Vapor–Liquid Equilibrium in Binary and Ternary Azeotropic Solutions Acetonitrile-Ethanol-Water with the Addition of Amino Esters of Boric Acid
Abstract
:1. Introduction
2. Experimental Part
2.1. Synthesis of Amino Esters of Boric Acid
Materials
2.2. Experimental Methods for Studying Phase Equilibrium
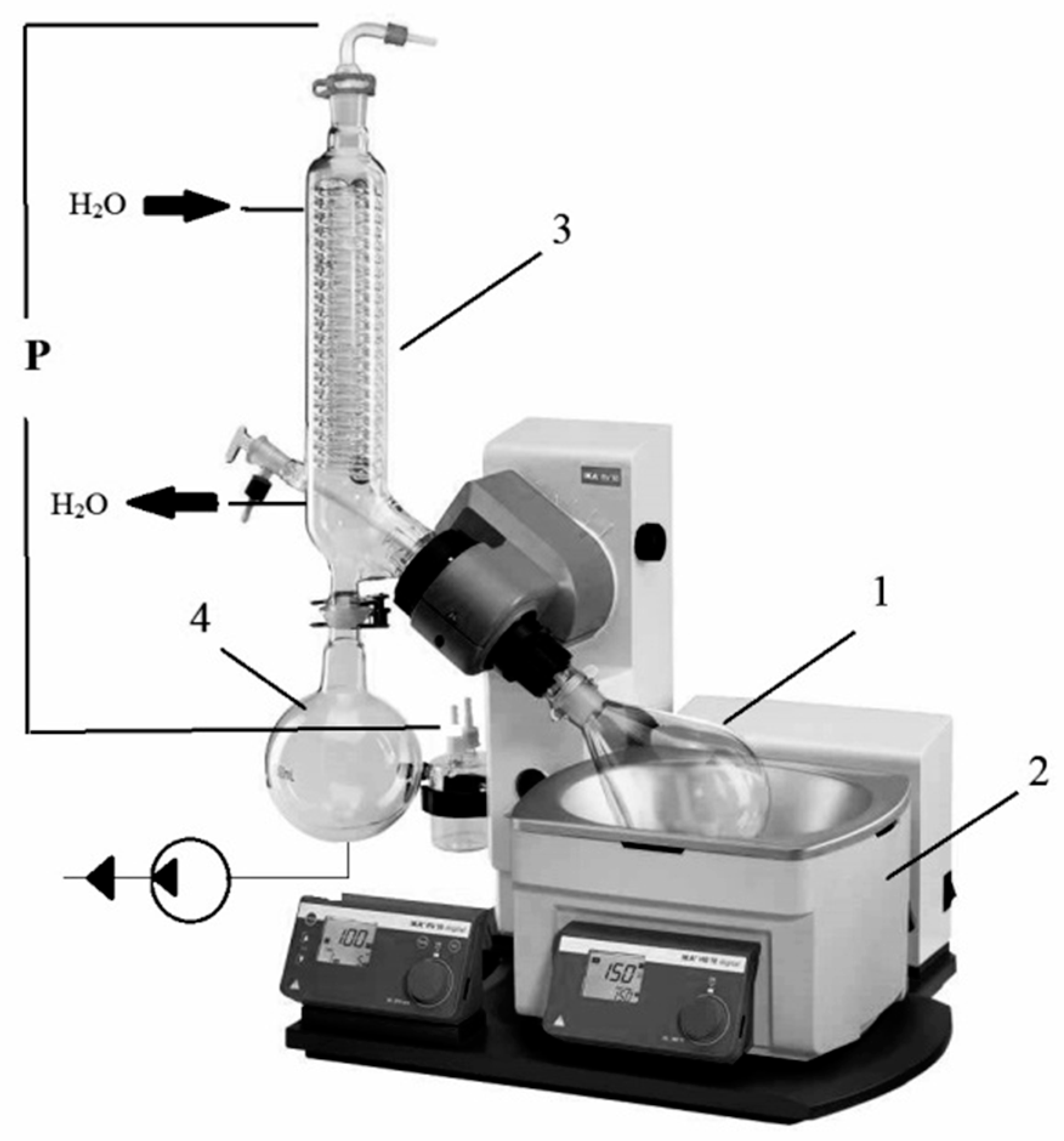
2.2.1. Method of Open Evaporation
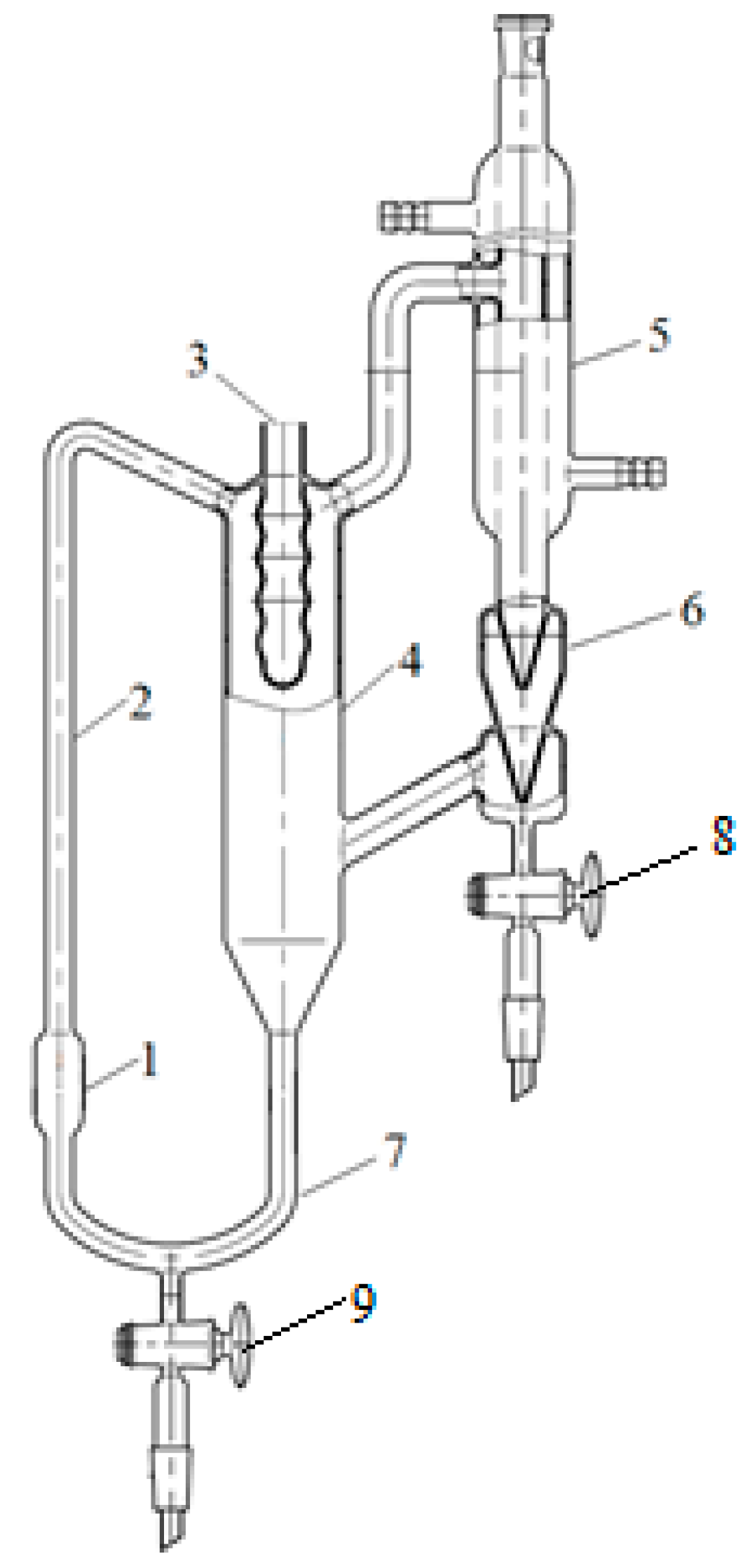
2.2.2. Study of Phase Equilibrium on the Swietoslavsky Ebulliometer
2.2.3. Simulation of Vapor–Liquid Phase Equilibrium Conditions
2.3. Methods and Equipment for Measuring Compositions
3. Results and Discussion
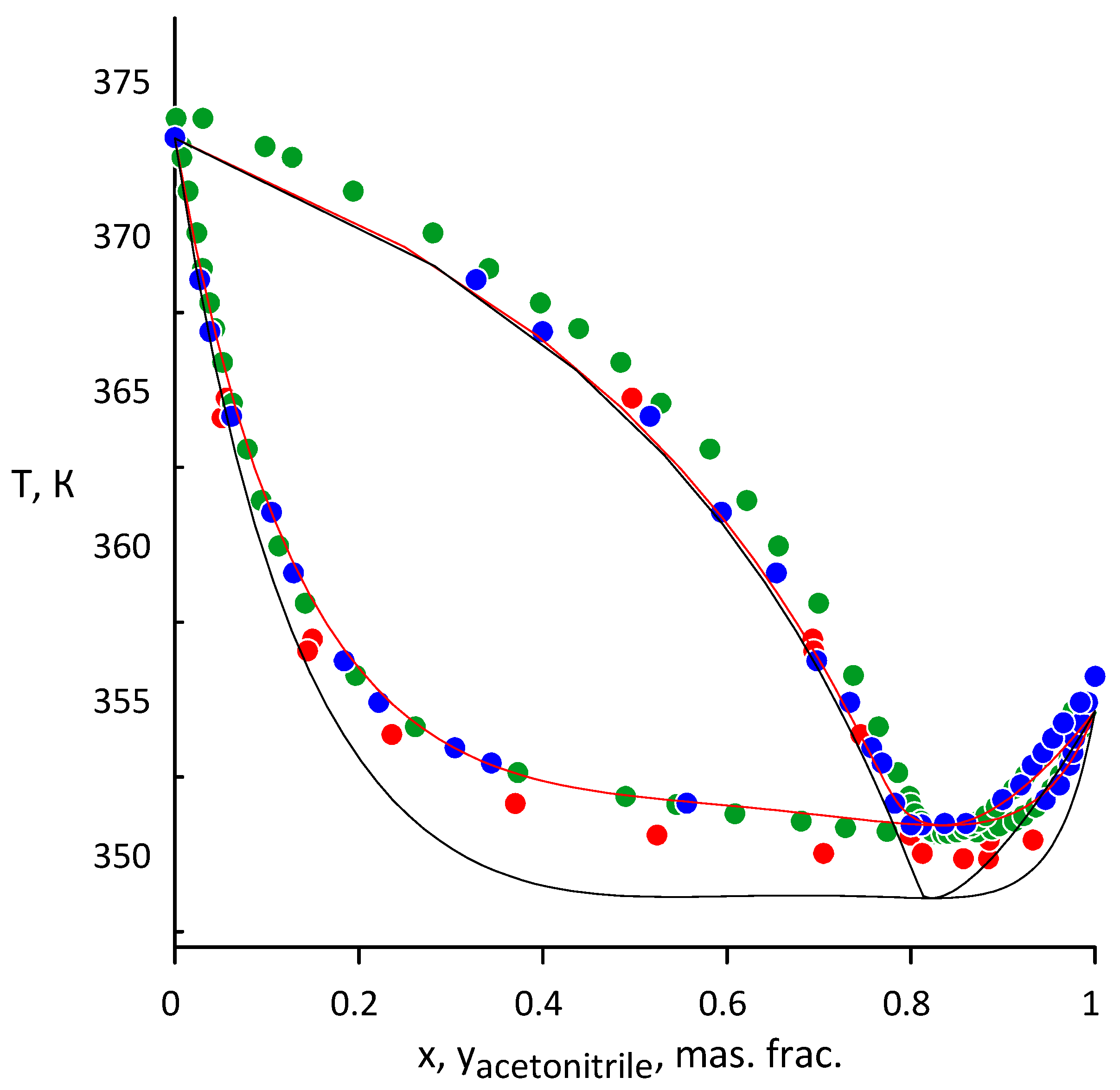
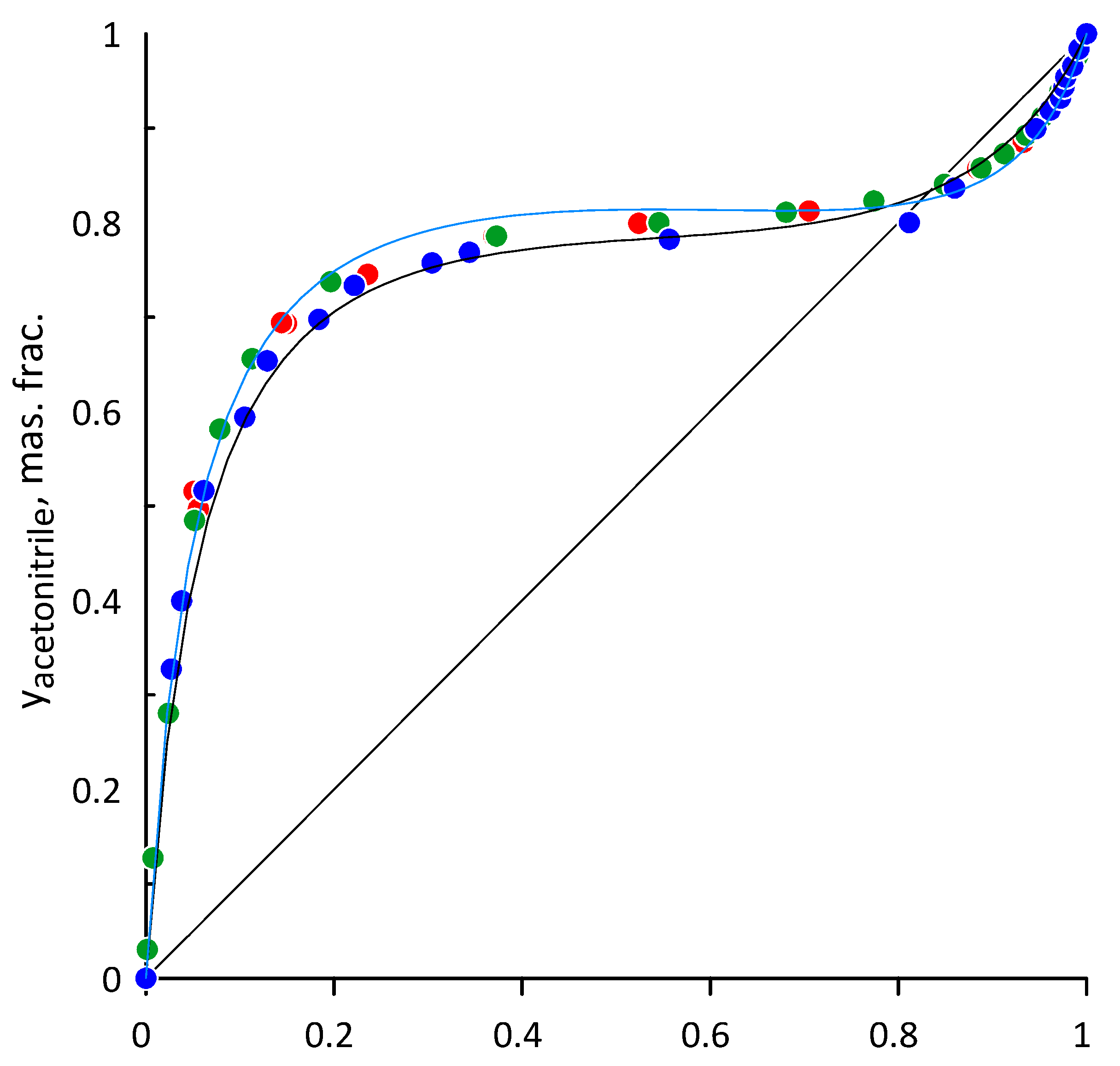
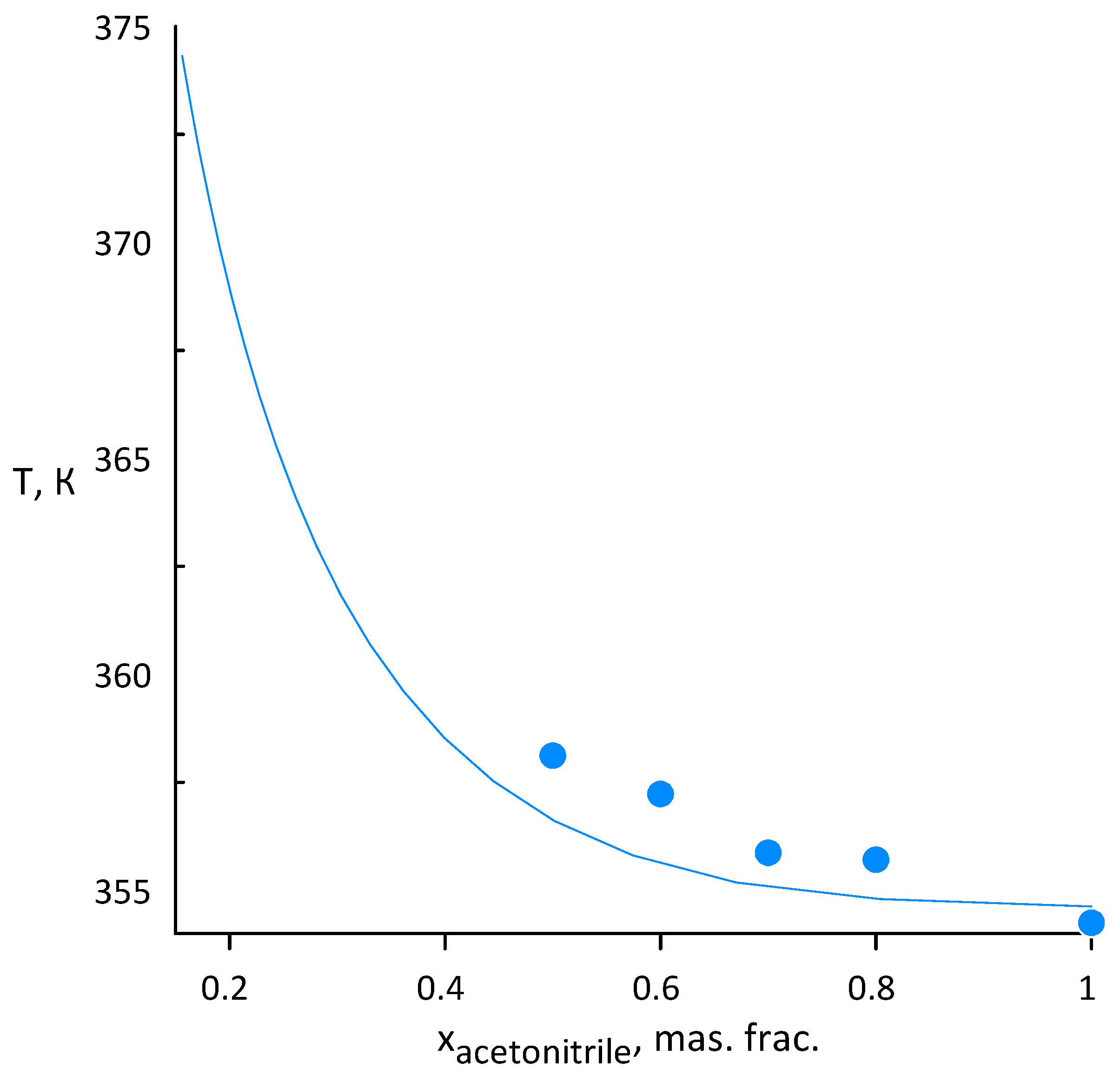
3.1. Acetonitrile–Water
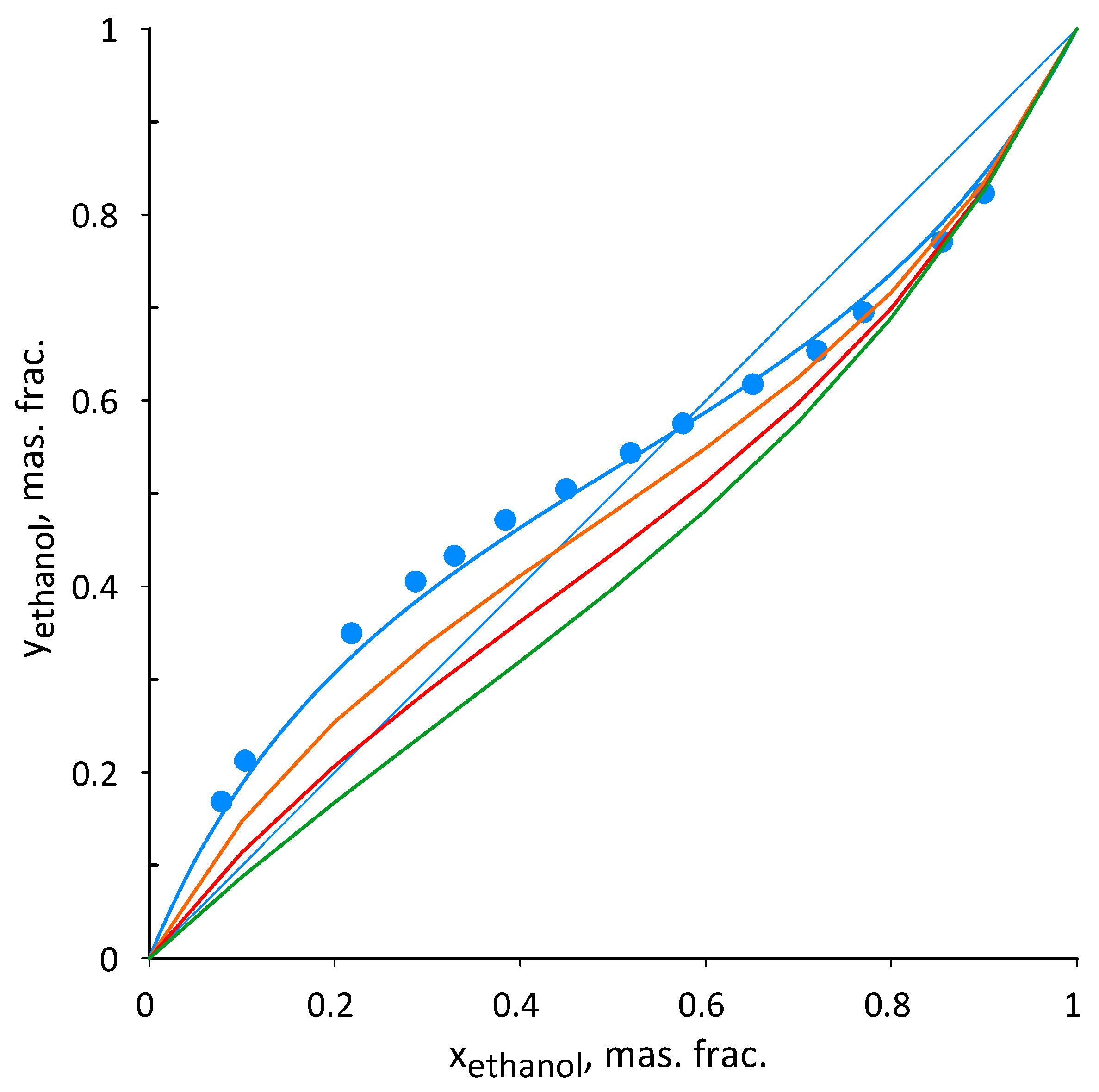
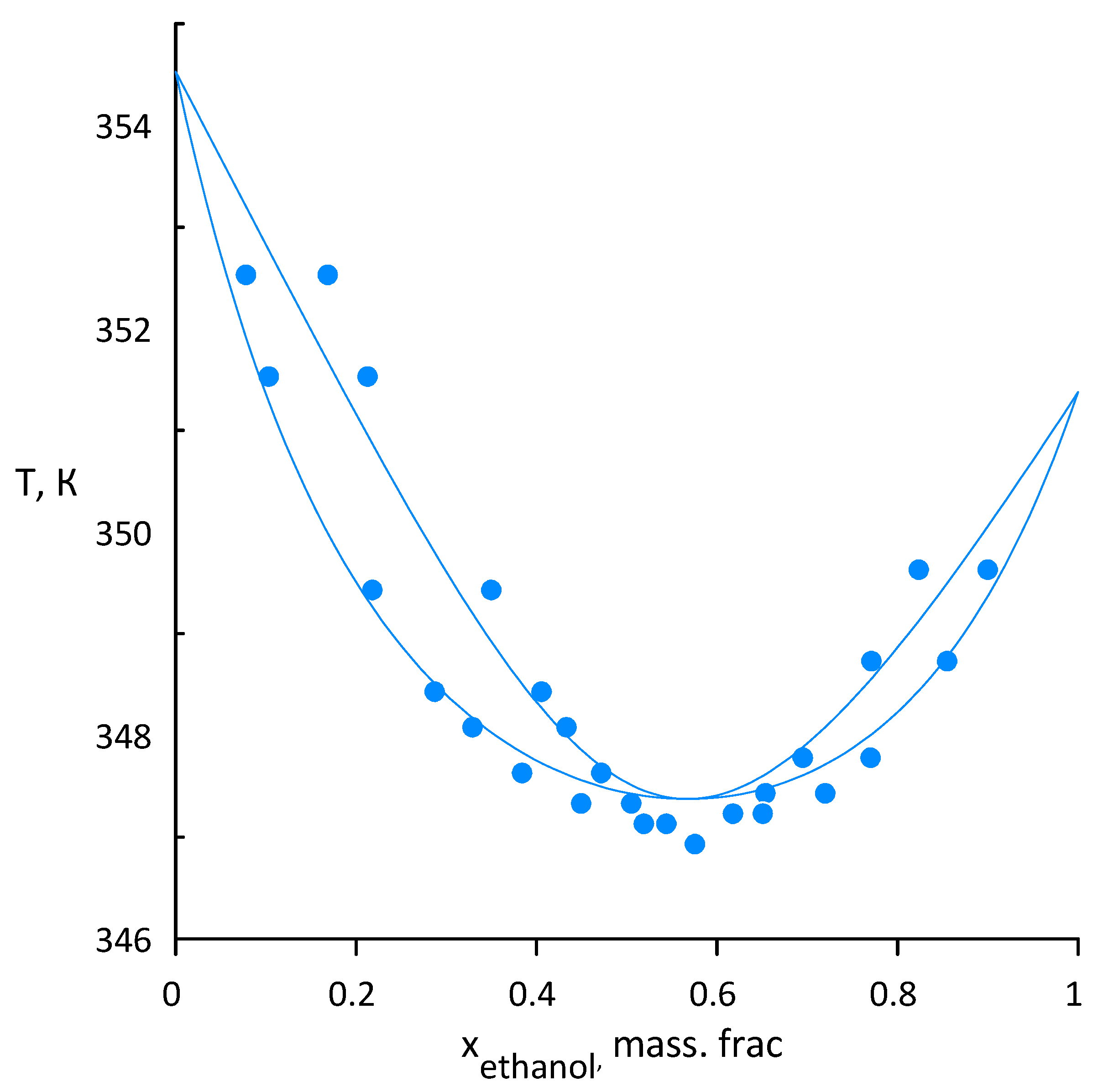
3.2. Ethanol–Acetonitrile
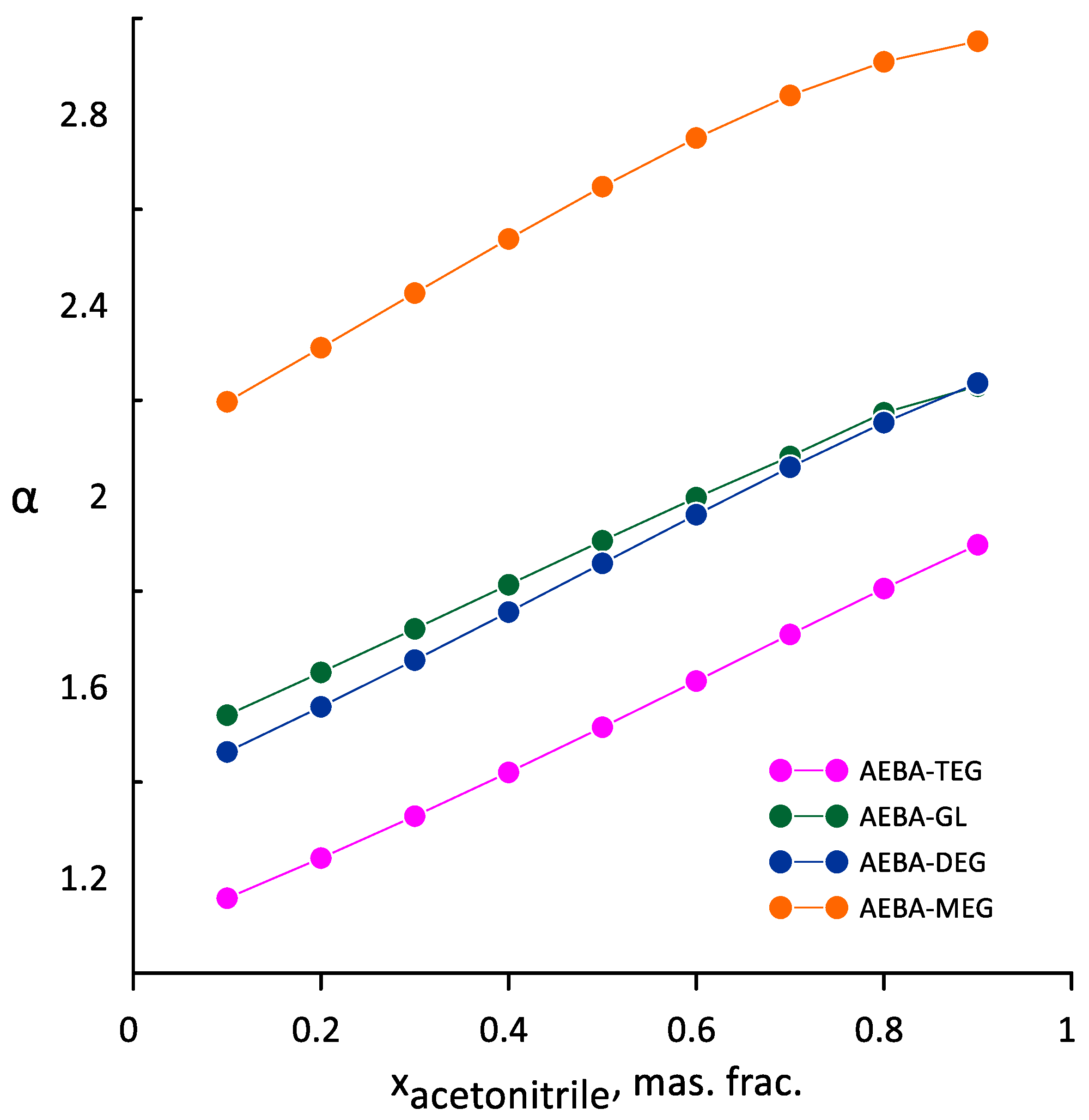
3.3. Ethanol-Acetonitrile-Water
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, K.X. Handbook of Fine Organic Chemical Raw Materials and Intermediates, 2nd ed.; Chemical Industry Press: Beijing, China, 2002. [Google Scholar]
- Mahdi, T.; Ahmad, A.; Nasef, M.M.; Ripin, A. State-of-the-Art Technologies for Separation of Azeotropic Mixtures. Sep. Purif. Rev. 2014, 44, 308–330. [Google Scholar] [CrossRef]
- Bajpai, P. Developments in Bioethanol. In Green Energy and Technology; Springer Nature Singapore Pte Ltd.: Kanpur, India, 2021. [Google Scholar] [CrossRef]
- Wang, B.; Feng, H.; Ezeji, T.; Blaschek, H. Sugaring-Out Separation of Acetonitrile from Its Aqueous Solution. Chem. Eng. Technol. 2008, 31, 1869–1874. [Google Scholar] [CrossRef]
- Liang, K.; Li, W.S.; Luo, H.T.; Xia, M.; Xu, C.J. Energy-efficient extractive distillation process by combining preconcentration column and entrainer recovery column. Ind. Eng. Chem. Res. 2014, 53, 7121–7131. [Google Scholar] [CrossRef]
- Keil, B. Laboratorní Technika Organické Chemie; NakladatelstvÌ CeskoslovenskÈ akademie ved: Praha, Czech Republic, 1963. [Google Scholar]
- Wang, Y.; Mei, X.; Ma, T.; Xue, C.; Wu, M.; Ji, M.; Li, Y. Green recovery of hazardous acetonitrile from high-salt chemical wastewater by pervaporation. J. Clean. Prod. 2018, 197, 742–749. [Google Scholar] [CrossRef]
- McConvey, I.F.; Woods, D.; Lewis, M.; Gan, Q.; Nancarrow, P. The Importance of Acetonitrile in the Pharmaceutical Industry and Opportunities for its Recovery from Waste. Org. Process Res. Dev. 2012, 16, 612–624. [Google Scholar] [CrossRef] [Green Version]
- Vane, L.M. Membrane Materials for the Removal of Water from Industrial Solvents by Pervaporation and Vapor Permeation. J. Chem. Technol. Biotechnol. 2018, 94, 343–365. [Google Scholar] [CrossRef] [PubMed]
- Horsley, L.H. Azeotropic Data, III; American Chemical Society: Washington, DC, USA, 1973. [Google Scholar]
- Ogorodnikov, S.; Lesteva, T.; Kogan, V. Azeotropic Mixtures; Khimia: Leningrad, Russia, 1971. [Google Scholar]
- Gmehling, J.; Bolts, R. Azeotropic data for binary and ternary systems at moderate. J. Chem. Eng. Data 1996, 41, 202–209. [Google Scholar] [CrossRef]
- Dean, J.A. Langes Handbook of Chemistry, 15th ed.; McGraw-Hill Book Co. Press. Inc.: New York, NY, USA, 1999. [Google Scholar]
- Sun, S.; Chun, W.; Yang, A.; Shen, W.; Cui, P.; Ren, J. The separation of ternary azeotropic mixture: Thermodynamic insight and improved multi-objective optimization. Energy 2020, 206, 118117. [Google Scholar] [CrossRef]
- Ruiz, R.A.; Borda, N.B.; Alexander, L.R.; Javier, R.; Guevara, L.; Ivan, D.; Gil, C. Control of an azeotropic distillation process to acetonitrile production. Comput. Aided Chem. Eng. 2011, 29, 833–838. [Google Scholar] [CrossRef]
- Chianese, A.; Zinnamosca, F. Ethanol dehydration by azeotropic distillation with mixed solvent entrainer. Chem. Eng. J. 1990, 43, 59–65. [Google Scholar] [CrossRef]
- Gomis, V.; Pedraza, R.; Frances, O.; Font, A.; Asensi, J. Dehydration of ethanol using azeotropic distillation with isooctane. Ind. Eng. Chem. Res. 2007, 46, 4572–4576. [Google Scholar] [CrossRef]
- Repke, J.-U.; Forner, F.; Klein, A. Separation of homogeneous azeotropic mixtures by pressure-swing distillation-analysis of the operation performance. Chem. Eng. Technol. 2005, 28, 1151–1157. [Google Scholar] [CrossRef]
- Sun, S.; Lü, L.; Yang, A.; Wei, S.; Shen, W. Extractive distillation: Advances in conceptual design, solvent selection, and separation strategies. Chin. J. Chem. Eng. 2018, 27, 1247. [Google Scholar] [CrossRef]
- Fu, J. Simulation of Salt-Containing Extractive Distillation for the System of Ethanol/Water/Ethanediol/KAc. 2. Simulation of Salt-Containing Extractive Distillation. Ind. Eng. Chem. Res. 2004, 43, 1279–1283. [Google Scholar] [CrossRef]
- Furter, W.F. Extractive distillation by salt effect. Chem. Eng. Commun. 1992, 116, 35–40. [Google Scholar] [CrossRef]
- Pinto, R.T.P.; Wolf-Maciel, M.R.; Lintomen, L. Saline extractive distillation process for ethanol purification. Comput. Chem. Eng. 2000, 24, 1689–1694. [Google Scholar] [CrossRef]
- Pan, Q.; Shang, X.; Sun, L. Energy-efficient separation process and control scheme for extractive distillation of ethanol-water using deep eutectic solvent. Sep. Purif. Technol. 2019, 219, 113–126. [Google Scholar] [CrossRef]
- Duan, C.; Li, C. Novel energy-saving methods to improve the three-column extractive distillation process for separating ethyl acetate and ethanol using furfural. Sep. Purif. Technol. 2021, 272, 118887. [Google Scholar] [CrossRef]
- Ryzhkin, D.A.; Raeva, V.M. Analysis of energy consumption of technological schemes of extraction distillation of a four-component solvent mixture. ChemChemTech 2021, 64, 47–55. [Google Scholar] [CrossRef]
- Rao, C.V.S.R.; Rao, K.V.; Ravlprasad, A.; Chiranjiv, C. Extraction of acetonitrile from aqueous solutions 1. Ternary liquid equilibria. J. Chem. Eng. Data 1978, 23, 23–25. [Google Scholar] [CrossRef]
- Rao, D.S.; Rao, K.V.; Prasad, A.R.; Chiranjivi, C. Extraction of acetonitrile from aqueous mixtures. 2. Ternary liquid equilibria. J. Chem. Eng. Data 1979, 24, 241–244. [Google Scholar] [CrossRef]
- Mandal, M.K.; Sant, S.B.; Bhattacharya, P.K. Dehydration of aqueous acetonitrile solution by pervaporation using PVA–iron oxide nanocomposite membrane. Colloids Surf. A Physicochem. Eng. Asp. 2011, 373, 11–21. [Google Scholar] [CrossRef]
- Khayet, M.; Cojocaru, C.; Zakrzewska-Trznadel, G. Studies on pervaporation separation of acetone, acetonitrile and ethanol from aqueous solutions. Sep. Purif. Technol. 2008, 63, 303–310. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W. Pervaporation membrane materials: Recent trends and perspectives. J. Membr. Sci. 2021, 636, 119557. [Google Scholar] [CrossRef]
- Ulrich, S.; Pavel, S. Design and operation of a pervaporation plant for ethanol dehydration. J. Membr. Sci. 1988, 36, 463–475. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, L.; Lu, Z.; Deng, J.; Wei, Y. Two-dimensional MXene membrane for ethanol dehydration. J. Membr. Sci. 2019, 590, 117300. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, C.; Wang, S.; Huang, A. Copper-exchanged LTA zeolite membranes with enhanced water flux for ethanol dehydration. Chin. Chem. Lett. 2019, 30, 1204–1206. [Google Scholar] [CrossRef]
- Soukup, J.; Jandera, P. Adsorption of water from aqueous acetonitrile on silica-based stationary phases in aqueous normal-phase liquid chromatography. J. Chromatogr. A. 2014, 1374, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, S.M.; Höltzel, A.; Seidel-Morgenstern, A.; Tallarek, U. Adsorption of water—Acetonitrile mixtures to model silica surfaces. J. Phys. Chem. C. 2013, 117, 6620–6631. [Google Scholar] [CrossRef]
- Shen, W.F.; Dong, L.C.; Wei, S.A.; Li, J.; Benyounes, H.; You, X.Q.; Gerbaud, V. Systematic design of extractive distillation for maximum-boiling azeotropes with heavy entrainers. AIChE J. 2015, 61, 3898–3910. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.T.; Ma, K.; Bai, W.T.; Du, D.Q.; Zhu, Z.Y.; Wang, Y.L.; Gao, J. Energy-saving thermally coupled ternary extractive distillation process by combining with mixed entrainer for separating ternary mixture containing bioethanol. Energy 2018, 148, 296–308. [Google Scholar] [CrossRef]
- Rodriguez-Donis, I.; Acosta-Esquijarosa, J.; Gerbaud, V.; Joulia, X. Heterogeneous batch-extractive distillation of minimum boiling azeotropic mixtures. AIChE J. 2003, 49, 3074–3083. [Google Scholar] [CrossRef]
- You, X.; Gu, J.; Gerbaud, V.; Peng, C.; Liu, H. Optimization of pre-concentration, entrainer recycle and pressure selection for the extractive distillation of acetonitrile-water with ethylene glycol. Chem. Eng. Sci. 2018, 177, 354–368. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.B.; Li, Y.; Feng, T.Y.; Sun, G.X.; Lin, L. Separation of acetonitrile and water by saline extractive distillation. Petrochem. Technol. 2007, 36, 1229–1233. [Google Scholar]
- Zhou, J.B.; Cui, X.B.; Dong, B.L.; Wang, Y.F.; Chen, Z.K. Separation of acetonitrile and water mixture by batch extractive distillation. Chem. Ind. Eng. 2009, 26, 482–486. [Google Scholar]
- Meirelles, A.; Weiss, S.; Herfurth, H. Ethanol dehydration by extractive distillation. J. Chem. Technol. Biotechnol. 2007, 53, 181–188. [Google Scholar] [CrossRef]
- García-Herreros, P.; Gomez, J. Optimization of the Design and Operation of an Extractive Distillation System for the Production of Fuel Grade Ethanol Using Glycerol as Entrainer. Ind. Eng. Chem. Res. 2011, 50, 3977. [Google Scholar] [CrossRef]
- Gil, I.D.; Gómez, J.M.; Rodríguez, G. Control of an extractive distillation process to dehydrate ethanol using glycerol as entrainer. Comput. Chem. Eng. 2012, 39, 129–142. [Google Scholar] [CrossRef]
- Acosta, J.; Rodríguez, I.; Jáuregui, U.; Nuevas, L.; Pardillo, E. Recovery of acetonitrile from aqueous waste by a combined process: Solvent extraction and batch distillation. Sep. Purif. Technol. 2006, 52, 95–101. [Google Scholar] [CrossRef]
- Rodriguez-Donis, I.; Papp, K.; Rev, E.; Lelkes, Z.; Gerbaud, V.; Joulia, X. Column configurations of continuous heterogeneous extractive distillation. AIChE J. 2007, 53, 1982–1993. [Google Scholar] [CrossRef]
- Sharma, B. Ammonium-based deep eutectic solvent as entrainer for separation of acetonitrile–water mixture by extractive distillation. J. Mol. Liq. 2019, 285, 185–193. [Google Scholar] [CrossRef]
- Abbott, A.P.; Ahmed, E.I.; Harrisa, R.C.; Rydera, K.S. Evaluating watermiscible deep eutectic solvents (DESs) and ionic liquids as potential lubricants. Green Chem. 2014, 16, 4156–4161. [Google Scholar] [CrossRef] [Green Version]
- Davletbaeva, I.M.; Klinov, A.V.; Khairullina, A.R.; Malygin, A.V.; Dulmaev, S.E.; Davletbaeva, A.R.; Mukhametzyanov, T.A. Organoboron Ionic Liquids as Extractants for Distillation Process of Binary Ethanol + Water Mixtures. Processes 2020, 8, 628. [Google Scholar] [CrossRef]
- Klinov, A.V.; Malygin, A.V.; Khairullina, A.R.; Dulmaev, S.E.; Davletbaeva, I.M. Alcohols dehydration by extractive distillation with use of aminoethers of boric acid. Processes 2020, 8, 1466. [Google Scholar] [CrossRef]
- Gmehling, J.; Kleiber, M.; Kolbe, B.; Rarey, J. Chemical Thermodynamics for Process Simulation; John Wiley & Sons: Hoboken, NJ, USA, 2019; p. 808. [Google Scholar]
- Swietoslawski, W. Azeotropy and Polyazeotropy; Macmillan Company: New York, NY, USA, 1963. [Google Scholar]
- Acosta, J.; Arce, A.; Rodil, E.; Soto, A. A thermodynamic study on binary and ternary mixtures of acetonitrile, water and butyl acetate. Fluid Phase Equilib. 2002, 203, 83–98. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, M.; Huang, D.; Jia, P.; Sun, D.; Li, W. Isobaric Vapor Liquid Equilibrium for the Extractive Distillation of Acetonitrile + Water Mixtures Using Dimethyl Sulfoxide at 101.3 kPa. J. Chem. Eng. Data 2013, 58, 3364–3369. [Google Scholar] [CrossRef]
- Online UNIFAC Source. Available online: http://unifac.ddbst.de/unifac-matrix.html (accessed on 12 September 2022).
- Gmehling, J.; Rasmussen, P.; Fredenslund, A. Vapor-liquid equilibriums by UNIFAC group contribution. Revision and extension. 2. Ind. Eng. Chem. Process Des. Dev. 1982, 21, 118–127. [Google Scholar] [CrossRef]
- Reid, R.C.; Prausnitz, J.M.; Poling, B.E. The Properties of Gases and Liquids, 4th ed.; McGraw-Hill: New York, NY, UAS, 1987; p. 753. [Google Scholar]
- Wittig, R.; Lohmann, J.; Gmehling, J. Vapor-Liquid Equilibria by UNIFAC Group Contribution. Revision and Extension. Ind. Eng. Chem. Res. 2003, 42, 183–188. [Google Scholar] [CrossRef]
- Abrams, D.S.; Prausnitz, J.M. Statistical thermodynamics of liquid mixtures: A new expression for the excess Gibbs energy of partly or completely miscible systems. AIChE J. 1975, 21, 116–128. [Google Scholar] [CrossRef]
- Bondi., A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441. [Google Scholar] [CrossRef]
- Mato, F.; Benito, G.G.A.; Quim, A. Liquid-Vapor Equilibrium of Binary Mixtures of Nitriles and Alcohols. An. Quim. Ser. A 1985, 81, 116. [Google Scholar]
- Serafimov, L.A.; Zharov, V.T.; Timofeev, V.S. Rectification of Multicomponent Mixtures. I. Topological Analyses of Liquid-Vapor Phase Equilibrium Diagrams. Acta Chim. Hungar. 1971, 69, 383–396. [Google Scholar]
- Kiva, V.N.; Hilmen, E.K.; Skogestad, S. Azeotropic phase equilibrium diagrams: A survey. Chem. Eng. Sci. 2003, 58, 1903–1953. [Google Scholar] [CrossRef]




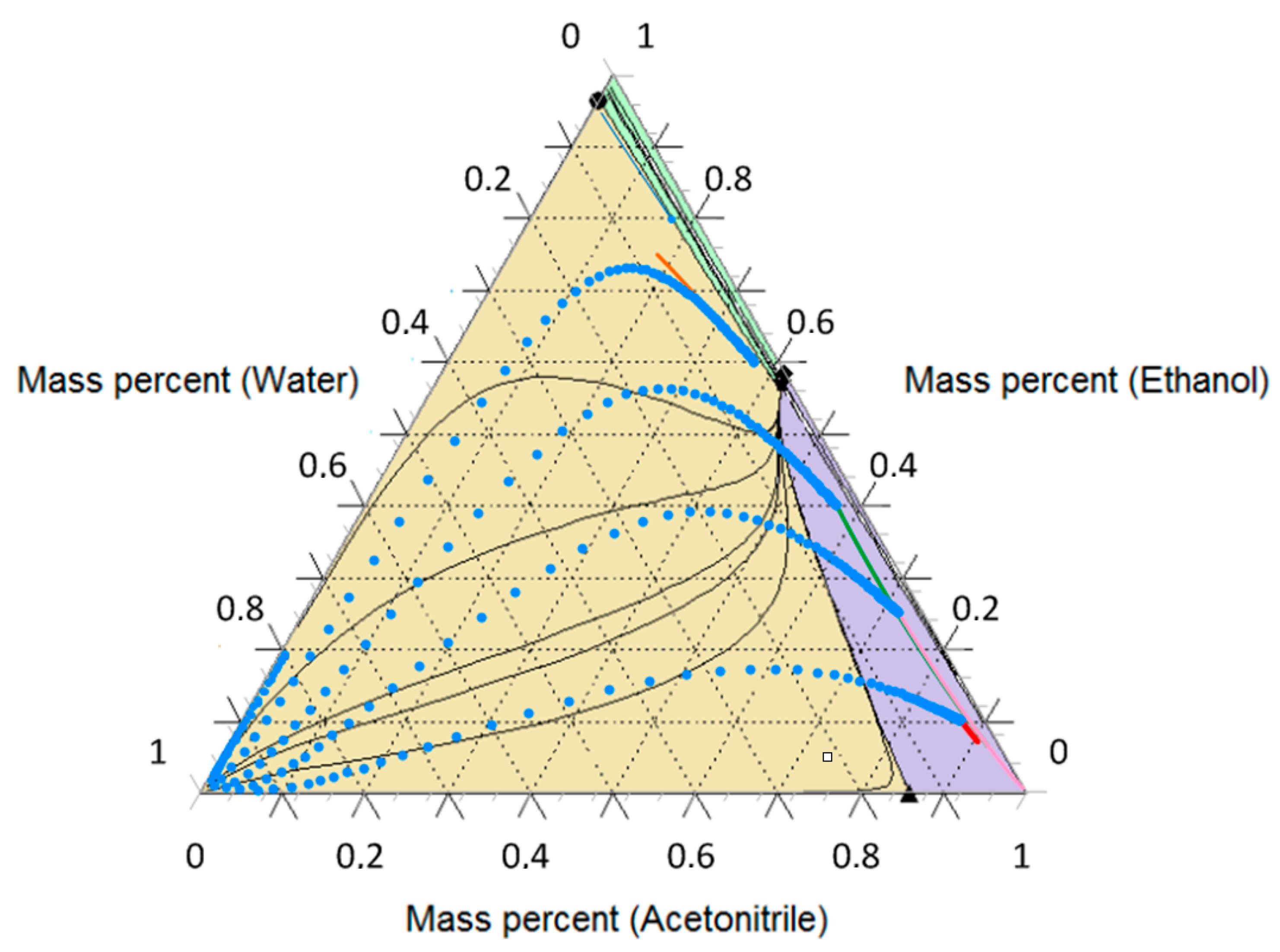
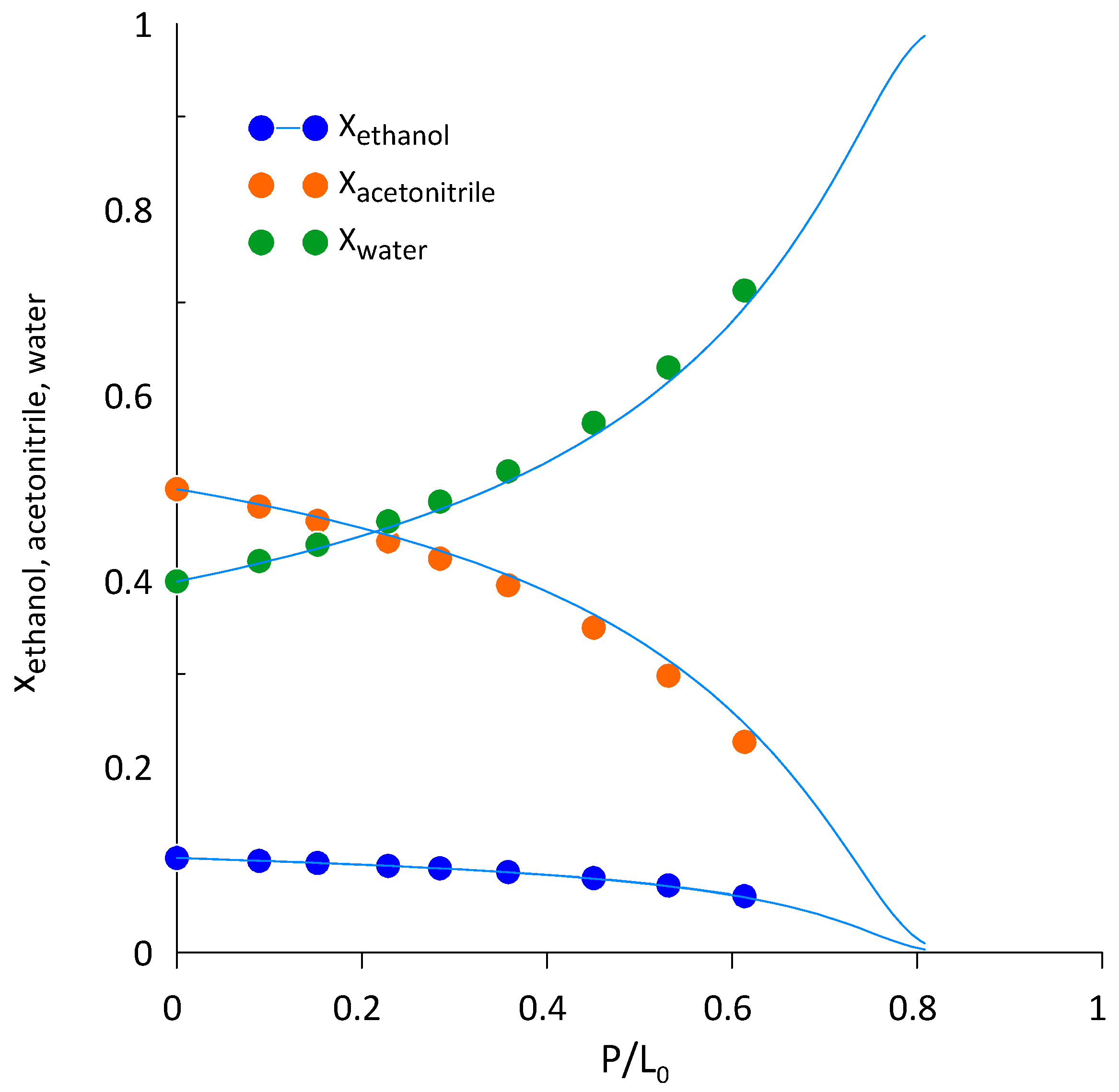
| T, K | wAEBA–TEG (Exactly) | xacetonitril | yacetonitril |
|---|---|---|---|
| AEBA–TEG 0.2 mas. frac. (approximately) | |||
| 367.36 360.9 356.8 354.22 352.99 350.8 352.65 350.48 353.73 | 0.222 0.224 0.228 0.217 0.223 0.216 0.229 0.223 0.231 | 0.054 0.146 0.228 0.336 0.451 0.761 0.815 0.885 0.922 | 0.451 0.658 0.718 0.767 0.797 0.840 0.855 0.901 0.925 |
| AEBA–TEG 0.4 mas. frac. (approximately) | |||
| 368.21 362.91 358.94 359.47 356.85 355.26 354.72 354.79 355.24 356.14 357.29 | 0.433 0.42 0.432 0.439 0.443 0.434 0.435 0.445 0.432 0.439 0.437 | 0.078 0.183 0.264 0.296 0.371 0.477 0.595 0.694 0.82 0.873 0.935 | 0.52 0.664 0.737 0.744 0.778 0.803 0.838 0.869 0.916 0.944 0.977 |
| AEBA–TEG 0.6 mas. frac. (approximately) | |||
| 380.25 374.73 370.86 366.95 363.99 363.06 362.06 361.7 362.32 361.71 | 0.661 0.644 0.637 0.644 0.636 0.647 0.630 0.634 0.654 0.639 | 0.038 0.102 0.170 0.291 0.378 0.513 0.635 0.769 0.799 0.918 | 0.291 0.507 0.629 0.733 0.775 0.840 0.873 0.937 0.949 0.984 |
| Group-m | Group-n | amn | anm |
|---|---|---|---|
| H2O | CH2 | 300.00 | 1318.00 |
| H2O | OH | −229.10 | 353.50 |
| H2O | CH2O | 540.50 | −314.70 |
| H2O | (C)3N | 304.00 | −598.80 |
| H2O | B | −237.83 | −1136.35 |
| H2O | CCN | 186.76 | 79.05 |
| CH2 | OH | 986.50 | 156.40 |
| CH2 | CH2O | 251.50 | 83.36 |
| CH2 | (C)3N | 206.60 | −83.98 |
| CH2 | B | 170.60 | −384.58 |
| CH2 | CCN | 597.00 | 24.82 |
| OH | CH2O | 28.06 | 237.70 |
| OH | (C)3N | −323.00 | 28.60 |
| OH | B | −281.82 | −722.30 |
| OH | CCN | 6.71 | 185.40 |
| CH2O | (C)3N | 5422.00 | −194.10 |
| CH2O | B | 405.99 | 1825.92 |
| CH2O | CCN | −18.51 | 38.81 |
| (C)3N0 | B | 113.96 | −13.53 |
| (C)3N | CCN | 834.46 | 322.39 |
| B | CCN | 1714.77 | −66.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davletbaeva, I.M.; Klinov, A.V.; Khairullina, A.R.; Malygin, A.V.; Madaminov, N.V. Vapor–Liquid Equilibrium in Binary and Ternary Azeotropic Solutions Acetonitrile-Ethanol-Water with the Addition of Amino Esters of Boric Acid. Processes 2022, 10, 2125. https://doi.org/10.3390/pr10102125
Davletbaeva IM, Klinov AV, Khairullina AR, Malygin AV, Madaminov NV. Vapor–Liquid Equilibrium in Binary and Ternary Azeotropic Solutions Acetonitrile-Ethanol-Water with the Addition of Amino Esters of Boric Acid. Processes. 2022; 10(10):2125. https://doi.org/10.3390/pr10102125
Chicago/Turabian StyleDavletbaeva, Ilsiya M., Alexander V. Klinov, Alina R. Khairullina, Alexander V. Malygin, and Nikolay V. Madaminov. 2022. "Vapor–Liquid Equilibrium in Binary and Ternary Azeotropic Solutions Acetonitrile-Ethanol-Water with the Addition of Amino Esters of Boric Acid" Processes 10, no. 10: 2125. https://doi.org/10.3390/pr10102125
APA StyleDavletbaeva, I. M., Klinov, A. V., Khairullina, A. R., Malygin, A. V., & Madaminov, N. V. (2022). Vapor–Liquid Equilibrium in Binary and Ternary Azeotropic Solutions Acetonitrile-Ethanol-Water with the Addition of Amino Esters of Boric Acid. Processes, 10(10), 2125. https://doi.org/10.3390/pr10102125







