Reaction of Chloroacetyl-Modified Peptides with Mercaptoundecahydrododecaborate (BSH) Is Accelerated by Basic Amino Acid Residues in the Peptide
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
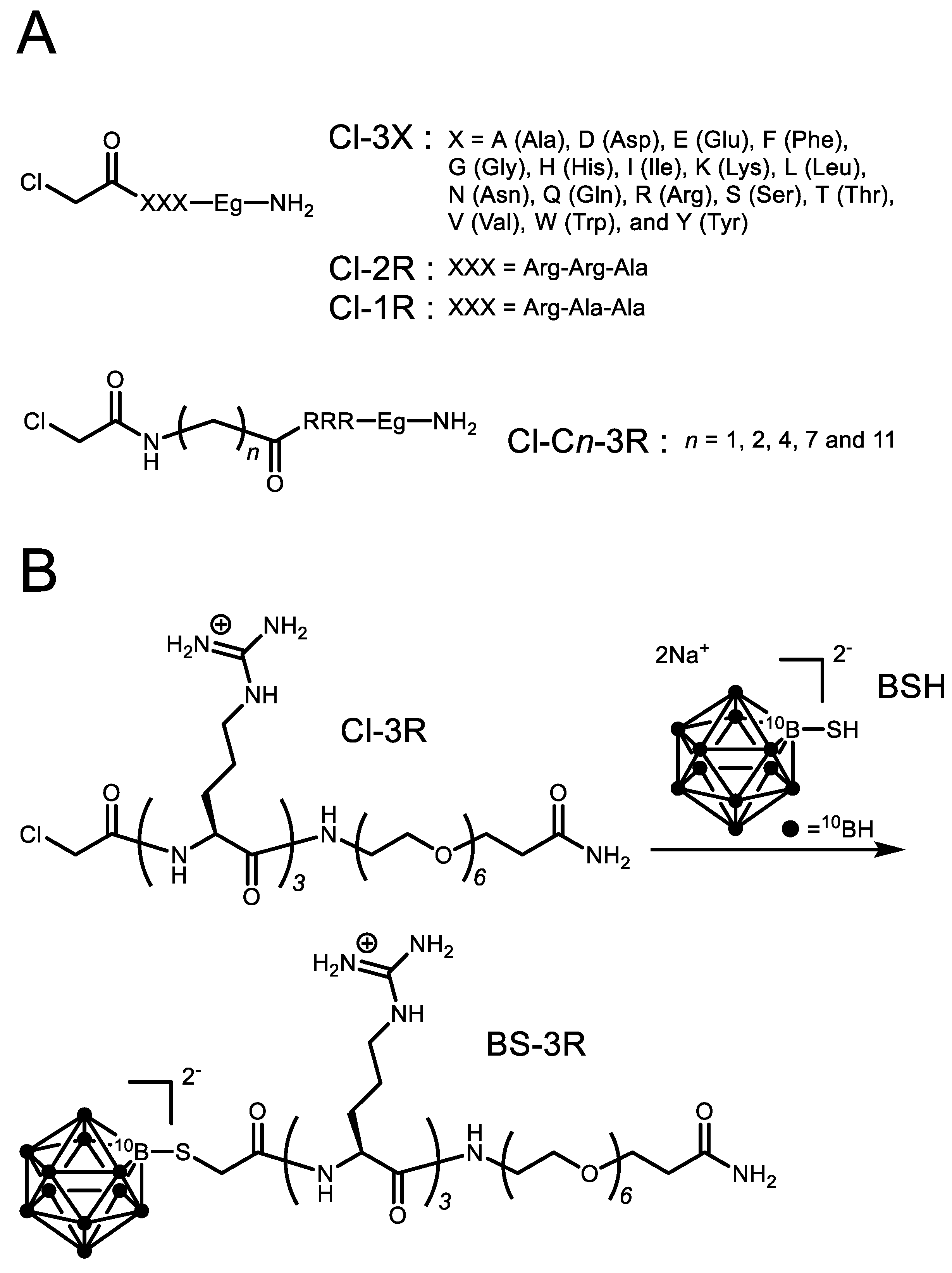
3.1. Reactivity of BSH with Cl-3X
3.2. Reactivity of BSH with Cl-3R, Cl-2R and Cl-1R
3.3. Reactivity of BSH with Cl-Cn-3R
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barth, R.F.; Vicente, M.G.H.; Harling, O.K.; Kiger, W.S., III.; Riley, K.J.; Binns, P.J.; Franz, M.; Wagner, F.M.; Suzuki, M.; Aihara, T.; et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat. Oncol. 2012, 7, 146–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, I.; Ono, K.; Sakurai, Y.; Ohmae, M.; Maruhashi, A.; Imahori, Y.; Kirihata, M.; Nakazawa, M.; Yura, Y. Effectiveness of BNCT for recurrent head and neck malignancies. Appl. Radiat. Isot. 2004, 61, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Yang, Z.; Zhang, L.; Xie, L.; Wang, L.; Xu, H.; Josephson, L.; Liang, S.H.; Zhang, M.-R. Boron agents for neutron capture therapy. Coord. Chem. Rev. 2020, 405, 213139–213158. [Google Scholar] [CrossRef]
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018, 38, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Lamba, M.; Goswami, A.; Bandyopadhyay, A. A periodic development of BPA and BSH based derivatives in boron neutron capture therapy (BNCT). Chem. Commun. 2021, 57, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Michiue, H.; Sakurai, Y.; Kondo, N.; Kitamatsu, M.; Bin, F.; Nakajima, K.; Hirota, Y.; Kawabata, S.; Nishiki, T.; Ohmori, I.; et al. The acceleration of boron neutron capture therapy using multi-linked mercaptoundecahydrododecaborate (BSH) fused cell-penetrating peptide. Biomaterials 2014, 35, 3396–3405. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, Y.; Michiue, H.; Kitamatsu, M.; Hayashi, Y.; Takenaka, F.; Nishiki, T.; Matsui, H. Tumor-specific delivery of BSH-3R for boron neutron capture therapy and positron emission tomography imaging in a mouse brain tumor model. Biomaterials 2015, 56, 10–17. [Google Scholar] [CrossRef]
- Nakase, I.; Katayama, M.; Hattori, Y.; Ishimura, M.; Inaura, S.; Fujiwara, D.; Takatani-Nakase, T.; Fujii, I.; Futaki, S.; Kirihata, M. Intracellular target delivery of cell-penetrating peptide-conjugated dodecaborate for boron neutron capture therapy (BNCT). Chem. Commun. 2019, 55, 13955–13958. [Google Scholar] [CrossRef]
- Kitamatsu, M.; Nakamura-Tachibana, A.; Ishikawa, Y.; Michiue, H. Improvement of water solubility of mercaptoundecahydrododecaborate (BSH)-peptides by conjugating with ethylene glycol linker and interaction with cyclodextrin. Processes 2020, 9, 167. [Google Scholar] [CrossRef]
- Kimura, S.; Masunaga, S.; Harada, T.; Kawamura, Y.; Ueda, S.; Okuda, K.; Nagasawa, H. Synthesis and evaluation of cyclic RGD-boron cluster conjugates to develop tumor-selective boron carriers for boron neutron capture therapy. Bioorg. Med. Chem. 2011, 19, 1721–1728. [Google Scholar] [CrossRef]
- Ol’shevskaya, V.A.; Alpatova, V.M.; Radchenko, A.S.; Ramonova, A.A.; Petrova, A.S.; Tatarskiy, V.V.; Zaitsev, A.V.; Kononova, E.G.; Ikonnikov, N.S.; Kostyukov, A.A.; et al. β-Maleimide substituted meso-arylporphyrins: Synthesis, transformations, physico-chemical and antitumor properties. Dyes Pigm. 2019, 171, 107760–107773. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Q.; Liu, M.; Lin, M.; Wang, T.; Zhang, Z.; Zhong, X.; Guo, N.; Lu, Y.; Xu, J.; et al. Remarkable boron delivery of iRGD-modified polymeric nanoparticles for boron neutron capture therapy. Int. J. Nanomed. 2019, 14, 8161–8177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakase, I.; Aoki, A.; Sakai, Y.; Hirase, S.; Ishimura, M.; Takatani-Nakase, T.; Hattori, Y.; Kirihata, M. Antibody-based receptor targeting using an Fc-binding peptide-dodecaborate conjugate and macropinocytosis induction for boron neutron capture therapy. ACS Omega 2020, 5, 22731–22738. [Google Scholar] [CrossRef] [PubMed]
- Ol’shevskaya, V.A.; Alpatova, V.M.; Makarenkov, A.V.; Kononova, E.G.; Smol’yakov, A.F.; Peregudov, A.S.; Rys, E.G. Synthesis of maleimide-functionalized carboranes and their utility in Michael addition reactions. New J. Chem. 2021, 45, 12159–12167. [Google Scholar] [CrossRef]
- Smith, S.W. Chiral toxicology: It’s the same thing…only different. Toxicol. Sci. 2009, 110, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Ueno, U.; Lee, J.D.; Ban, H.S.; Justus, E.; Fan, P.; Gabel, D. Synthesis of dodecaborate-conjugated cholesterols for efficient boron delivery in neutron capture therapy. Tetrahedron Lett. 2007, 48, 3151–3154. [Google Scholar] [CrossRef]
- Justus, E.; Awad, D.; Hohnholt, M.; Schaffran, T.; Edwards, K.; Karlsson, G.; Damian, L.; Gabel, D. Synthesis, liposomal preparation, and in vitro toxicity of two novel dodecaborate cluster lipids for boron neutron capture therapy. Bioconjugate Chem. 2007, 18, 1287–1293. [Google Scholar] [CrossRef]
- Lee, J.D.; Ueno, M.; Miyajima, Y.; Nakamura, H. Synthesis of boron cluster lipids: Closo-dodecaborate as an alternative hydrophilic function of boronated liposomes for neutron capture therapy. Org. Lett. 2007, 9, 323–326. [Google Scholar] [CrossRef]
- El-Zaria, M.E.; Nakamura, H. New strategy for synthesis of mercaptoundecahydrododecaborate derivatives via click chemistry: Possible boron carriers and visualization in cells for neutron capture therapy. Inorg. Chem. 2009, 48, 11896–11902. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Sivaev, I.B.; Petrovskii, P.V.; Bregadze, V.I. Synthesis of monosubstituted functional derivatives of carboranes from 1-mercapto-ortho-carborane: 1-HOOC(CH2)nS-1,2-C2B10H11 and [7-HOOC(CH2)nS-7,8-C2B9H11]- (n = 1–4). Dalton Trans. 2010, 39, 1817–1822. [Google Scholar] [CrossRef]
- Kusaka, S.; Hattori, Y.; Uehara, K.; Asano, T.; Tanimori, S.; Kirihata, M. Synthesis of optically active dodecaborate-containing L-amino acids for BNCT. Appl. Radiat. Isot. 2011, 69, 1768–1770. [Google Scholar] [CrossRef]
- Hattori, Y.; Kusaka, S.; Mukumoto, M.; Uehara, K.; Asano, T.; Suzuki, M.; Masunaga, S.; Ono, K.; Tanimori, S.; Kirihata, M. Biological evaluation of dodecaborate-containing L-amino acids for boron neutron capture therapy. J. Med. Chem. 2012, 55, 6980–6984. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Ueda, N.; Ban, H.S.; Ueno, M.; Tachikawa, S. Design and synthesis of fluorescence-labeled closo-dodecaborate lipid: Its liposome formation and in vivo imaging targeting of tumors for boron neutron capture therapy. Org. Biomol. Chem. 2012, 10, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Asano, R.; Nagami, A.; Fukumoto, Y.; Miura, K.; Yazama, F.; Ito, H.; Sakata, I.; Tai, A. Synthesis and biological evaluation of new BSH-conjugated chlorin derivatives as agents for both photodynamic therapy and boron neutron capture therapy of cancer. J. Photochem. Photobiol. B Biol. 2014, 140, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Genady, A.R.; Ioppolo, J.A.; Azaam, M.M.; El-Zaria, M.E. New functionalized mercaptoundecahydrododecaborate derivatives for potential application in boron neutron capture therapy: Synthesis, characterization and dynamic visualization in cells. Eur. J. Med. Chem. 2015, 93, 574–583. [Google Scholar] [CrossRef]
- Worm, D.J.; Els-Heindl, S.; Kellert, M.; Kuhnert, R.; Saretz, S.; Koebberling, J.; Riedl, B.; Hey-Hawkins, E.; Beck-Sickinger, A.G. A stable meta-carborane enables the generation of boron-rich peptide agonists targeting the ghrelin receptor. J. Pept. Sci. 2018, 24, e3119. [Google Scholar] [CrossRef]
- Mochizuki, M.; Sato, S.; Asatyas, S.; Leśnikowski, Z.J.; Hayashi, T.; Nakamura, H. Raman cell imaging with boron cluster molecules conjugated with biomolecules. RSC Adv. 2019, 9, 23973–23978. [Google Scholar] [CrossRef] [Green Version]
- Worm, D.J.; Hoppenz, P.; Els-Heindl, S.; Kellert, M.; Kuhnert, R.; Saretz, S.; Köbberling, J.; Riedl, B.; Hey-Hawkins, E.; Beck-Sickinger, A.G. Selective neuropeptide Y conjugates with maximized carborane loading as promising boron delivery agents for boron neutron capture therapy. J. Med. Chem. 2020, 63, 2358–2371. [Google Scholar] [CrossRef]
- Takeuchi, K.; Hattori, Y.; Kawabata, S.; Futamura, G.; Hiramatsu, R.; Wanibuchi, M.; Tanaka, H.; Masunaga, S.; Ono, K.; Miyatake, S.; et al. Synthesis and evaluation of dodecaboranethiol containing kojic acid (KA-BSH) as a novel agent for boron neutron capture therapy. Cells 2020, 9, 1551. [Google Scholar] [CrossRef]
- Kubasov, A.S.; Turyshev, E.S.; Novikov, I.V.; Gurova, O.M.; Starodubets, P.A.; Golubev, A.V.; Zhizhin, K.Y.; Kuznetsov, N.T. Theoretical and experimental comparison of the reactivity of the sulfanyl-closo-decaborate and sulfanyl-closo-dodecaborate anions and their mono-S-substituted derivatives. Polyhedron 2021, 206, 115347–115356. [Google Scholar] [CrossRef]
- Woods, M.; Sherry, A.D. An improved and versatile synthetic route to 6,7:13,14-dibenzo-1,8,4,11-dioxadiazacyclotetradecane. Inorg. Chim. Acta. 2003, 351, 395–398. [Google Scholar] [CrossRef]
- Kawamura, A.; Münzel, M.; Kojima, T.; Yapp, C.; Bhushan, B.; Goto, Y.; Tumber, A.; Katoh, T.; King, O.N.F.; Passioura, T.; et al. Highly selective inhibition of histone demethylases by de novo macrocyclic peptides. Nat. Commun. 2017, 8, 14773–14782. [Google Scholar] [CrossRef] [PubMed]



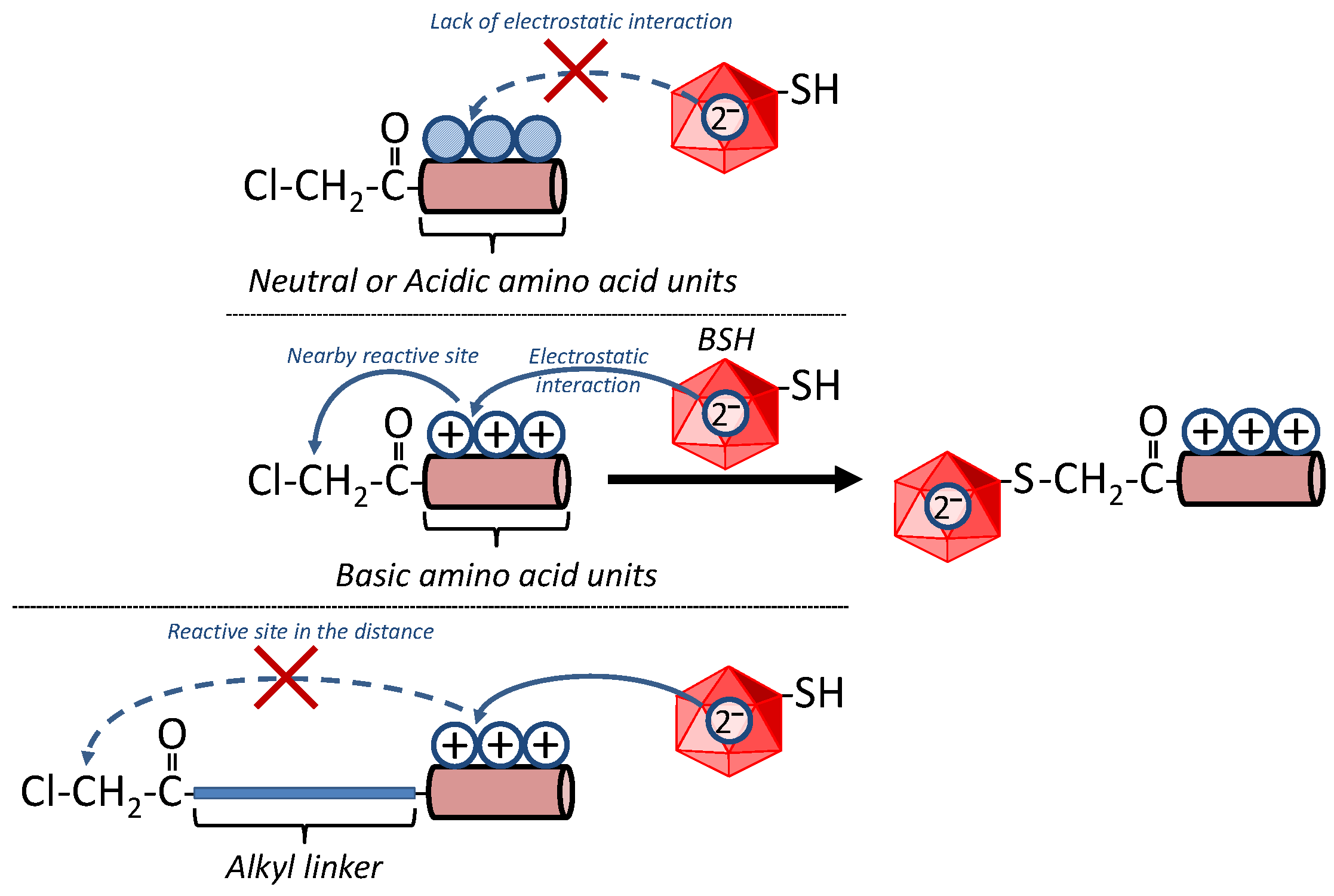
| Tripeptides for Which the Reaction Proceeded | Tripeptides for Which the Reaction Did Not Proceed |
|---|---|
| BS-3X | BSH/Cl-3X |
| X = His, Lys, Arg | X = Ala, Asp, Glu, Phe, Gly, Ile, Leu, Asn, Gln, Ser, Thr, Val, Trp, Tyr |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitamatsu, M.; Inoue, K.; Yamagata, N.; Michiue, H. Reaction of Chloroacetyl-Modified Peptides with Mercaptoundecahydrododecaborate (BSH) Is Accelerated by Basic Amino Acid Residues in the Peptide. Processes 2022, 10, 2200. https://doi.org/10.3390/pr10112200
Kitamatsu M, Inoue K, Yamagata N, Michiue H. Reaction of Chloroacetyl-Modified Peptides with Mercaptoundecahydrododecaborate (BSH) Is Accelerated by Basic Amino Acid Residues in the Peptide. Processes. 2022; 10(11):2200. https://doi.org/10.3390/pr10112200
Chicago/Turabian StyleKitamatsu, Mizuki, Ken Inoue, Naoki Yamagata, and Hiroyuki Michiue. 2022. "Reaction of Chloroacetyl-Modified Peptides with Mercaptoundecahydrododecaborate (BSH) Is Accelerated by Basic Amino Acid Residues in the Peptide" Processes 10, no. 11: 2200. https://doi.org/10.3390/pr10112200
APA StyleKitamatsu, M., Inoue, K., Yamagata, N., & Michiue, H. (2022). Reaction of Chloroacetyl-Modified Peptides with Mercaptoundecahydrododecaborate (BSH) Is Accelerated by Basic Amino Acid Residues in the Peptide. Processes, 10(11), 2200. https://doi.org/10.3390/pr10112200






