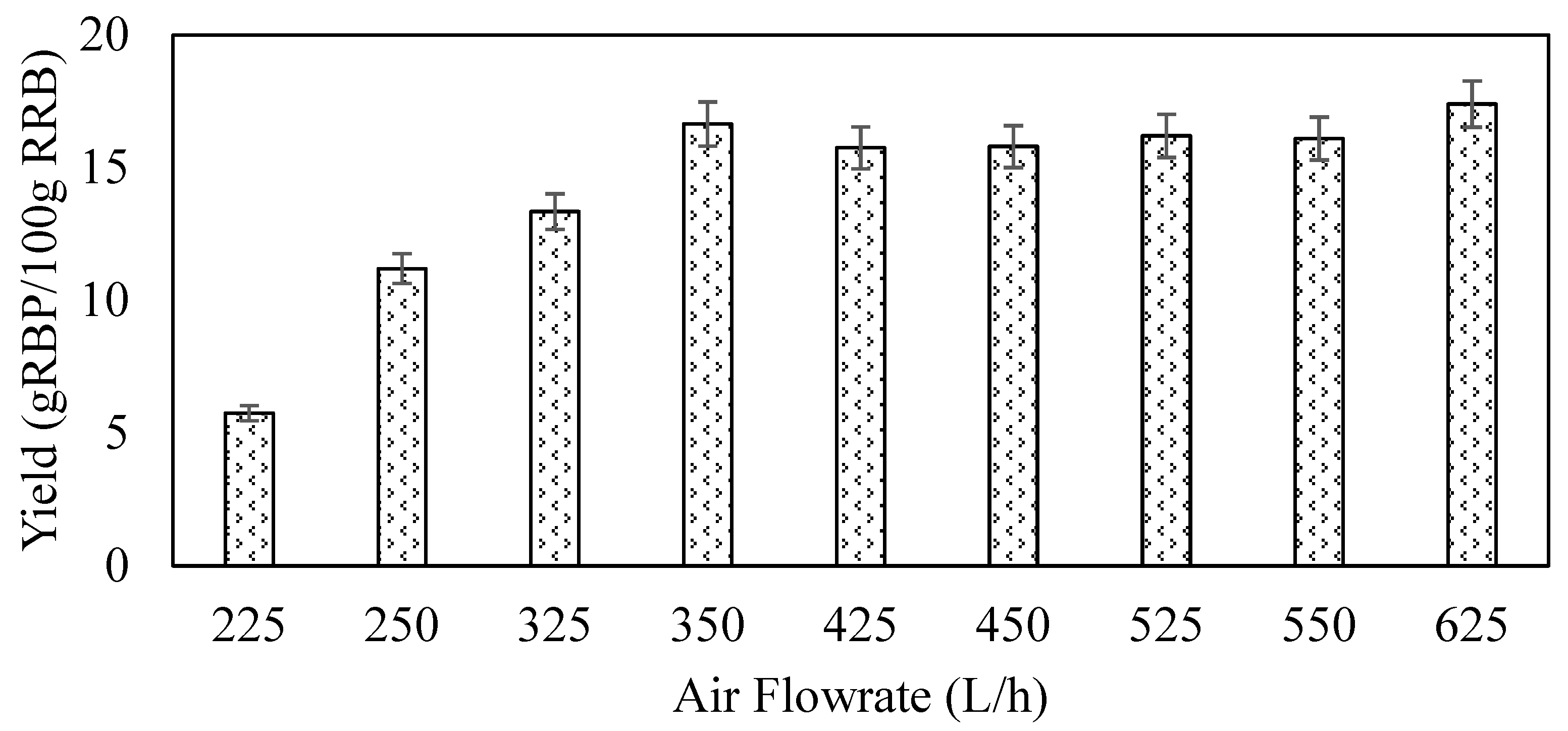
3.3. Effect of Air Flowrate on Spray-Drying Process on the RBP Powder Yield
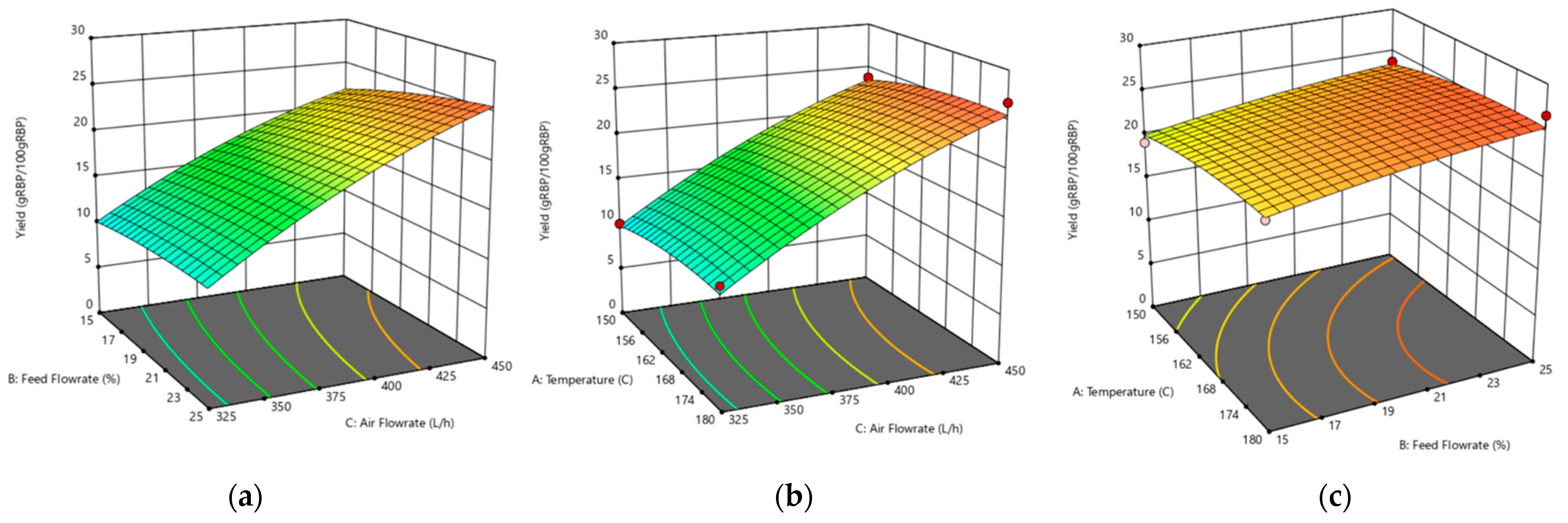
3.4. Optimization of the Spray-Drying Process for RBP Powder Production
- A = Inlet temperature (°C);
- B = Feed flowrate (%); and
- C = Air flowrate (L/h).
3.5. Comparison between Other Drying Methods
3.6. Protein Identification Analysis Based on Amino Acid Profiling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gul, K.; Yousuf, B.; Singh, A.K.; Singh, P.; Wani, A.A. Rice bran: Nutritional values and its emerging potential for development of functional food—A review. Bioact. Carbohydr. Diet. Fibre 2015, 6, 24–30. [Google Scholar] [CrossRef]
- Rajamoorthy, Y.; Abdul Rahim, K.; Munusamy, S. Rice Industry in Malaysia: Challenges, Policies and Implications. Procedia Econ. Financ. 2015, 31, 861–867. [Google Scholar] [CrossRef] [Green Version]
- Malekian, F.; Rao, R.M.; Prinyawiwatkul, W.; Marshall, W.E.; Windhauser, M.; Ahmedna, M.; Rogers, R.L. Lipase and Lipoxygenase Activity, Functionality, and Nutrient Losses in Rice Bran During Storage; Bulletin Number; Louisiana State University: Baton Rouge, LA, USA, 2000; Volume 870. [Google Scholar]
- Tadayyon, A.; Hill, G.A.; Ingledew, W.M.; Sokhansanj, S. Contact-sorption drying of Penicillium bilaii in a fluidized bed dryer. J. Chem. Technol. Biotechnol. 1997, 68, 277–282. [Google Scholar] [CrossRef]
- Caparino, O.A.; Tang, J.; Nindo, C.I.; Sablani, S.S.; Powers, J.R.; Fellman, J.K. Effect of drying methods on the physical properties and microstructures of mango (Philippine “Carabao” var.) powder. J. Food Eng. 2012, 111, 135–148. [Google Scholar] [CrossRef]
- Barbosa-C´anovas, G.V.; Ortega-Rivas, E.; Juliano, P.; Yan, H. Food Powders: Physical Properties, Processing, and Functionality, Chapter 2; Kluwer Academic Publishers: New York, NY, USA, 2005; pp. 19–54. [Google Scholar] [CrossRef]
- Emami, F.; Vatanara, A.; Park, E.J.; Na, D.H. Drying technologies for the stability and bioavailability of biopharmaceuticals. Pharmaceutics 2018, 10, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, R.; Shrivastava, S.L. Comparative evaluation of rice bran oil obtained with two-step microwave assisted extraction and conventional solvent extraction. J. Food Eng. 2018, 218, 106–114. [Google Scholar] [CrossRef]
- Izzah, N.; Rahim, A.; Morad, N.A.; Long, K. Rice Bran Water Extraction through Autoclaving and Sonication: Protein Content and Amino Acid Profile. J. Appl. Sci. Agric. 2015, 10, 97–103. [Google Scholar]
- McDonald, W.H.; Ohi, R.; Miyamoto, D.T.; Mitchison, T.J.; Yates, J.R. Comparison of three directly coupled HPLC MS/MS strategies for identification of proteins from complex mixtures: Single-dimension LC-MS/MS, 2-phase MudPIT, and 3-phase MudPIT. Int. J. Mass Spectrom. 2002, 219, 245–251. [Google Scholar] [CrossRef]
- Saisavoey, T.; Sangtanoo, P.; Reamtong, O.; Karnchanatat, A. Antioxidant and Anti-Inflammatory Effects of Defatted Rice Bran (Oryza Sativa L.) Protein Hydrolysates on Raw 264.7 Macrophage Cells. J. Food Biochem. 2016, 40, 731–740. [Google Scholar] [CrossRef]
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; Maier, C.S.; Stevens, J.F. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016, 192, 156–162. [Google Scholar] [CrossRef]
- de Oliveira, A.H.; Mario Eduardo, R.M.; Mata, C.; Fortes, M.; Duarte, M.E.M.; Pasquali, M.; Lisboa, H.M. Influence of spray drying conditions on the properties of whole goat milk. Dry. Technol. 2020, 39, 726–737. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. Spray drying of tomato pulp in dehumidified air: II. The effect on powder properties. J. Food Eng. 2005, 66, 35–42. [Google Scholar]
- Quek, S.Y.; Chok, N.K.; Swedlund, P. The physicochemical properties of spray-dried watermelon powders. Chem. Eng. Process. Process Intensif. 2007, 46, 386–392. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Fazaeli, M.; Emam-Djomeh, Z.; Kalbasi Ashtari, A.; Omid, M. Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food Bioprod. Process. 2012, 90, 667–675. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C.; Padma Ishwarya, S. Spray Drying Techniques for Food Ingredient Encapsulation; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Chegini, G.R.; Khazaei, J.; Ghobadian, B.; Goudarzi, A.M. Prediction of process and product parameters in an orange juice spray dryer using artificial neural networks. J. Food Eng. 2008, 84, 534–543. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Chen, W. Trends of spray drying: A critical review on drying of fruit and vegetable juices. Trends Food Sci. Technol. 2017, 65, 49–67. [Google Scholar] [CrossRef]
- Ghosal, S.; Indira, T.N.; Bhattacharya, S. Agglomeration of a model food powder: Effect of maltodextrin and gum Arabic dispersions on flow behavior and compacted mass. J. Food Eng. 2010, 96, 222–228. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V. Influence of process conditions on the physicochemical properties of pomegranate juice in spray drying process: Modelling and optimization. J. Saudi Soc. Agric. Sci. 2017, 16, 358–366. [Google Scholar] [CrossRef]
- Sadegh, P.; Ecevit, B. Scale-up of pharmaceutical spray drying using scale up rules: A review. Int. J. Pharm. 2019, 562, 271–292. [Google Scholar]
- Filková, I.; Huang, L.X.; Mujumdar, A.S. Industrial spray drying systems. In Handbook of Industrial Drying; CRC Press: Boca Raton, FL, USA; Taylor and Francis: New York, NY, USA, 2006; Volume 3, pp. 215–256. [Google Scholar]
- Hanley, K.J.; Cronin, K.; Sullivan, C.O.; Fenelon, M.A.; Mahony JA, O.; Byrne, E.P. Effect of composition on the mechanical response of agglomerates of infant formulae. J. Food Eng. 2011, 107, 71–79. [Google Scholar] [CrossRef]
- Muzaffar, K.; Kumar, P. Parameter optimization for spray drying of tamarind pulp using response surface methodology. Powder Technol. 2015, 279, 179–184. [Google Scholar] [CrossRef]
- Vardin, H.; Yasar, M. Original article Optimisation of pomegranate (Punica Granatum L.) juice spray-drying as affected by temperature and maltodextrin content. Int. J. Food Sci. Technol. 2012, 47, 167–176. [Google Scholar] [CrossRef]
- Ngamnikom, P.; Songsermpong, S. The effects of freeze, dry, and wet grinding processes on rice flour properties and their energy consumption. J. Food Eng. 2011, 104, 632–638. [Google Scholar] [CrossRef]
- Wang, S.; Xing, T.; Liu, A.P.; He, Z.; Yan, Y.; Daly, T.J.; Li, N. Simple Approach for Improved LC-MS Analysis of Protein Biopharmaceuticals via Modification of Desolvation Gas. Anal. Chem. 2019, 91, 3156–3162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabian, C.; Ju, Y.-H. A Review on Rice Bran Protein: Its Properties and Extraction Methods. Crit. Rev. Food Sci. Nutr. 2011, 51, 816–827. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Physical and flow properties of rice protein powders. J. Food Eng. 2016, 190, 1–9. [Google Scholar] [CrossRef]
- Kim, S.-M.; Chung, H.-J.; Lim, S.-T. Effect of various heat treatments on rancidity and some bioactive compounds of rice bran. J. Cereal Sci. 2014, 60, 243–248. [Google Scholar] [CrossRef]
- Bakthisaran, R.; Tangirala, R.; Rao, C.M. Biochimica et Biophysica Acta Small heat shock proteins: Role in cellular functions and pathology. BBA—Proteins Proteom. 2015, 1854, 291–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishimaru, T.; Miyazaki, M.; Shigemitsu, T.; Nakata, M.; Kuroda, M.; Kondo, M.; Masumura, T. Effect of high temperature stress during ripening on the accumulation of key storage compounds among Japanese highly palatable rice cultivars. J. Cereal Sci. 2020, 95, 103018. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-Y.; Yoon, U.-H.; Lim, S.-H.; Kim, Y.-M. Effects of Reduced Prolamin on Seed Storage Protein Composition and the Nutritional Quality of Rice. Int. J. Mol. Sci. 2013, 14, 17073–17084. [Google Scholar] [CrossRef]



| Response (R1) | Experimental Data (g RBP/100 g RRB) | Predicted Value (g RBP/100 g RRB) | Error AARD (%) | |
|---|---|---|---|---|
| Average | AAD | |||
| Yield | 25.66 | 0.55 | 24.9 | 2.96 |
| Protein | Raw Rice Bran (RRB) | Rice Bran Protein (RBP) |
|---|---|---|
| Globulin | D | D |
| Glutelin | D | D |
| Prolamin | D | ND |
| Glucose | ND | D |
| Fructose | ND | D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansor, M.R.; Md Sarip, M.S.; Nik Daud, N.M.A.; Saidi, S.A.; Mohd Nawi, M.A.H.; Jamlos, M.A. Preparation of Rice Bran Protein (RBP) Powder Using Spray Drying Method at the Optimal Condition and Its Protein Quality. Processes 2022, 10, 2026. https://doi.org/10.3390/pr10102026
Mansor MR, Md Sarip MS, Nik Daud NMA, Saidi SA, Mohd Nawi MAH, Jamlos MA. Preparation of Rice Bran Protein (RBP) Powder Using Spray Drying Method at the Optimal Condition and Its Protein Quality. Processes. 2022; 10(10):2026. https://doi.org/10.3390/pr10102026
Chicago/Turabian StyleMansor, Mohd Rizuan, Mohd Sharizan Md Sarip, Nik Muhammad Azhar Nik Daud, Syahrul Affandi Saidi, Mohd Al Hafiz Mohd Nawi, and Mohd Aminudin Jamlos. 2022. "Preparation of Rice Bran Protein (RBP) Powder Using Spray Drying Method at the Optimal Condition and Its Protein Quality" Processes 10, no. 10: 2026. https://doi.org/10.3390/pr10102026






