An Intensified Green Process for the Coproduction of DMC and DMO by the Oxidative Carbonylation of Methanol
Abstract
:1. Introduction
2. Thermodynamics and Physical Properties
3. Reaction Kinetics and Reactor Design
3.1. Reactions and Kinetic Model
3.2. Fixed-Bed Reactor Design
4. Flammability Analysis
5. Process Development
5.1. Process Flowsheet Simulation
5.2. Separation of Azeotropic Mixture
6. Heat Integration
7. Profitability Analysis
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Ac | Cross-sectional area of the reactor (m2) |
| Bo | Constant that depends on the properties of the fixed bed (MPa/m) |
| CBM | Bare module cost |
| Dp | Diameter of particles in the bed (m) |
| Effective diffusivity (m2/s) | |
| E | Activation energy (J/mol) |
| Fi | Molar flow rate for each component (kmol/h) |
| Total inlet flow rates (kmol/h) | |
| FT | Total outlet flow rate (kmol/h) |
| Superficial mass velocity ) | |
| kn | Constant rate of reaction |
| Mass transfer coefficient (m/s) | |
| P | Final pressure (MPa) |
| pi,n | Partial pressure of components (MPa) |
| Po | Initial pressure (2 MPa) |
| Reaction rate (mol∙g−1 h−1) | |
| Actual rates of reaction (mol∙g−1 h−1) | |
| R | Universal gas constant (8.314 J∙mol−1 K−1) |
| T | Reaction temperature (403.15 K) |
| V | Volume of the reactor (m3) |
| Greek letters | |
| Overall effectiveness factor | |
| Porosity of the catalyst | |
| Biot number | |
| Voidage of the reactor | |
| Viscosity of the mixture (Pa∙s) | |
| Inlet mixture density | |
| Indices | |
| i | Component |
| a, b, c | Power exponents of the reaction rate equations |
| Reaction number | |
References
- Varma, R.S. Greener and Sustainable Chemistry. Appl. Sci. 2014, 4, 493–497. [Google Scholar] [CrossRef] [Green Version]
- Crawford, S.E.; Hartung, T.; Hollert, H.; Mathes, B.; van Ravenzwaay, B.; Steger-Hartmann, T.; Studer, C.; Krug, H.F. Green Toxicology: A strategy for sustainable chemical and material development. Environ. Sci. Eur. 2017, 29, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Pu, Y.; Yang, J.; Wang, T.; Chen, L.; Zhao, N.; Xiao, F. Process design and economic optimization for the indirect synthesis of dimethyl carbonate from urea and methanol. Chin. J. Chem. Eng. 2018, 27, 1879–1887. [Google Scholar] [CrossRef]
- Tundo, P.; Selva, M. The Chemistry of Dimethyl Carbonate. Accounts Chem. Res. 2002, 35, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, G.; Perosa, A.; Selva, M. Dimethyl carbonate: A versatile reagent for a sustainable valorization of renewables. Green Chem. 2018, 20, 288–322. [Google Scholar] [CrossRef]
- Tan, H.-Z.; Wang, Z.-Q.; Xu, Z.-N.; Sun, J.; Xu, Y.-P.; Chen, Q.-S.; Chen, Y.; Guo, G.-C. Review on the synthesis of dimethyl carbonate. Catal. Today 2018, 316, 2–12. [Google Scholar] [CrossRef]
- Saada, R.; Aboelazayem, O.; Kellici, S.; Heil, T.; Morgan, D.; Lampronti, G.I.; Saha, B. Greener synthesis of dimethyl carbonate using a novel tin-zirconia/graphene nanocomposite catalyst. Appl. Catal. B Environ. 2018, 226, 451–462. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, B.; Alvaro, M.; Garcia, H. Application of Dimethyl Carbonate as Solvent and Reagent; Springer: Cham, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Park, J.H.; Jeon, J.Y.; Lee, J.J.; Jang, Y.; Varghese, J.K.; Lee, B.Y. Preparation of High-Molecular-Weight Aliphatic Polycarbonates by Condensation Polymerization of Diols and Dimethyl Carbonate. Macromolecules 2013, 46, 3301–3308. [Google Scholar] [CrossRef]
- Gift, J.S.; McGaughy, R.; Singh, D.V.; Sonawane, B. Health assessment of phosgene: Approaches for derivation of reference concentration. Regul. Toxicol. Pharmacol. 2008, 51, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Kongpanna, P.; Pavarajarn, V.; Gani, R.; Assabumrungrat, S. Techno-economic evaluation of different CO2-based processes for dimethyl carbonate production. Chem. Eng. Res. Des. 2015, 93, 496–510. [Google Scholar] [CrossRef]
- Esan, A.O.; Adeyemi, A.D.; Ganesan, S. A review on the recent application of dimethyl carbonate in sustainable biodiesel production. J. Clean. Prod. 2020, 257, 120561. [Google Scholar] [CrossRef]
- Garcia-Herrero, I.; Cuéllar-Franca, R.M.; Enríquez-Gutiérrez, V.M.; Alvarez-Guerra, M.; Irabien, A.; Azapagic, A. Environmental Assessment of Dimethyl Carbonate Production: Comparison of a Novel Electrosynthesis Route Utilizing CO2 with a Commercial Oxidative Carbonylation Process. ACS Sustain. Chem. Eng. 2016, 4, 2088–2097. [Google Scholar] [CrossRef] [Green Version]
- Fang, D.; Cao, F. Intrinsic kinetics of direct oxidative carbonylation of vapour phase methanol to dimethyl carbonate over Cu-based catalysts. Chem. Eng. J. 2000, 78, 237–241. [Google Scholar] [CrossRef]
- Curnutt, G.L. Catalytic vapor phase process for producing dihydrocarbyl carbonates. U.S. Patent 5004827, 2 April 1991. [Google Scholar]
- Wang, J.; Fu, T.; Meng, F.; Zhao, D.; Chuang, S.S.; Li, Z. Highly active catalysis of methanol oxidative carbonylation over nano Cu2O supported on micropore-rich mesoporous carbon. Appl. Catal. B Environ. 2021, 303, 120890. [Google Scholar] [CrossRef]
- Ren, X.; Quan, Y.; Yang, W.; Zhao, J.; Shi, R.; Ren, J. Highly efficient super activated carbon supported ultra-low loading copper catalyst for the oxidative carbonylation of methanol to dimethyl carbonate. Mol. Catal. 2022, 531, 112694. [Google Scholar] [CrossRef]
- Lee, D.-H.; You, J.; Woo, J.-M.; Seo, J.Y.; Park, Y.C.; Lee, J.-S.; Kim, H.; Moon, J.-H.; Bin Park, S. Influence of dehydrating agents on the oxidative carbonylation of methanol for dimethyl carbonate synthesis over a Cu/Y-zeolite catalyst. Chin. J. Chem. Eng. 2018, 26, 1059–1063. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Z.; Chen, R.; Sun, B.; Qiao, L.; Yao, Y.; Wu, K. Insights into the reaction mechanism of CO oxidative coupling to dimethyl oxalate over palladium: A combined DFT and IR study. Phys. Chem. Chem. Phys. 2015, 17, 9126–9134. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, J.; Lv, J.; Zhao, Y.; Ma, X. Hydrogenation of Dimethyl Oxalate over Copper-Based Catalysts: Acid–Base Properties and Reaction Paths. Ind. Eng. Chem. Res. 2015, 54, 9699–9707. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, S. Hydrogenation of dimethyl oxalate to ethylene glycol over Cu/SiO2 catalysts. In Proceedings of the 2011 International Conference on Electronics, Communications and Control (ICECC), Ningbo, China, 9–11 September 2011; pp. 4344–4347. [Google Scholar] [CrossRef]
- Niu, Y.; Li, C.; Shen, J.; Wei, X. Absorption of dilute sulfur dioxide in ethanediamine with ethylene glycol or polyethylene glycol 400 plus water system. J. Clean. Prod. 2018, 171, 506–511. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, S.H. Transfer Hydrogenation of Organic Formates and Cyclic Carbonates: An Alternative Route to Methanol from Carbon Dioxide. ACS Catal. 2014, 4, 3630–3636. [Google Scholar] [CrossRef]
- Lee, W.; Lee, J.W. Concurrent Production of Carbon Monoxide and Manganese(II) Oxide through the Reaction of Carbon Dioxide with Manganese. ACS Sustain. Chem. Eng. 2014, 2, 1503–1509. [Google Scholar] [CrossRef]
- Gmehling, J.; Möllmann, C. Synthesis of Distillation Processes Using Thermodynamic Models and the Dortmund Data Bank. Ind. Eng. Chem. Res. 1998, 37, 3112–3123. [Google Scholar] [CrossRef]
- Fredenslund, A.; Jones, R.L.; Prausnitz, J.M. Group-contribution estimation of activity coefficients in nonideal liquid mixtures. AIChE J. 1975, 21, 1086–1099. [Google Scholar] [CrossRef]
- Shen, W.F.; Benyounes, H.; Song, J. A review of ternary azeotropic mixtures advanced separation strategies. Theor. Found. Chem. Eng. 2016, 50, 28–40. [Google Scholar] [CrossRef] [Green Version]
- Fogler, H.S. Essentials of Chemical Reaction Engineering; Prentice-Hall: Upper Saddle River, NJ, USA, 2017. [Google Scholar]
- Zabetakis, M.G.; Scott, G.S.; Jones, G.W. Limits of Flammability of Paraffin Hydrocarbons in Air. Ind. Eng. Chem. 1951, 43, 2120–2124. [Google Scholar] [CrossRef]
- Chang, Y.-M.; Tseng, J.-M.; Shu, C.-M.; Hu, K.-H. Flammability studies of benzene and methanol with various vapor mixing ratios at 150 °C. Korean J. Chem. Eng. 2005, 22, 803–812. [Google Scholar] [CrossRef]
- Carlson, E.C. Don’t gamble with physical properties. Chem. Eng. Prog. 1996, 92, 35. [Google Scholar]
- Wei, H.-M.; Wang, F.; Zhang, J.-L.; Liao, B.; Zhao, N.; Xiao, F.-K.; Wei, W.; Sun, Y.-H. Design and Control of Dimethyl Carbonate–Methanol Separation via Pressure-Swing Distillation. Ind. Eng. Chem. Res. 2013, 52, 11463–11478. [Google Scholar] [CrossRef]
- Li, M.; Xu, X.; Li, X.; Ma, K.; Qin, B.; Zhu, Z.; Wang, Y. Phase Behavior and Thermodynamic Model Parameters in Simulations of Extractive Distillation for Azeotrope Separation. Sci. Rep. 2017, 7, 9497. [Google Scholar] [CrossRef] [PubMed]
- Umbach, J.S.; Nitsche, L.S. Online Pinch Analysis Tool Pinch Analysis. Available online: http://www.uic-che.org/pinch/ (accessed on 12 December 2021).
- Holtbruegge, J.; Kuhlmann, H.; Lutze, P. Process analysis and economic optimization of intensified process alternatives for simultaneous industrial scale production of dimethyl carbonate and propylene glycol. Chem. Eng. Res. Des. 2015, 93, 411–431. [Google Scholar] [CrossRef]
- El-Halwagi, M.M. Process Integration, 1st ed.; Elsevier: Oxford, UK, 2006; Volume 7. [Google Scholar]
- Olsen, D.; Abdelouadoud, Y.; Liem, P.; Wellig, B. The Role of Pinch Analysis for Industrial ORC Integration. Energy Procedia 2017, 129, 74–81. [Google Scholar] [CrossRef]
- Ebrada, L.C.; De Luna, M.D.G.; Manegdeg, F.G.; Grisdanurak, N. The effect of minimum temperature difference in the design and optimization of heat exchanger networks of a brewery based on Pinch Methodology. Lect. Notes Eng. Comput. Sci. 2014, 2, 952–957. [Google Scholar]
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A.; Bhattacharyya, D. Analysis, Synthesis, and Design of Chemical Processes; Pearson: Upper Saddle River, NJ, USA, 2012. [Google Scholar]
- Jenkins, S. 2019 Chemical Engineering Plant Cost Index Annual Average. 2020. Available online: https://www.chemengonline.com/2020-annual-cepci-average-value/ (accessed on 20 February 2022).


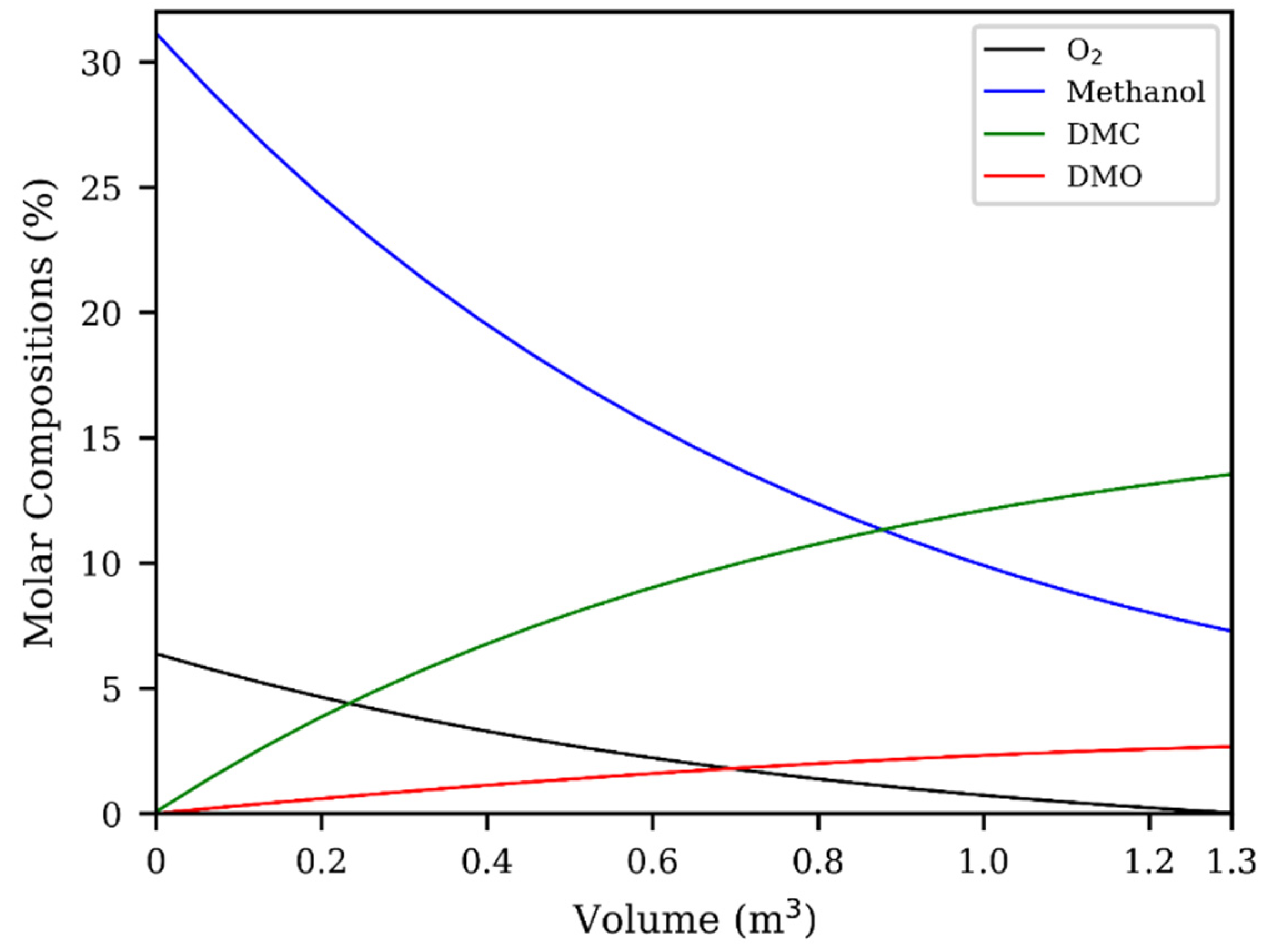

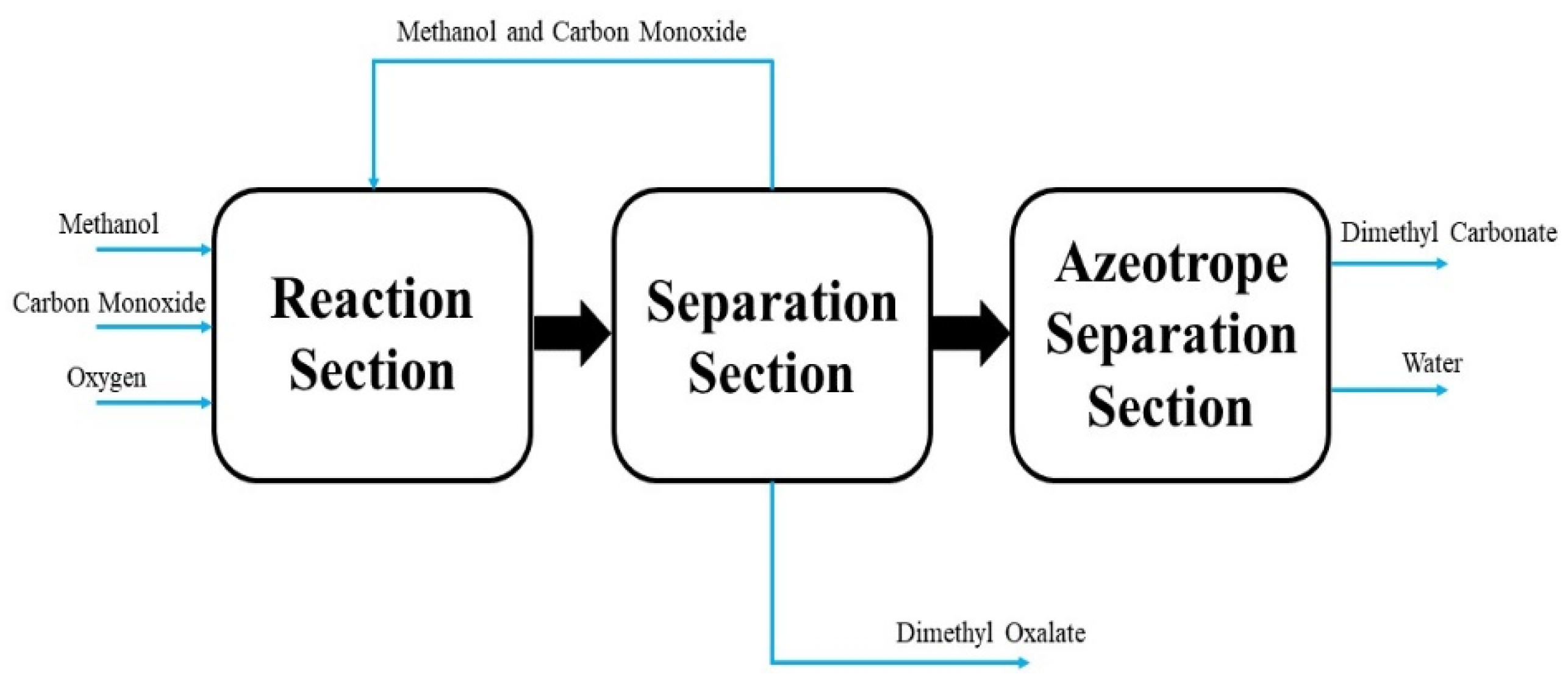
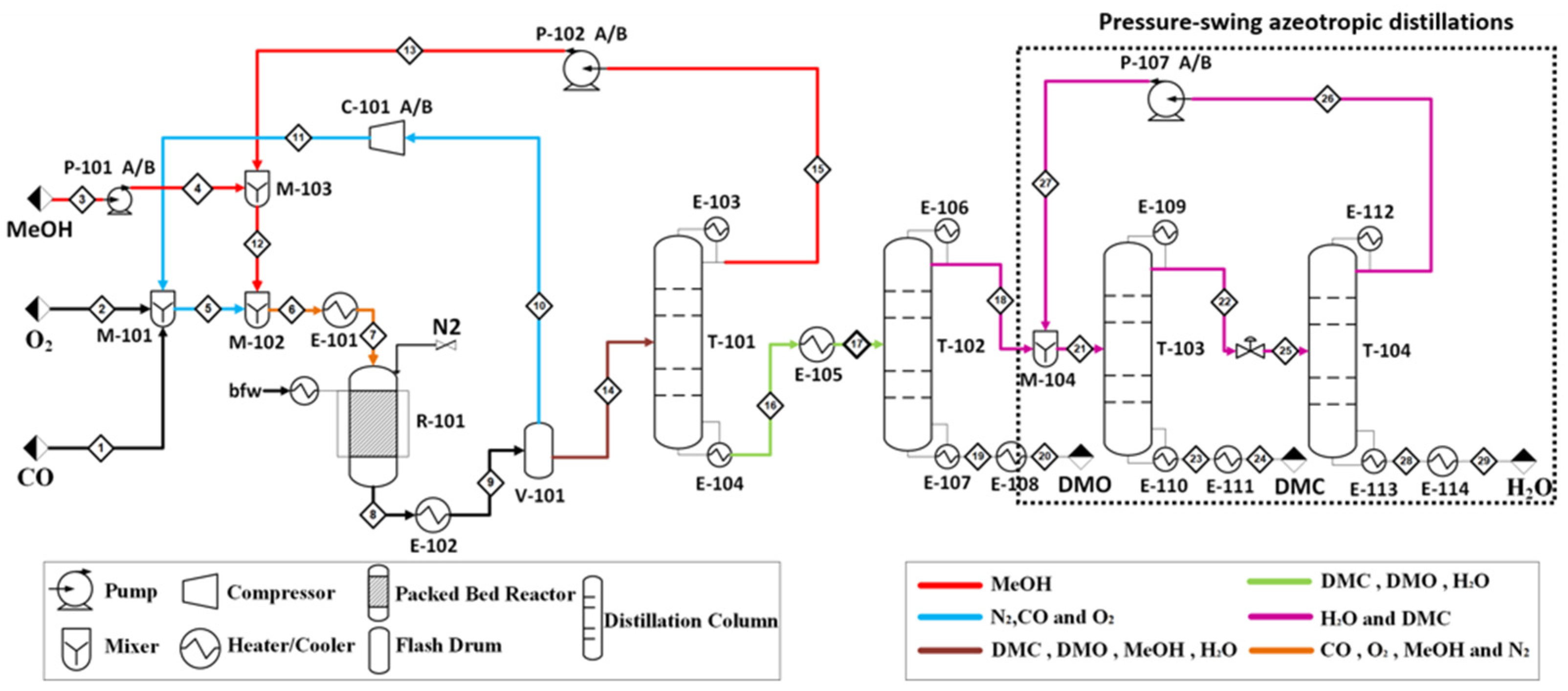


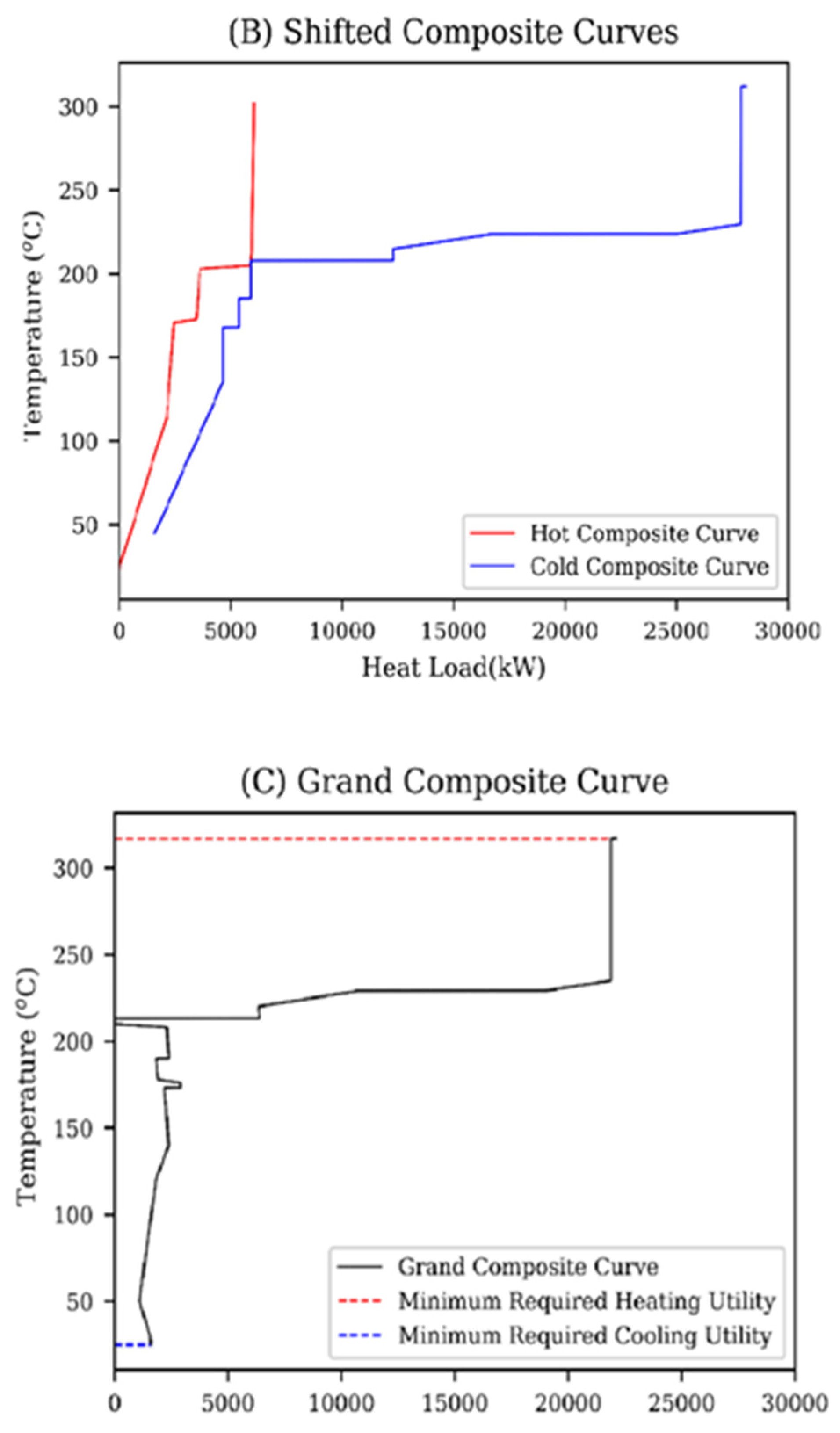
| Reaction No. | Constant Rate of Reaction | Activation Energy, E (J∙mol−1) | Power Exponents |
|---|---|---|---|
| a | |||
| b | |||
| c | |||
| 1 | 0.3674 × 107 | 0.1589 × 105 | 1.402 |
| 0.953 | |||
| 0.005 | |||
| 2 | 0.1613 × 105 | 0.4038 × 104 | 0.728 |
| 1.031 | |||
| 0.172 |
| Component | Vol.% | Yi | LFL% at 25 °C | UFL% at 25 °C | LFL% at 130 °C | UFL% at 130 °C | LFL% mix. | UFL% mix. |
|---|---|---|---|---|---|---|---|---|
| MeOH | 31.21 | 0.36 | 7.3 | 36.00 | 6.74 | 36.56 | - | - |
| CO | 55.52 | 0.64 | 12.5 | 74.00 | 11.34 | 75.16 | - | - |
| O2 | 6.37 | - | - | - | - | - | - | - |
| N2 | 6.90 | - | - | - | - | - | - | - |
| Total | 100 | - | - | - | - | - | 9.10 | 83.62 |
| Stream No. | 1 | 2 | 3 | 7 | 9 | 11 |
| Temperature (°C) | 25 | 25 | 25 | 130 | 30 | 38.4 |
| Pressure (bar) | 1 | 1 | 1 | 20 | 19.2 | 20.3 |
| Vapor mole fraction | 1 | 1 | 0 | 1 | 0.6059 | 1 |
| Total flow (kg/h) | 3830.4 | 1878.8 | 7525.1 | 27,208 | 27,208.1 | 12,346.6 |
| Total flow (kmol/h) | 136.8 | 58.7 | 234.9 | 920.5 | 725 | 439.4 |
| Component flow rates (kmol/h) | ||||||
| O2 | 0 | 58.7 | 0 | 58.7 | 0 | 0 |
| N2 | 0 | 0 | 0 | 63.6 | 63.6 | 63.6 |
| H2O | 0 | 0 | 0 | 0.5 | 118 | 0.4 |
| CO | 136.8 | 0 | 0 | 510.2 | 373.4 | 373.4 |
| DMC | 0 | 0 | 0 | 0.6 | 98.6 | 0.6 |
| DMO | 0 | 0 | 0 | 0 | 19.4 | 0 |
| MeOH | 0 | 0 | 234.9 | 286.9 | 52 | 1.4 |
| Stream No. | 12 | 13 | 14 | 17 | 18 | 20 |
| Temperature (°C) | 55.3 | 164.2 | 30 | 225.2 | 208.3 | 1.5 |
| Pressure (bar) | 20.3 | 20.3 | 19.2 | 19.7 | 19.7 | 20 |
| Vapor mole fraction | 0 | 0 | 0 | 0.948 | 0 | 0 |
| Total flow (kg/h) | 9150.6 | 1625.5 | 14,861.5 | 13,236 | 10,948.4 | 2287.6 |
| Total flow (kmol/h) | 285.6 | 50.8 | 285.6 | 234.9 | 215.5 | 19.4 |
| Component flow rates (kmol/h) | ||||||
| O2 | 0 | 0 | 0 | 0 | 0 | 0 |
| N2 | 0 | 0 | 0 | 0 | 0 | 0 |
| H2O | 0.1 | 0.1 | 117.5 | 117.4 | 117.4 | 0 |
| CO | 0 | 0 | 0 | 0 | 0 | 0 |
| DMC | 0 | 0 | 98 | 98 | 98 | 0 |
| DMO | 0 | 0 | 19.4 | 19.4 | 0 | 19.4 |
| MeOH | 285.5 | 50.7 | 50.7 | 0 | 0 | 0 |
| Stream No. | 21 | 22 | 24 | 27 | 29 | |
| Temperature (°C) | 81.9 | 201.1 | 30 | 170.9 | 30 | |
| Pressure (bar) | 19 | 19 | 1.5 | 19 | 1.5 | |
| Vapor mole fraction | 0 | 0 | 0 | 0 | 0 | |
| Total flow (kg/h) | 16,895.4 | 8065.5 | 8829.8 | 5949.2 | 2116.3 | |
| Total flow (kmol/h) | 348 | 249.8 | 98.2 | 132.6 | 117.3 | |
| Component flow rates (kmol/h) | ||||||
| O2 | 0 | 0 | 0 | 0 | 0 | |
| N2 | 0 | 0 | 0 | 0 | 0 | |
| H2O | 195.6 | 195.4 | 0.2 | 78.2 | 117.3 | |
| CO | 0 | 0 | 0 | 0 | 0 | |
| DMC | 146.2 | 48.3 | 98 | 48.2 | 0 | |
| DMO | 0 | 0 | 0 | 0 | 0 | |
| MeOH | 6.2 | 6.2 | 0 | 6.2 | 0 | |
| Components | Variables | Specifications |
|---|---|---|
| Reactor (R-101) | Type | FBR |
| Volume | 1.3 m3 | |
| Length | 1.5 m | |
| Diameter | 0.53 m | |
| Distillation column (T-101) | Reflux ratio | 4.709 |
| Number of stages | 44 | |
| Condenser duty | −7508 MJ/h | |
| Column diameter | 1.18 m | |
| Distillation column (T-102) | Reflux ratio | 0.4794 |
| Number of stages | 28 | |
| Condenser duty | −9326 MJ/h | |
| Column diameter | 0.82 m | |
| Distillation column (T-103) | Reflux ratio | 6.18 |
| Number of stages | 73 | |
| Condenser duty | −57,863 MJ/h | |
| Column diameter | 0.95 m | |
| Distillation column (T-104) | Reflux ratio | 0.52 |
| Number of stages | 24 | |
| Condenser duty | −6684 MJ/h | |
| Column diameter | 0.6 m |
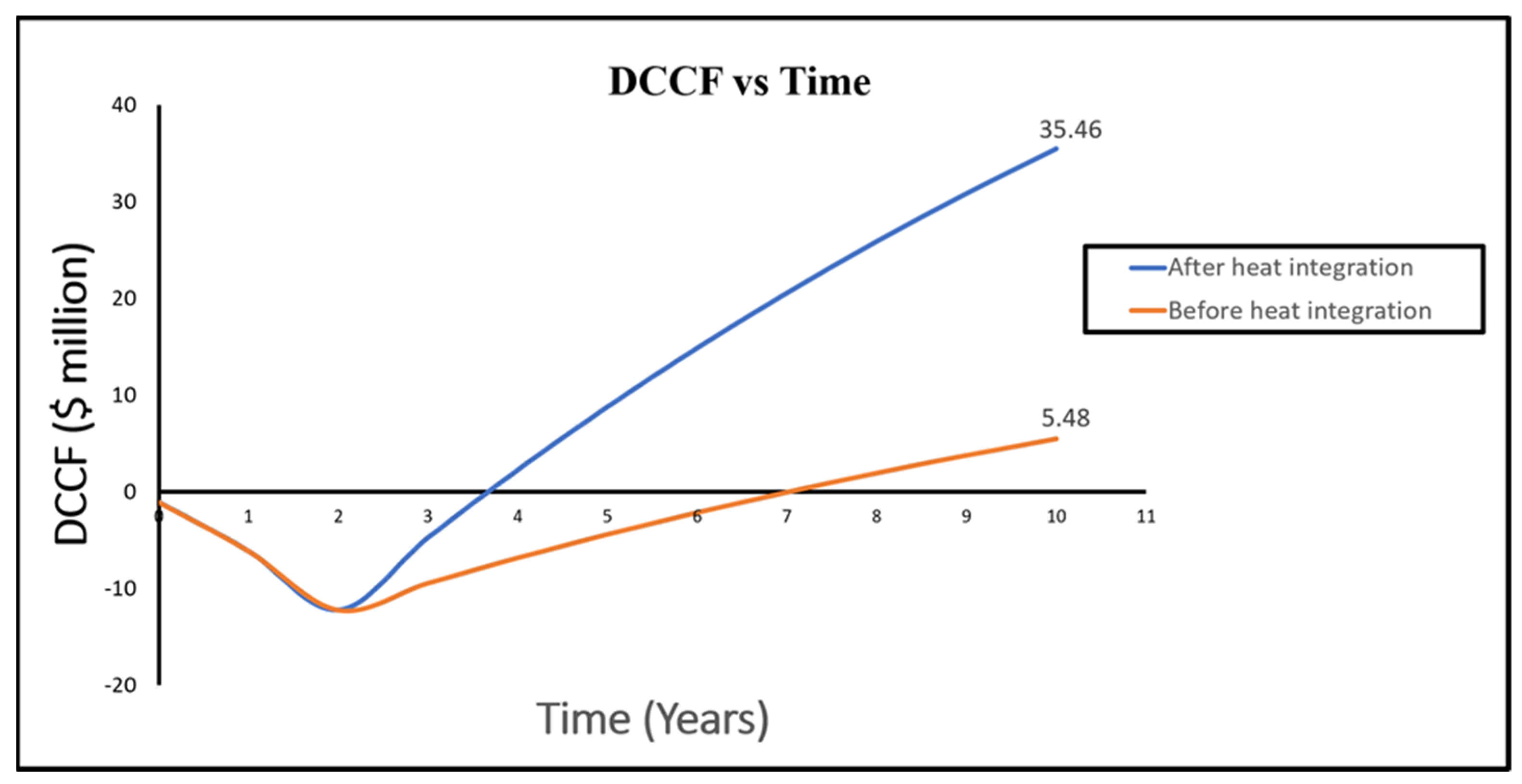
| Index | Before Process Integration | After Process Integration |
|---|---|---|
| NPV | USD 5.48 million | USD 35.46 million |
| Payback period | 7 years | 3.7 years |
| DCFROR | 17% | 56.4% |
| PVR | 1.4 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Rabiah, A.A.; Almutlaq, A.M.; Bashth, O.S.; Alyasser, T.M.; Alshehri, F.A.; Alofai, M.S.; Alshehri, A.S. An Intensified Green Process for the Coproduction of DMC and DMO by the Oxidative Carbonylation of Methanol. Processes 2022, 10, 2094. https://doi.org/10.3390/pr10102094
Al-Rabiah AA, Almutlaq AM, Bashth OS, Alyasser TM, Alshehri FA, Alofai MS, Alshehri AS. An Intensified Green Process for the Coproduction of DMC and DMO by the Oxidative Carbonylation of Methanol. Processes. 2022; 10(10):2094. https://doi.org/10.3390/pr10102094
Chicago/Turabian StyleAl-Rabiah, Abdulrahman A., Abdulaziz M. Almutlaq, Omar S. Bashth, Taher M. Alyasser, Fayez A. Alshehri, Mohammed S. Alofai, and Abdulelah S. Alshehri. 2022. "An Intensified Green Process for the Coproduction of DMC and DMO by the Oxidative Carbonylation of Methanol" Processes 10, no. 10: 2094. https://doi.org/10.3390/pr10102094
APA StyleAl-Rabiah, A. A., Almutlaq, A. M., Bashth, O. S., Alyasser, T. M., Alshehri, F. A., Alofai, M. S., & Alshehri, A. S. (2022). An Intensified Green Process for the Coproduction of DMC and DMO by the Oxidative Carbonylation of Methanol. Processes, 10(10), 2094. https://doi.org/10.3390/pr10102094






