Effect of Operational Variables on the Yield of Chemoenzymatic Oxidation of 2,5-Furandicarboxaldehyde to 2,5-Furandicarboxylic Acid in Fed-Batch and Continuous Packed-Bed Millibioreactor
Abstract
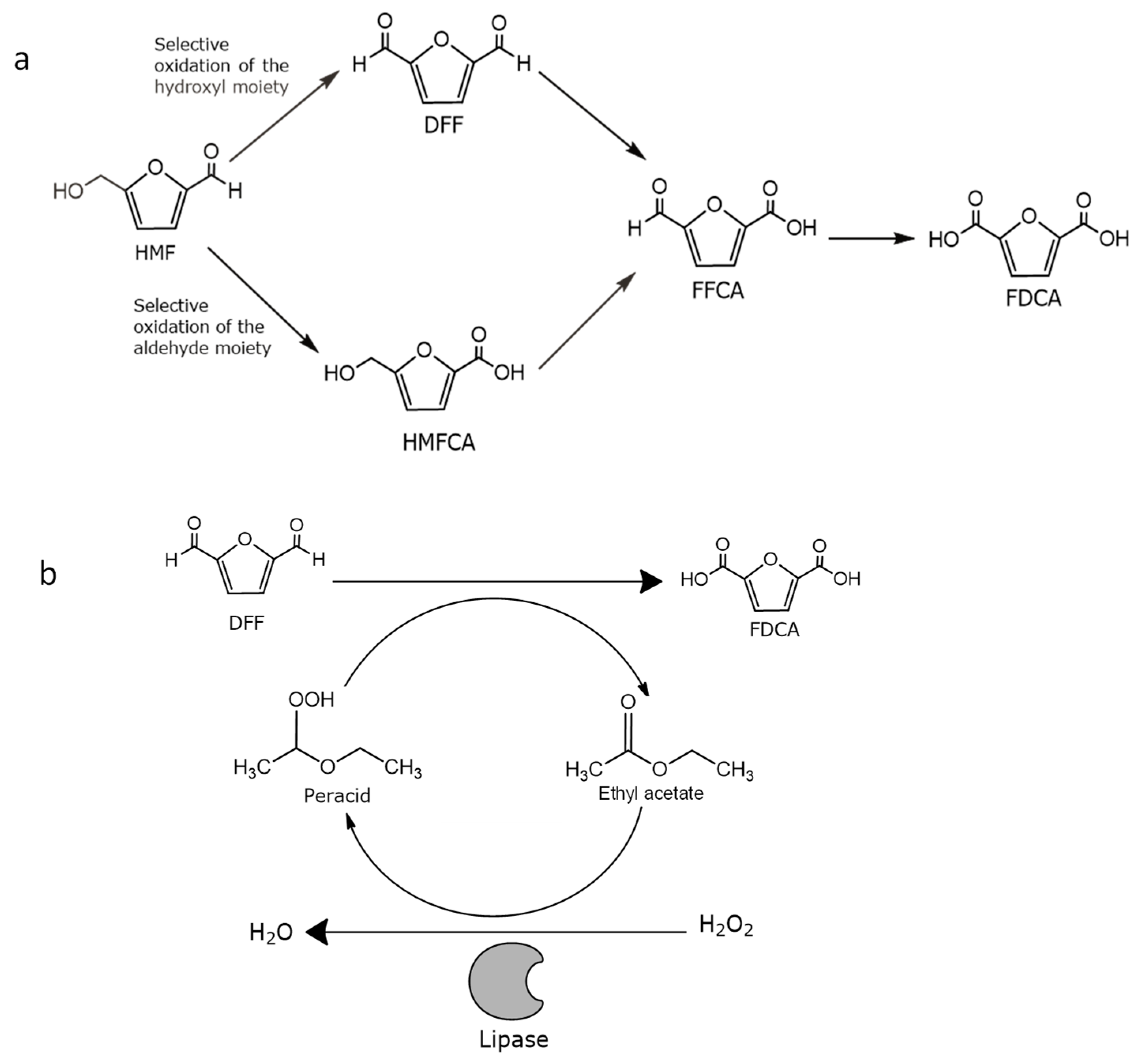
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Analytical Method for the Quantification of FDCA
2.3. Chemoenzymatic Synthesis of FDCA in a Batch Bioreactor
2.4. Chemoenzymatic Synthesis of FDCA in Fed-Batch Bioreactor
2.5. Chemoenzymatic Synthesis of FDCA in a Continuous Packed-Bed Millibioreactor
2.6. Operational Stability of the Biocatalyst
3. Results and Discussion
3.1. Chemoenzymatic Synthesis of FDCA in a Batch Reactor
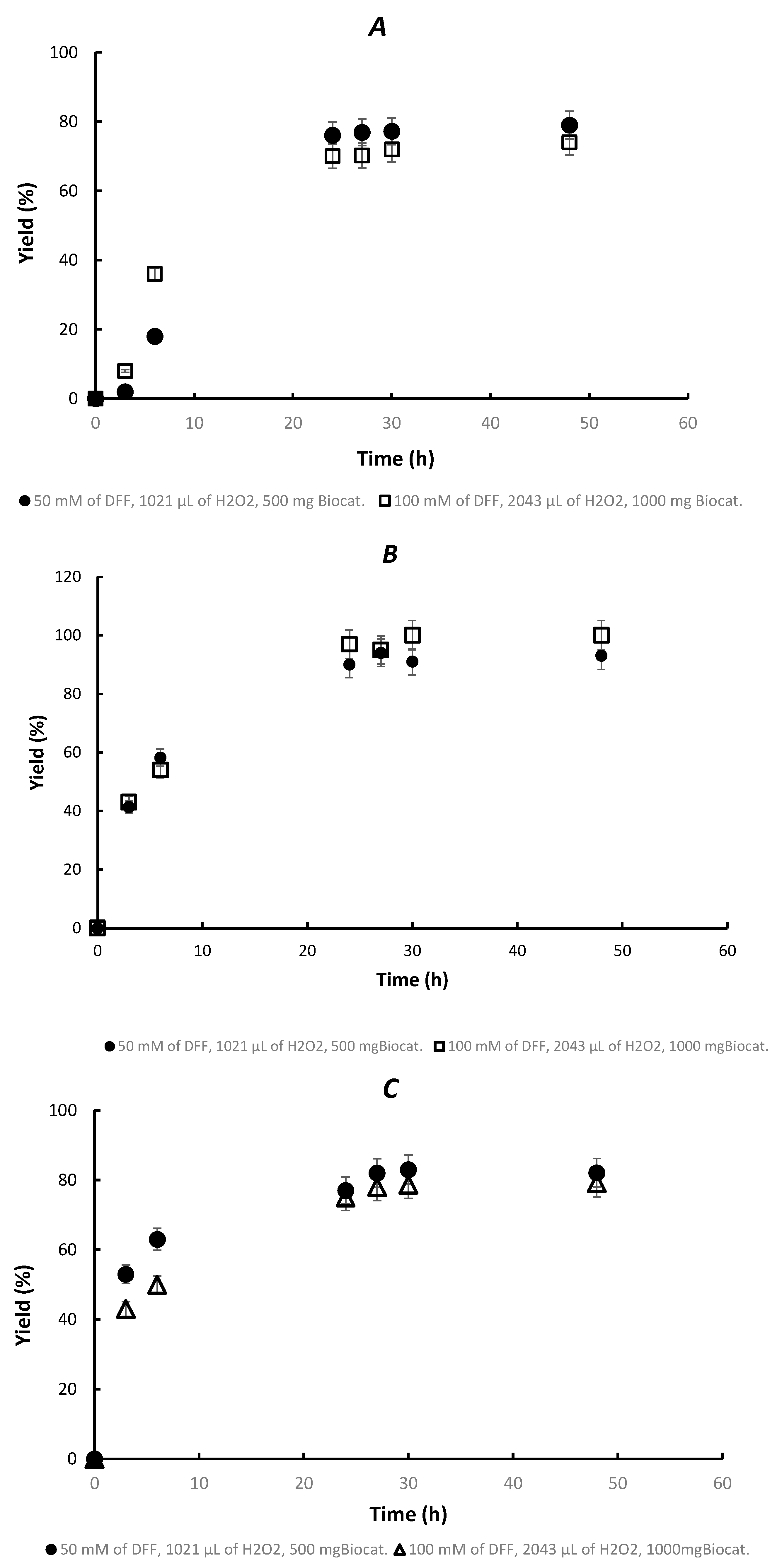
3.2. Chemoenzymatic Synthesis of FDCA in Fed-Batch Bioreactor
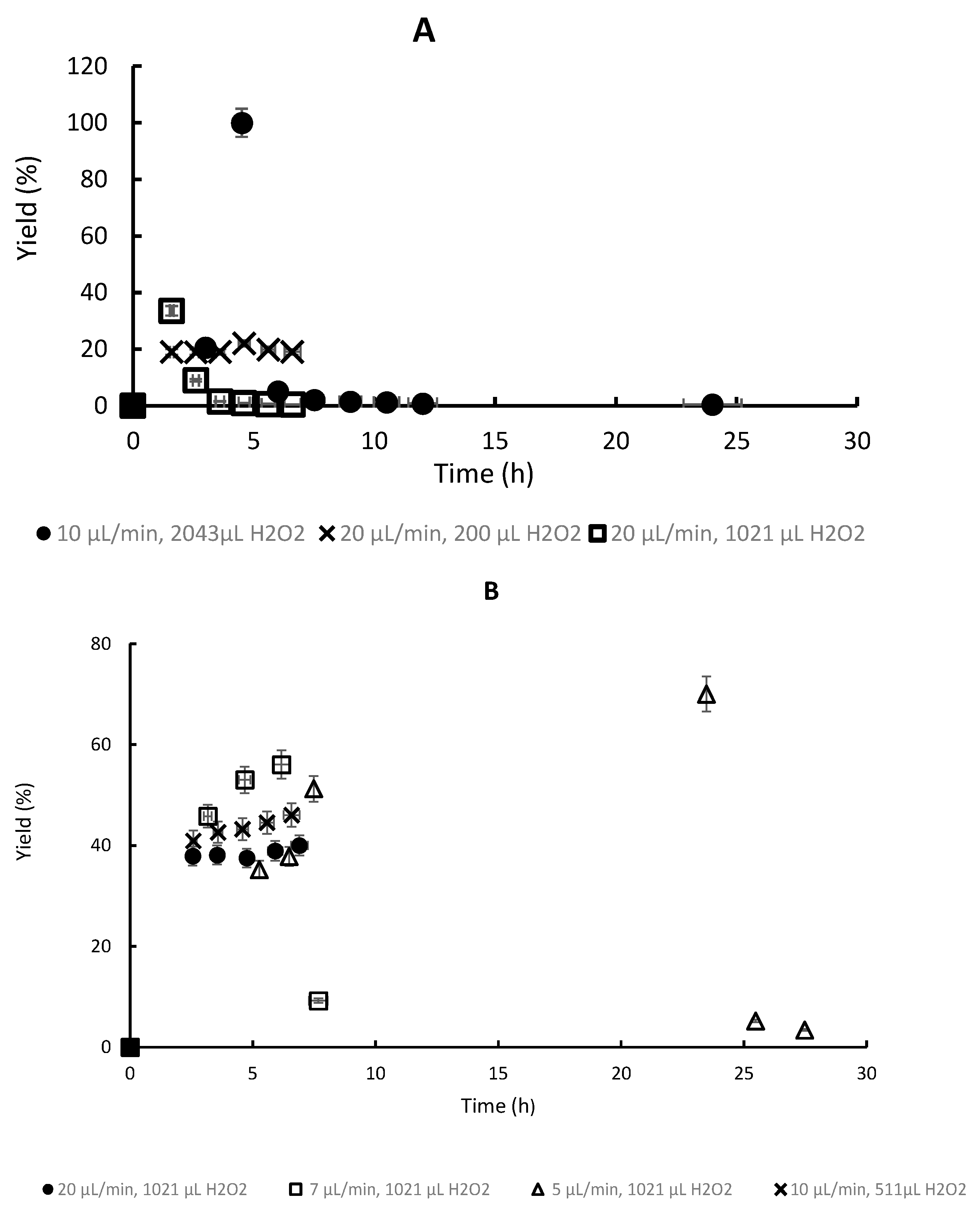
3.3. Chemoenzymatic Synthesis of FDCA in a Continuous Packed-Bed (CPBR) Bioreactor
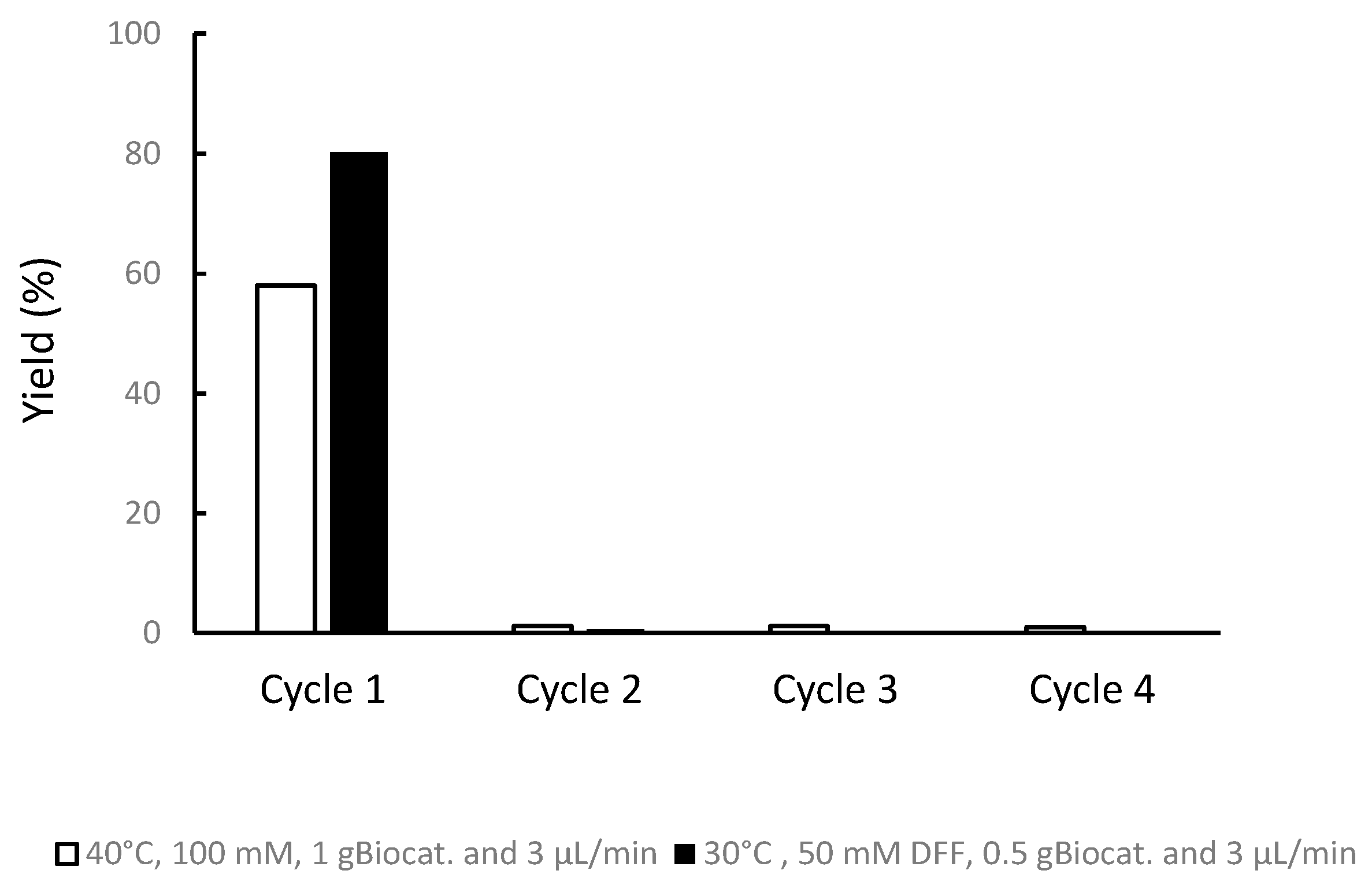
3.4. Operationalss Stability of the Biocatalyst in a Fed-Batch Bioreactor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Domínguez de María, P. Industrial Biorenewables: A Practical Viewpoint; Wiley: Hoboken, NJ, USA, 2016; ISBN 978-1-118-84372-7. [Google Scholar]
- Dong, C.; Wang, Y.; Wang, H.; Lin, C.S.K.; Hsu, H.Y.; Leu, S.Y. New Generation Urban Biorefinery toward Complete Utilization of Waste Derived Lignocellulosic Biomass for Biofuels and Value-Added Products. Energy Procedia 2019, 158, 918–925. [Google Scholar] [CrossRef]
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; da Silva, S.S. The Path Forward for Lignocellulose Biorefineries: Bottlenecks, Solutions, and Perspective on Commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Loizidou, M.; Moustakas, K.; Rehan, M.; Nizami, A.S.; Tabatabaei, M. New Developments in Sustainable Waste-to-Energy Systems. Renew. Sustain. Energy Rev. 2021, 151, 111581. [Google Scholar] [CrossRef]
- Hou, Q.; Qi, X.; Zhen, M.; Qian, H.; Nie, Y.; Bai, C.; Zhang, S.; Bai, X.; Ju, M. Biorefinery Roadmap Based on Catalytic Production and Upgrading 5-Hydroxymethylfurfural. Green Chem. 2021, 23, 119–231. [Google Scholar] [CrossRef]
- Galkin, K.I.; Ananikov, V.P. When Will 5-Hydroxymethylfurfural, the “Sleeping Giant” of Sustainable Chemistry, Awaken? ChemSusChem 2019, 12, 2976–2982. [Google Scholar] [CrossRef] [PubMed]
- Tacacima, J.; Derenzo, S.; Poco, J.G.R. Synthesis of HMF from Fructose Using Purolite® Strong Acid Catalyst: Comparison between BTR and PBR Reactor Type for Kinetics Data Acquisition. Mol. Catal. 2018, 458, 180–188. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtárová, K. Terephthalic Acid from Waste PET: An Efficient and Reusable Catalyst for Xylose Conversion into Furfural. Catal. Today 2019, 324, 27–32. [Google Scholar] [CrossRef]
- Lange, J.P.; Van Der Heide, E.; Van Buijtenen, J.; Price, R. Furfural-A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.; Li, X.; Liu, X.; Fan, J.; Clark, J.H.; Hu, C. The Production of Furfural Directly from Hemicellulose in Lignocellulosic Biomass: A Review. Catal. Today 2019, 319, 14–24. [Google Scholar] [CrossRef]
- Van Putten, R.J.; Van Der Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, a Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Moreau, C.; Belgacem, M.N.; Gandini, A. Recent Catalytic Advances in the Chemistry of Substituted Furans from Carbohydrates and in the Ensuing Polymers. Top. Catal. 2004, 27, 11–30. [Google Scholar] [CrossRef]
- Tang, X.; Wei, J.; Ding, N.; Sun, Y.; Zeng, X.; Hu, L.; Liu, S.; Lei, T.; Lin, L. Chemoselective Hydrogenation of Biomass Derived 5-Hydroxymethylfurfural to Diols: Key Intermediates for Sustainable Chemicals, Materials and Fuels. Renew. Sustain. Energy Rev. 2017, 77, 287–296. [Google Scholar] [CrossRef]
- Dijkman, W.P.; Groothuis, D.E.; Fraaije, M.W. Enzyme-Catalyzed Oxidation of 5-Hydroxymethylfurfural to Furan-2,5-Dicarboxylic Acid. Angew. Chem. Int. Ed. 2014, 53, 6515–6518. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xu, H.; Liu, Y. Aerobic Oxidation of 5-hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over Co/Mn-Lignin Coordination Complexes-Derived Catalysts. Appl. Catal. B: Environ. 2019, 244, 965–973. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased Polyesters and Other Polymers from 2,5-Furandicarboxylic Acid: A Tribute to Furan Excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Weidener, D.; Klose, H.; Leitner, W.; Schurr, U.; Usadel, B.; de María, P.D.; Grande, P.M. One-Step Lignocellulose Fractionation by Using 2,5-Furandicarboxylic Acid as a Biogenic and Recyclable Catalyst. ChemSusChem 2018, 11, 2051–2056. [Google Scholar] [CrossRef]
- Kong, Q.S.; Li, X.L.; Xu, H.J.; Fu, Y. Conversion of 5-Hydroxymethylfurfural to Chemicals: A Review of Catalytic Routes and Product Applications. Fuel Process. Technol. 2020, 209, 106528. [Google Scholar] [CrossRef]
- Zhong, X.; Wei, Y.; Sadjadi, S.; Liu, D.; Li, M.; Yu, T.; Zhuang, G.; Yuan, P. Base-Free Oxidation of 5-Hydroxymethylfurfural to 2, 5-Furandicarboxylic Acid over Palygorskite-Supported Bimetallic Pt–Pd Catalyst. Appl. Clay Sci. 2022, 226, 106574. [Google Scholar] [CrossRef]
- Tuercke, T.; Panic, S.; Loebbecke, S. Microreactor Process for the Optimized Synthesis of 5-Hydroxymethylfurfural: A Promising Building Block Obtained by Catalytic Dehydration of Fructose. Chem. Eng. Technol. 2009, 32, 1815–1822. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Dumesic, J.A. Solvent Effects on Fructose Dehydration to 5-Hydroxymethylfurfural in Biphasic Systems Saturated with Inorganic Salts. Top. Catal. 2009, 52, 297–303. [Google Scholar] [CrossRef]
- Chheda, J.N.; Dumesic, J. a Production of Hydroxymethylfurfural from Fructose. Science 2006, 312, 1933. [Google Scholar]
- Al Ghatta, A.; Wilton-Ely, J.D.E.T.; Hallett, J.P. From Sugars to FDCA: A Techno-Economic Assessment Using a Design Concept Based on Solvent Selection and Carbon Dioxide Emissions. Green Chem. 2021, 23, 1716–1733. [Google Scholar] [CrossRef]
- Besson, M.; Essayem, N.; RASS, H. Method for Preparing 2,5-Furandicarboxylic Acid. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=WO2014122319 (accessed on 13 October 2022).
- Cai, J.; Ma, H.; Zhang, J.; Song, Q.; Du, Z.; Huang, Y.; Xu, J. Gold Nanoclusters Confined in a Supercage of Y Zeolite for Aerobic Oxidation of HMF under Mild Conditions. Chem. - A Eur. J. 2013, 19, 14215–14223. [Google Scholar] [CrossRef]
- Siankevich, S.; Mozzettini, S.; Bobbink, F.; Ding, S.; Fei, Z.; Yan, N.; Dyson, P.J. Influence of the Anion on the Oxidation of 5-Hydroxymethylfurfural by Using Ionic-Polymer-Supported Platinum Nanoparticle Catalysts. ChemPlusChem 2018, 83, 19–23. [Google Scholar] [CrossRef]
- Kumar, H.; Fraaije, M.W. Conversion of Furans by Baeyer-Villiger Monooxygenases. Catalysts 2017, 7, 179. [Google Scholar] [CrossRef] [Green Version]
- Carro, J.; Ferreira, P.; Rodríguez, L.; Prieto, A.; Serrano, A.; Balcells, B.; Ardá, A.; Jiménez-Barbero, J.; Gutiérrez, A.; Ullrich, R.; et al. 5-Hydroxymethylfurfural Conversion By Fungal Aryl-Alcohol Oxidase and Unspecific Peroxygenase. FEBS J. 2015, 282, 3218–3229. [Google Scholar] [CrossRef] [Green Version]
- Krystof, M.; Pérez-Sánchez, M.; Domínguez de María, P. Lipase-Mediated Selective Oxidation of Furfural and 5-Hydroxymethylfurfural. ChemSusChem 2013, 6, 826–830. [Google Scholar] [CrossRef]
- Qin, Y.Z.; Zong, M.H.; Lou, W.Y.; Li, N. Biocatalytic Upgrading of 5-Hydroxymethylfurfural (HMF) with Levulinic Acid to HMF Levulinate in Biomass-Derived Solvents. ACS Sustain. Chem. Eng. 2016, 4, 4050–4054. [Google Scholar] [CrossRef]
- Van Deurzen, M.P.J.; Van Rantwijk, F.; Sheldon, R.A. Selective Oxidations Catalyzed by Peroxidases. Tetrahedron 1997, 53, 13183–13220. [Google Scholar] [CrossRef]
- Yuan, H.; Li, J.; Shin, H.D.; Du, G.; Chen, J.; Shi, Z.; Liu, L. Improved Production of 2,5-Furandicarboxylic Acid by Overexpression of 5-Hydroxymethylfurfural Oxidase and 5-Hydroxymethylfurfural/Furfural Oxidoreductase in Raoultella Ornithinolytica BF60. Bioresour. Technol. 2018, 247, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Koopman, F.; Wierckx, N.; de Winde, J.H.; Ruijssenaars, H.J. Efficient Whole-Cell Biotransformation of 5-(Hydroxymethyl)Furfural into FDCA, 2,5-Furandicarboxylic Acid. Bioresource Technol. 2010, 101, 6291–6296. [Google Scholar] [CrossRef]
- Decarpigny, C.; Ponchel, A.; Monflier, E.; Bleta, R. Effect of Functional Group on the Catalytic Activity of Lipase B from Candida Antarctica Immobilized in a Silica-Reinforced Pluronic F127/α-Cyclodextrin Hydrogel. Gels 2022, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.Z.; Li, Y.M.; Zong, M.H.; Wu, H.; Li, N. Enzyme-Catalyzed Selective Oxidation of 5-Hydroxymethylfurfural (HMF) and Separation of HMF and 2,5-Diformylfuran Using Deep Eutectic Solvents. Green Chem. 2015, 17, 3718–3722. [Google Scholar] [CrossRef]
- Törnvall, U.; Orellana-Coca, C.; Hatti-Kaul, R.; Adlercreutz, D. Stability of Immobilized Candida Antarctica Lipase B during Chemo-Enzymatic Epoxidation of Fatty Acids. Enzym. Microb. Technol. 2007, 40, 447–451. [Google Scholar] [CrossRef]
- Chávez, G.; Hatti-Kaul, R.; Sheldon, R.A.; Mamo, G. Baeyer-Villiger Oxidation with Peracid Generated in Situ by CaLB-CLEA Catalyzed Perhydrolysis. J. Mol. Catal. B: Enzym. 2013, 89, 67–72. [Google Scholar] [CrossRef]
- Guajardo, N.; Domínguez de María, P.; Canales, R. Integrating Biocatalysis with Viscous Deep Eutectic Solvents in Lab-On-A-Chip Microreactors. ChemSusChem 2022, 15, e202102674. [Google Scholar] [CrossRef]
- Guajardo, N.; Ahumada, K.; Domínguez de María, P. Immobilization of Pseudomonas Stutzeri Lipase through Cross-Linking Aggregates (CLEA) for Reactions in Deep Eutectic Solvents. J. Biotechnol. 2021, 337, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, N.; Schrebler, R.A.; Domínguez de María, P. From Batch to Fed-Batch and to Continuous Packed-Bed Reactors: Lipase-Catalyzed Esterifications in Low Viscous Deep-Eutectic-Solvents with Buffer as Cosolvent. Bioresour. Technol. 2019, 273, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, N.; Domínguez de María, H.P.; Ahumada, K.; Schrebler, R.A.; Ramírez-Tagle, R.; Crespo, F.A.; Carlesi, C. Water as Cosolvent: Nonviscous Deep Eutectic Solvents for Efficient Lipase-Catalyzed Esterifications. ChemCatChem 2017, 9, 1393–1396. [Google Scholar] [CrossRef]
- Guajardo, N.; Domínguez de María, P. Continuous Biocatalysis in Environmentally-Friendly Media: A Triple Synergy for Future Sustainable Processes. ChemCatChem 2019, 11, 3128–3137. [Google Scholar] [CrossRef]
- Benyahia, F.; O’Neill, K.E. Enhanced Voidage Correlations for Packed Beds of Various Particle Shapes and Sizes. Part. Sci. Technol. 2005, 23, 169–177. [Google Scholar] [CrossRef]




| Reaction Conditions | Range |
|---|---|
| Concentration of DFF (mM) | 10–50 mM |
| Biocatalyst loading (mg)/Activity (U) | 100–835 mg/20–167 U |
| Amount of H2O2 30% (µL) | 120–1021 µL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balboa, C.; Schrebler, R.A.; Lienqueo, M.E.; Guajardo, N. Effect of Operational Variables on the Yield of Chemoenzymatic Oxidation of 2,5-Furandicarboxaldehyde to 2,5-Furandicarboxylic Acid in Fed-Batch and Continuous Packed-Bed Millibioreactor. Processes 2022, 10, 2095. https://doi.org/10.3390/pr10102095
Balboa C, Schrebler RA, Lienqueo ME, Guajardo N. Effect of Operational Variables on the Yield of Chemoenzymatic Oxidation of 2,5-Furandicarboxaldehyde to 2,5-Furandicarboxylic Acid in Fed-Batch and Continuous Packed-Bed Millibioreactor. Processes. 2022; 10(10):2095. https://doi.org/10.3390/pr10102095
Chicago/Turabian StyleBalboa, Cristian, Rodrigo A. Schrebler, María Elena Lienqueo, and Nadia Guajardo. 2022. "Effect of Operational Variables on the Yield of Chemoenzymatic Oxidation of 2,5-Furandicarboxaldehyde to 2,5-Furandicarboxylic Acid in Fed-Batch and Continuous Packed-Bed Millibioreactor" Processes 10, no. 10: 2095. https://doi.org/10.3390/pr10102095