Potential Predictors for Cognitive Decline in Vascular Dementia: A Machine Learning Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Machine Learning
2.1.1. LASSO
2.1.2. RIDGE
2.1.3. Elastic Net
2.1.4. CART
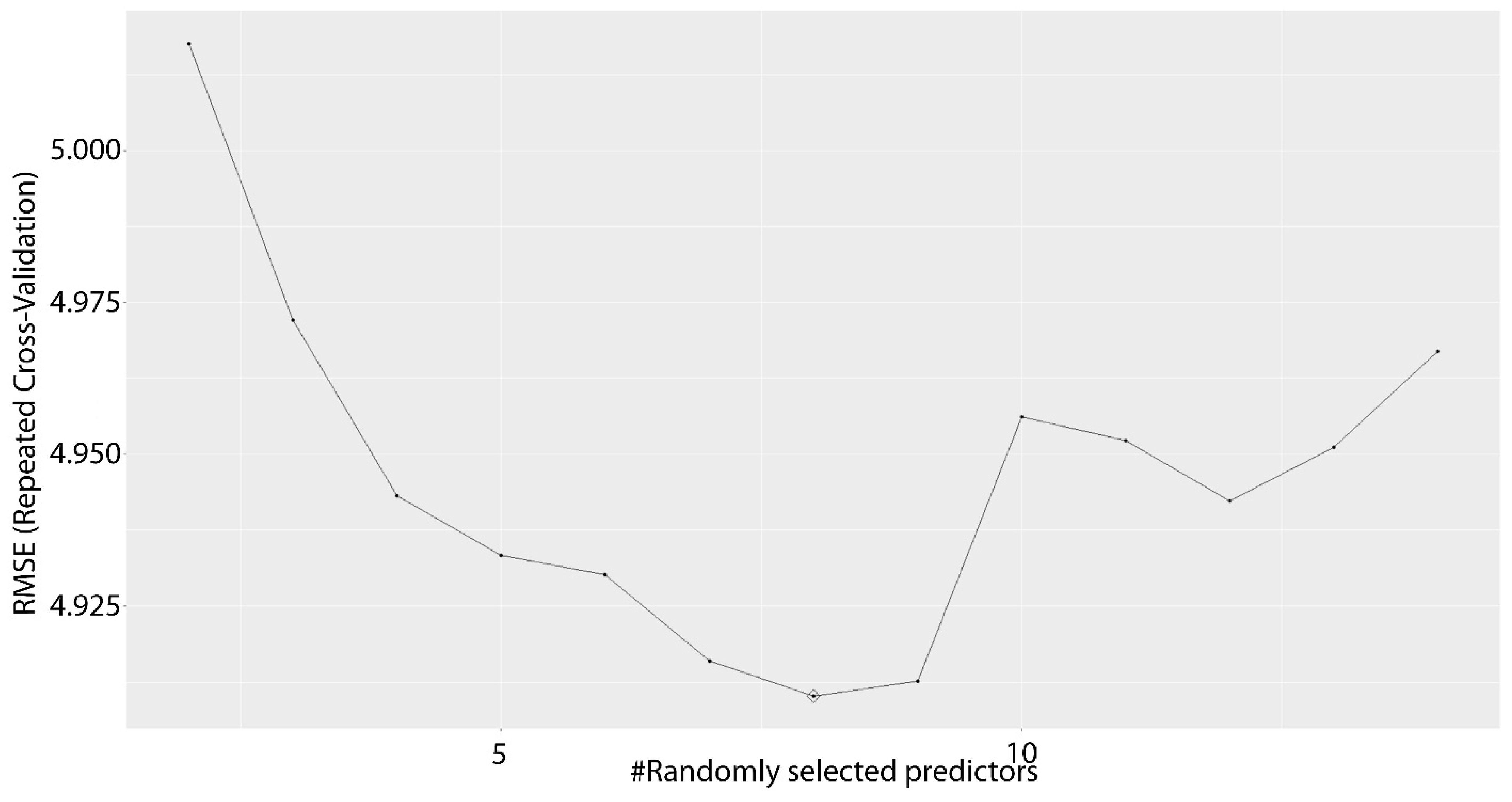
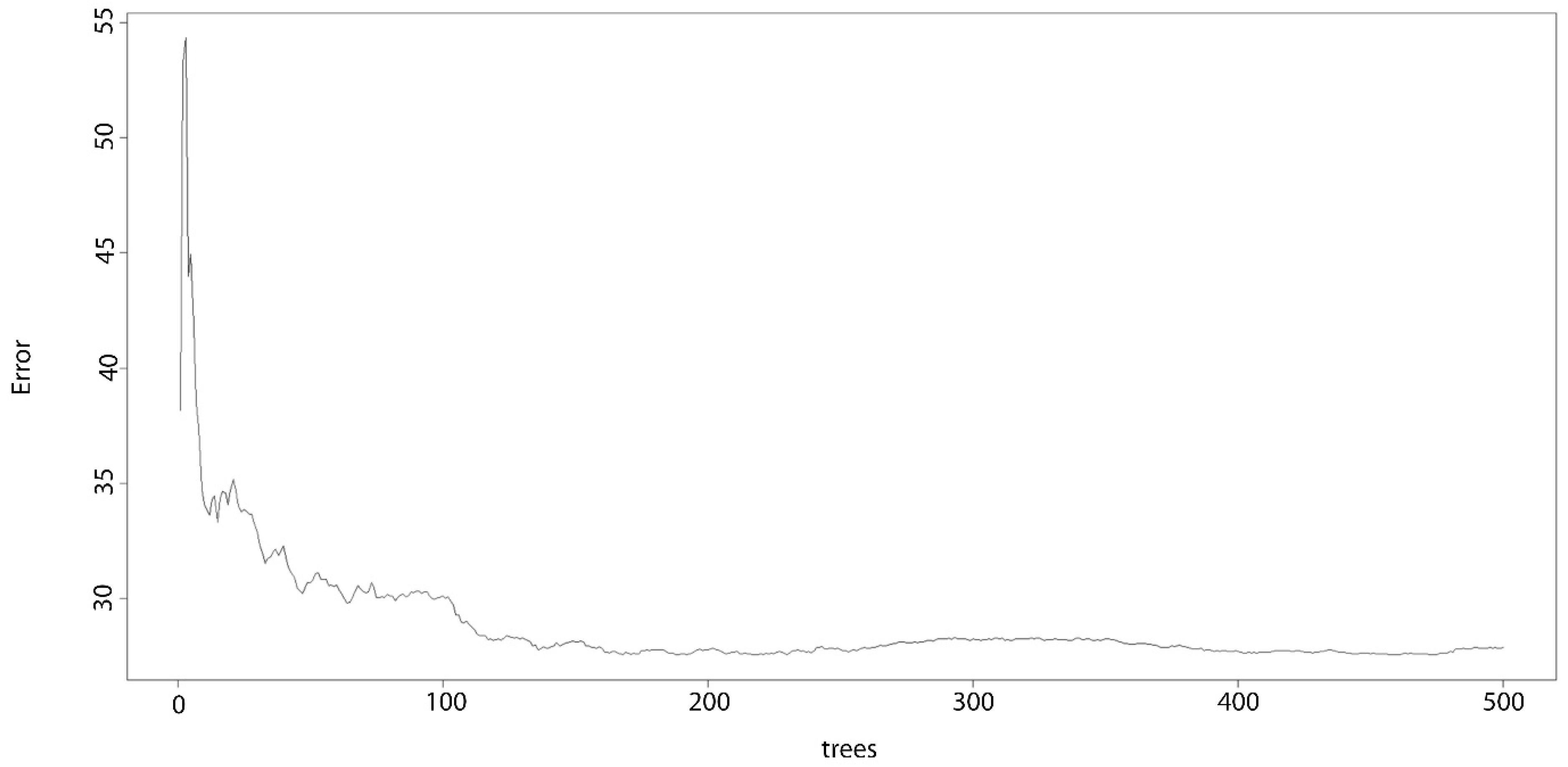
2.1.5. Random Forest
2.2. Machine Learning Approach—Data Augmentation
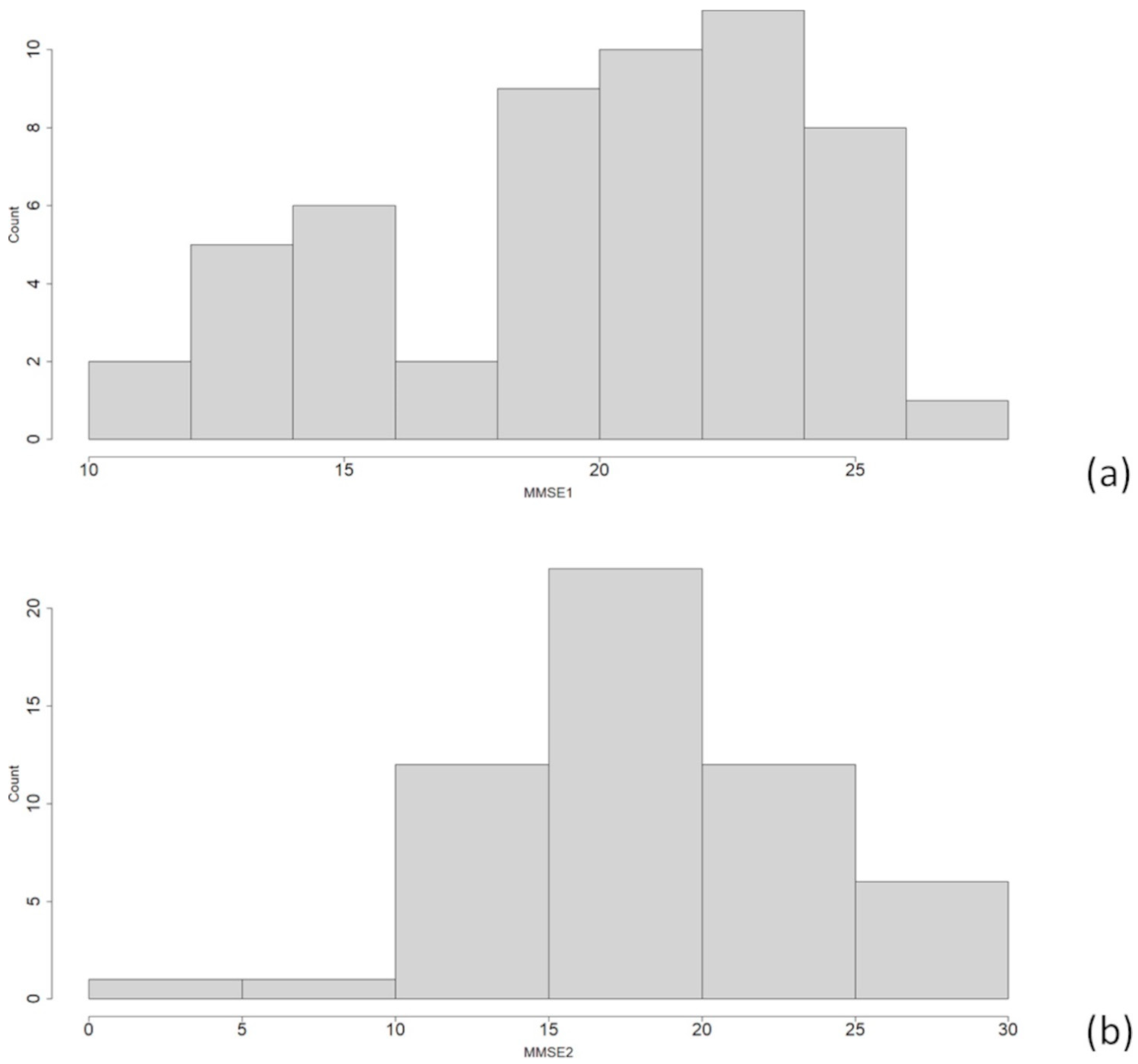
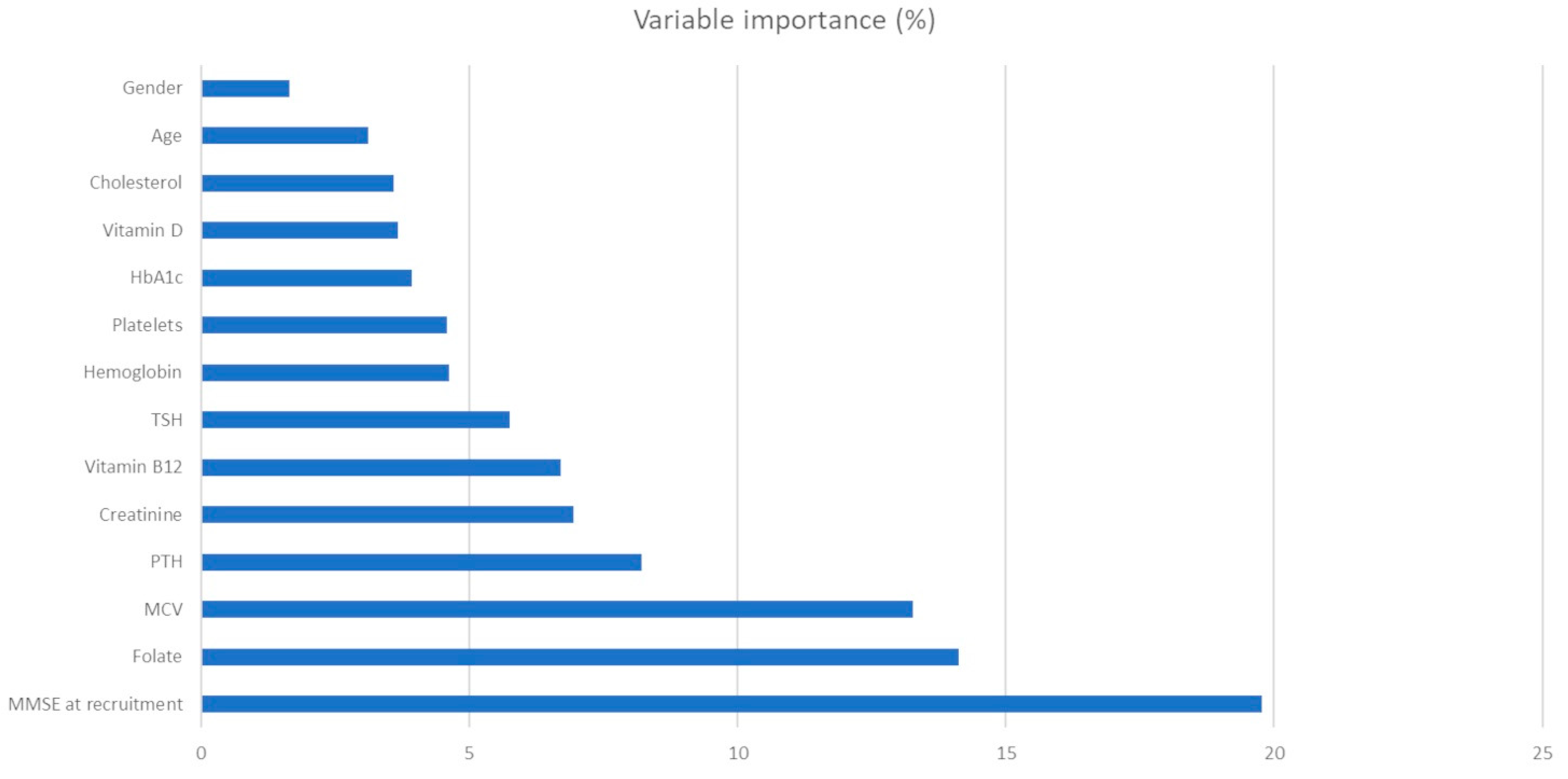
3. Results
4. Discussion
4.1. Hematic Values as Predictors of Cognitive Impairment
4.2. Study Limitations
5. Conclusions
5.1. Findings
5.2. Future Works
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Brien, J.T.; Thomas, A. Vascular Dementia. Lancet Lond. Engl. 2015, 386, 1698–1706. [Google Scholar] [CrossRef]
- Uwagbai, O.; Kalish, V.B. Vascular Dementia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tariq, S.; Barber, P.A. Dementia Risk and Prevention by Targeting Modifiable Vascular Risk Factors. J. Neurochem. 2018, 144, 565–581. [Google Scholar] [CrossRef] [PubMed]
- van der Flier, W.M.; Scheltens, P. Epidemiology and Risk Factors of Dementia. J. Neurol. Neurosurg. Psychiatry 2005, 76, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Venkat, P.; Chopp, M.; Chen, J. Models and Mechanisms of Vascular Dementia. Exp. Neurol. 2015, 272, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Duering, M.; Hachinski, V.; Joutel, A.; Pendlebury, S.T.; Schneider, J.A.; Dichgans, M. Vascular Cognitive Impairment and Dementia: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 3326–3344. [Google Scholar] [CrossRef] [PubMed]
- Korczyn, A.D.; Vakhapova, V.; Grinberg, L.T. Vascular Dementia. J. Neurol. Sci. 2012, 322, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; DeCarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular Contributions to Cognitive Impairment and Dementia. Stroke J. Cereb. Circ. 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Howes, M.-J.R.; Perry, N.S.L.; Vásquez-Londoño, C.; Perry, E.K. Role of Phytochemicals as Nutraceuticals for Cognitive Functions Affected in Ageing. Br. J. Pharmacol. 2020, 177, 1294–1315. [Google Scholar] [CrossRef]
- Mecocci, P.; Tinarelli, C.; Schulz, R.J.; Polidori, M.C. Nutraceuticals in Cognitive Impairment and Alzheimer’s Disease. Front. Pharmacol. 2014, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.; Solon-Biet, S.M.; Cogger, V.C.; Fontana, L.; Simpson, S.J.; Le Couteur, D.G.; Ribeiro, R.V. Aging, Lifestyle and Dementia. Neurobiol. Dis. 2019, 130, 104481. [Google Scholar] [CrossRef]
- de la Fuente Garcia, S.; Ritchie, C.W.; Luz, S. Artificial Intelligence, Speech, and Language Processing Approaches to Monitoring Alzheimer’s Disease: A Systematic Review. J. Alzheimers Dis. 2020, 78, 1547–1574. [Google Scholar] [CrossRef] [PubMed]
- Popuri, K.; Ma, D.; Wang, L.; Beg, M.F. Using machine learning to quantify structural MRI neurodegeneration patterns of Alzheimer’s disease into dementia score: Independent validation on 8834 images from ADNI, AIBL, OASIS, and MIRIAD databases. Hum. Brain. Mapp. 2020, 41, 4127–4147. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Brusini, I.; Andersson, O.; Ouellette, R.; Piehl, F.; Wang, C.; Granberg, T. Deep Learning Corpus Callosum Segmentation as a Neurodegenerative Marker in Multiple Sclerosis. J. Neuroimaging 2021, 31, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, P.; Stuke, H.; Kastrup, A.; Stuke, H.; Hildebrandt, H. Neuropsychological Testing and Machine Learning Distinguish Alzheimer’s Disease from Other Causes for Cognitive Impairment. Front. Aging Neurosci. 2017, 9, 114. [Google Scholar] [CrossRef]
- Zheng, Y.; Guo, H.; Zhang, L.; Wu, J.; Li, Q.; Lv, F. Machine Learning-Based Framework for Differential Diagnosis between Vascular Dementia and Alzheimer’s Disease Using Structural MRI Features. Front. Neurol. 2019, 10, 1097. [Google Scholar] [CrossRef] [PubMed]
- Castellazzi, G.; Cuzzoni, M.G.; Cotta Ramusino, M.; Martinelli, D.; Denaro, F.; Ricciardi, A.; Vitali, P.; Anzalone, N.; Bernini, S.; Palesi, F.; et al. A Machine Learning Approach for the Differential Diagnosis of Alzheimer and Vascular Dementia Fed by MRI Selected Features. Front. Neuroinform. 2020, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Banchero, S.; Tonacci, A.; Nencioni, A.; Monacelli, F.; Gangemi, S. Vitamin D and Folate as Predictors of MMSE in Alzheimer’s Disease: A Machine Learning Analysis. Diagnostics 2021, 11, 940. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Danieli, M.G.; Tonacci, A.; Paladini, A.; Longhi, E.; Moroncini, G.; Allegra, A.; Sansone, F.; Gangemi, S. A machine learning analysis to predict the response to intravenous and subcutaneous immunoglobulin in inflammatory myopathies. A proposal for a future multi-omics approach in autoimmune diseases. Autoimmun. Rev. 2022, 21, 103105. [Google Scholar] [CrossRef]
- Kuhn, M. Caret: Classification and Regression Training. R Package Version 6.0-73. Available online: https://CRAN.R-Project.Org/Package=caret (accessed on 10 March 2021).
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Hoerl, A.E.; Kennard, R.W. Ridge Regression: Biased Estimation for Nonorthogonal Problems. Technometrics 1970, 12, 55–67. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and Variable Selection via the Elastic Net. J. R. Stat. Soc. B 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees, 1st ed.; Routledge: Milton Park, UK, 1984. [Google Scholar] [CrossRef]
- Ho, T.K. The Random Subspace Method for Constructing Decision Forests. IEEE Trans. Pattern Anal. Mach. Intell. 1998, 20, 832–844. [Google Scholar]
- Conlin, A.K.; Martin, E.B.; Morris, A.J. Data Augmentation: An Alternative Approach to the Analysis of Spectroscopic Data. Chemom. Intell. Lab. Syst. 1998, 44, 161–173. [Google Scholar] [CrossRef]
- Beinecke, J.; Heider, D. Gaussian Noise Up-Sampling Is Better Suited than SMOTE and ADASYN for Clinical Decision Making. BioData Min. 2021, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Finch, W.; Finch, M. Regularization Methods for Fitting Linear Models with Small Sample Sizes: Fitting the Lasso Estimator Using R. Pract. Assess. Res. Eval. 2019, 21, 7. [Google Scholar] [CrossRef]
- Gaubert, S.; Houot, M.; Raimondo, F.; Ansart, M.; Corsi, M.-C.; Naccache, L.; Sitt, J.D.; Habert, M.-O.; Dubois, B.; De Vico Fallani, F.; et al. A Machine Learning Approach to Screen for Preclinical Alzheimer’s Disease. Neurobiol. Aging 2021, 105, 205–216. [Google Scholar] [CrossRef]
- Shojaie, M.; Tabarestani, S.; Cabrerizo, M.; DeKosky, S.T.; Vaillancourt, D.E.; Loewenstein, D.; Duara, R.; Adjouadi, M. PET Imaging of Tau Pathology and Amyloid-β, and MRI for Alzheimer’s Disease Feature Fusion and Multimodal Classification. J. Alzheimers Dis. 2021, 84, 1497–1514. [Google Scholar] [CrossRef]
- Andrade de Oliveira, A.; Carthery-Goulart, M.T.; Oliveira Júnior, P.P.d.M.; Carrettiero, D.C.; Sato, J.R. Defining Multivariate Normative Rules for Healthy Aging Using Neuroimaging and Machine Learning: An Application to Alzheimer’s Disease. J. Alzheimers Dis. 2015, 43, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Ben, O.; Melamed, M.; Granovsky, L.; Nieuwboer, A.; Rochester, L.; Del, D.; Avanzino, L.; Pelosin, E.; Bloem, B.R.; et al. Detecting Sensitive Mobility Features for Parkinson Disease Stages via Machine Learning. Mov. Disord. 2021, 36, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Rizk-Jackson, A.; Stoffers, D.; Sheldon, S.; Kuperman, J.; Dale, A.; Goldstein, J.; Corey-Bloom, J.; Poldrack, R.A.; Aron, A.R. Evaluating Imaging Biomarkers for Neurodegeneration in Pre-Symptomatic Huntington’s Disease Using Machine Learning Techniques. NeuroImage 2011, 56, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Caballero, H.S.; McFall, G.P.; Zheng, Y.; Dixon, R.A. Data-Driven Approaches to Executive Function Performance and Structure in Aging: Integrating Person-Centered Analyses and Machine Learning Risk Prediction. Neuropsychology 2021, 35, 889–903. [Google Scholar] [CrossRef] [PubMed]
- Maffei, L.; Picano, E.; Andreassi, M.G.; Angelucci, A.; Baldacci, F.; Baroncelli, L.; Begenisic, T.; Bellinvia, P.F.; Berardi, N.; Biagi, L.; et al. Randomized Trial on the Effects of a Combined Physical/Cognitive Training in Aged MCI Subjects: The Train the Brain Study. Sci. Rep. 2017, 7, 39471. [Google Scholar] [CrossRef]
- Tonacci, A.; Bruno, R.M.; Ghiadoni, L.; Pratali, L.; Berardi, N.; Tognoni, G.; Cintoli, S.; Volpi, L.; Bonuccelli, U.; Sicari, R.; et al. Olfactory Evaluation in Mild Cognitive Impairment: Correlation with Neurocognitive Performance and Endothelial Function. Eur. J. Neurosci. 2017, 45, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Brai, E.; Tonacci, A.; Brugada-Ramentol, V.; D’Andrea, F.; Alberi, L. Intercepting Dementia: Awareness and Innovation as Key Tools. Front. Aging Neurosci. 2021, 13, 730727. [Google Scholar] [CrossRef]
- Er, F.; Iscen, P.; Sahin, S.; Çinar, N.; Karsidag, S.; Goularas, D. Distinguishing Age-Related Cognitive Decline from Dementias: A Study Based on Machine Learning Algorithms. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2017, 42, 186–192. [Google Scholar] [CrossRef]
- Emrani, S.; Lamar, M.; Price, C.C.; Wasserman, V.; Matusz, E.; Au, R.; Swenson, R.; Nagele, R.; Heilman, K.M.; Libon, D.J. Alzheimer’s/Vascular Spectrum Dementia: Classification in Addition to Diagnosis. J. Alzheimers Dis. 2020, 73, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Vinters, H.V.; Zarow, C.; Borys, E.; Whitman, J.D.; Tung, S.; Ellis, W.G.; Zheng, L.; Chui, H.C. Review: Vascular Dementia: Clinicopathologic and Genetic Considerations. Neuropathol. Appl. Neurobiol. 2018, 44, 247–266. [Google Scholar] [CrossRef]
- Gofir, A.; Wibowo, S.; Hakimi, M.; Putera, D.D.; Satriotomo, I. Mustofa Folic Acid Treatment for Patients with Vascular Cognitive Impairment: A Systematic Review and Meta-Analysis. Int. J. Neuropsychopharmacol. 2022, 25, 136–143. [Google Scholar] [CrossRef]
- Block, G.A.; Klassen, P.S.; Lazarus, J.M.; Ofsthun, N.; Lowrie, E.G.; Chertow, G.M. Mineral Metabolism, Mortality, and Morbidity in Maintenance Hemodialysis. J. Am. Soc. Nephrol. 2004, 15, 2208–2218. [Google Scholar] [CrossRef]
- Garcia de la Torre, N.; Wass, J.A.H.; Turner, H.E. Parathyroid Adenomas and Cardiovascular Risk. Endocr. Relat. Cancer 2003, 10, 309–322. [Google Scholar] [CrossRef][Green Version]
- Hagström, E.; Kilander, L.; Nylander, R.; Larsson, E.-M.; Michaëlsson, K.; Melhus, H.; Ahlström, H.; Johansson, L.; Lind, L.; Ärnlöv, J. Plasma Parathyroid Hormone Is Associated with Vascular Dementia and Cerebral Hyperintensities in Two Community-Based Cohorts. J. Clin. Endocrinol. Metab. 2014, 99, 4181–4189. [Google Scholar] [CrossRef] [PubMed]
- Lourida, I.; Thompson-Coon, J.; Dickens, C.M.; Soni, M.; Kuźma, E.; Kos, K.; Llewellyn, D.J. Parathyroid Hormone, Cognitive Function and Dementia: A Systematic Review. PLoS ONE 2015, 10, e0127574. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Han, X.; Wang, Y.; Hou, T.; Cong, L.; Tang, S.; Han, X.; Ngandu, T.; Kivipelto, M.; Winblad, B.; et al. Red Cell Distribution Width and Dementia Among Rural-Dwelling Older Adults: The MIND-China Study. J. Alzheimers Dis. 2021, 83, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Koenig, A.M.; Nobuhara, C.K.; Williams, V.J.; Arnold, S.E. Biomarkers in Alzheimer’s, Frontotemporal, Lewy Body, and Vascular Dementias. Focus (Am. Psychiatr. Publ.) 2018, 16, 164–172. [Google Scholar] [CrossRef]
- Kurella Tamura, M.; Gaussoin, S.A.; Pajewski, N.M.; Chelune, G.J.; Freedman, B.I.; Gure, T.R.; Haley, W.E.; Killeen, A.A.; Oparil, S.; Rapp, S.R.; et al. Kidney Disease, Intensive Hypertension Treatment, and Risk for Dementia and Mild Cognitive Impairment: The Systolic Blood Pressure Intervention Trial. J. Am. Soc. Nephrol. 2020, 31, 2122–2132. [Google Scholar] [CrossRef]
- Hong, C.H.; Falvey, C.; Harris, T.B.; Simonsick, E.M.; Satterfield, S.; Ferrucci, L.; Metti, A.L.; Patel, K.V.; Yaffe, K. Anemia and Risk of Dementia in Older Adults: Findings from the Health ABC Study. Neurology 2013, 81, 528–533. [Google Scholar] [CrossRef]
- Brai, E.; Hummel, T.; Alberi, L. Smell, an Underrated Early Biomarker for Brain Aging. Front. Neurosci. 2020, 14, 792. [Google Scholar] [CrossRef]
- Tonacci, A.; Baldus, G.; Corda, D.; Piccaluga, E.; Andreassi, M.G.; Cremonesi, A.; Guagliumi, G.; Picano, E. Olfactory non-cancer effects of exposure to ionizing radiation in staff working in the cardiac catheterization laboratory. Int. J. Cardiol. 2014, 171, 461–463. [Google Scholar] [CrossRef]


| Approach | Train/Test (%) | LASSO | RIDGE | E-Net | CART | Random Forest |
| Original Dataset | 80/20 | 3.992 | 4.220 | 3.967 | 4.370 | 3.710 |
| 70/30 | 3.321 | 3.364 | 3.326 | 3.971 | 1.979 | |
| Data Augmentation by 100% | 80/20 | 3.939 | 4.430 | 4.181 | 5.433 | 4.212 |
| 70/30 | 4.720 | 4.777 | 4.734 | 7.197 | 5.001 | |
| Data Augmentation by 200% | 80/20 | 3.757 | 3.756 | 3.801 | 3.135 | 3.948 |
| 70/30 | 5.386 | 5.527 | 5.351 | 5.862 | 5.419 |
| Approach | Train/Test (%) | LASSO | RIDGE | E-Net | CART | Random Forest |
| Original Dataset | 70/30 | 3.295 | 3.833 | 3.292 | 3.307 | 2.864 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murdaca, G.; Banchero, S.; Casciaro, M.; Tonacci, A.; Billeci, L.; Nencioni, A.; Pioggia, G.; Genovese, S.; Monacelli, F.; Gangemi, S. Potential Predictors for Cognitive Decline in Vascular Dementia: A Machine Learning Analysis. Processes 2022, 10, 2088. https://doi.org/10.3390/pr10102088
Murdaca G, Banchero S, Casciaro M, Tonacci A, Billeci L, Nencioni A, Pioggia G, Genovese S, Monacelli F, Gangemi S. Potential Predictors for Cognitive Decline in Vascular Dementia: A Machine Learning Analysis. Processes. 2022; 10(10):2088. https://doi.org/10.3390/pr10102088
Chicago/Turabian StyleMurdaca, Giuseppe, Sara Banchero, Marco Casciaro, Alessandro Tonacci, Lucia Billeci, Alessio Nencioni, Giovanni Pioggia, Sara Genovese, Fiammetta Monacelli, and Sebastiano Gangemi. 2022. "Potential Predictors for Cognitive Decline in Vascular Dementia: A Machine Learning Analysis" Processes 10, no. 10: 2088. https://doi.org/10.3390/pr10102088
APA StyleMurdaca, G., Banchero, S., Casciaro, M., Tonacci, A., Billeci, L., Nencioni, A., Pioggia, G., Genovese, S., Monacelli, F., & Gangemi, S. (2022). Potential Predictors for Cognitive Decline in Vascular Dementia: A Machine Learning Analysis. Processes, 10(10), 2088. https://doi.org/10.3390/pr10102088











