Auto-Ignition Delay Characteristics of Ammonia Substitution on Methane
Abstract
:1. Introduction
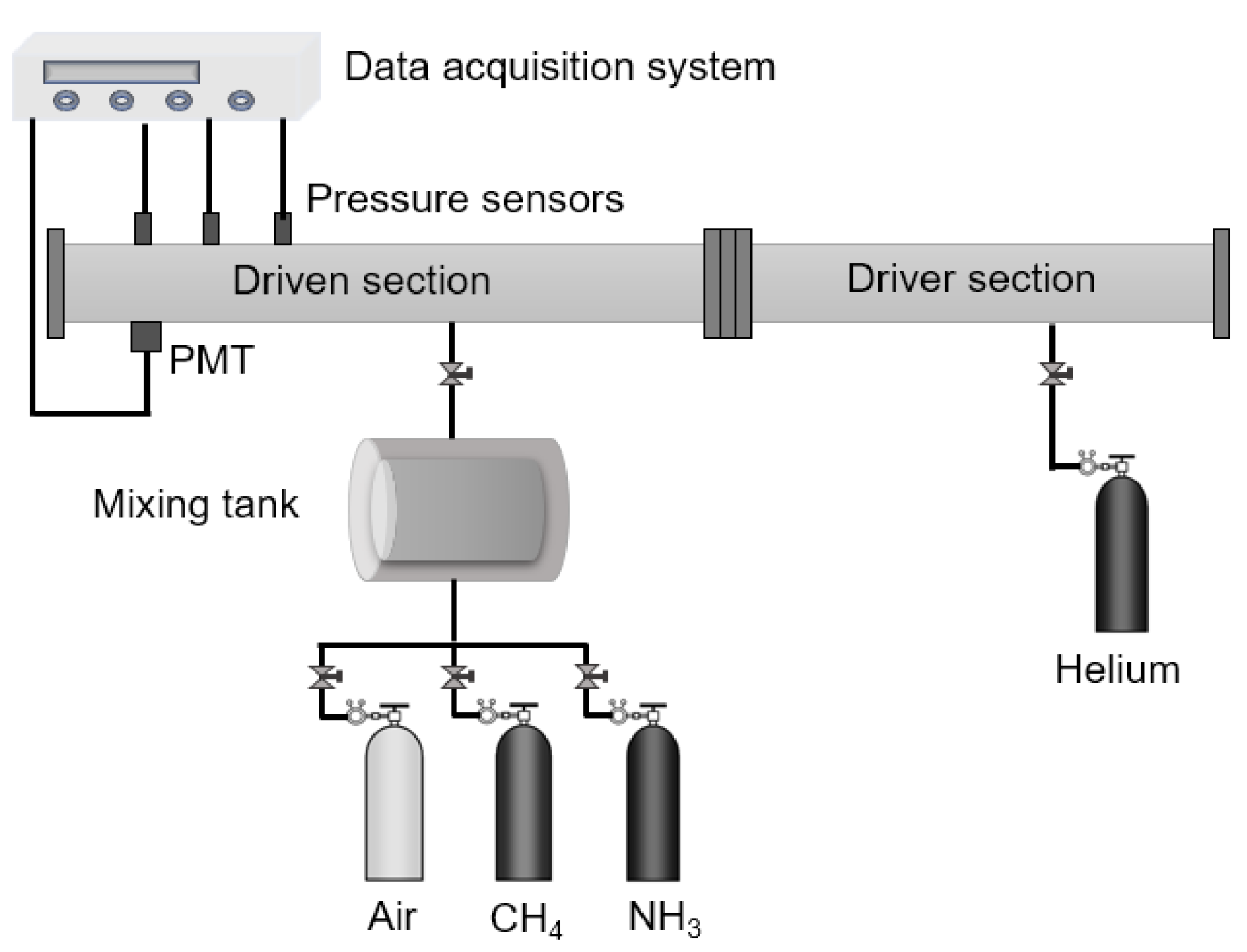
2. Experimental Setup and Numerical Analysis
3. Results and Discussion
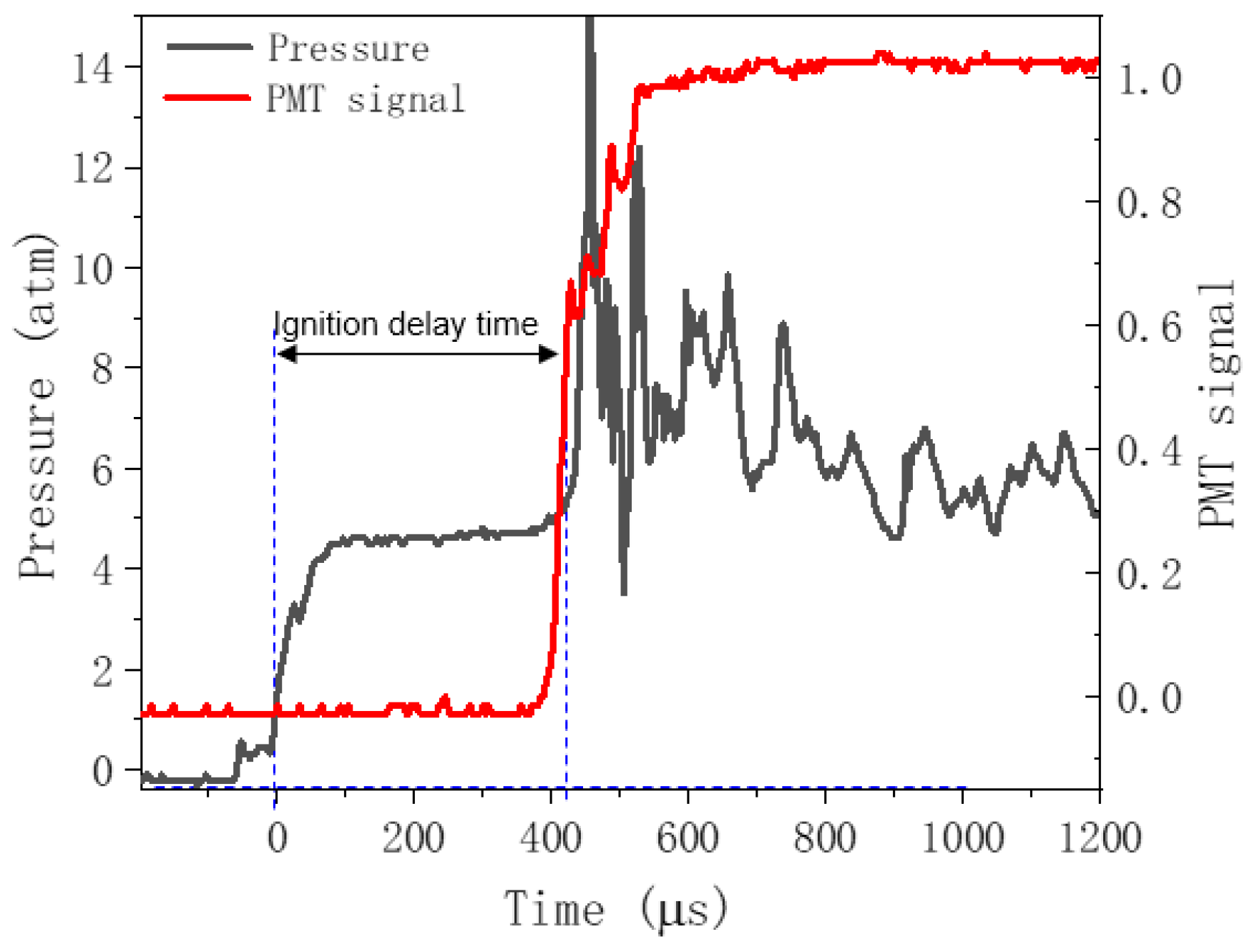
3.1. Validation of the Model
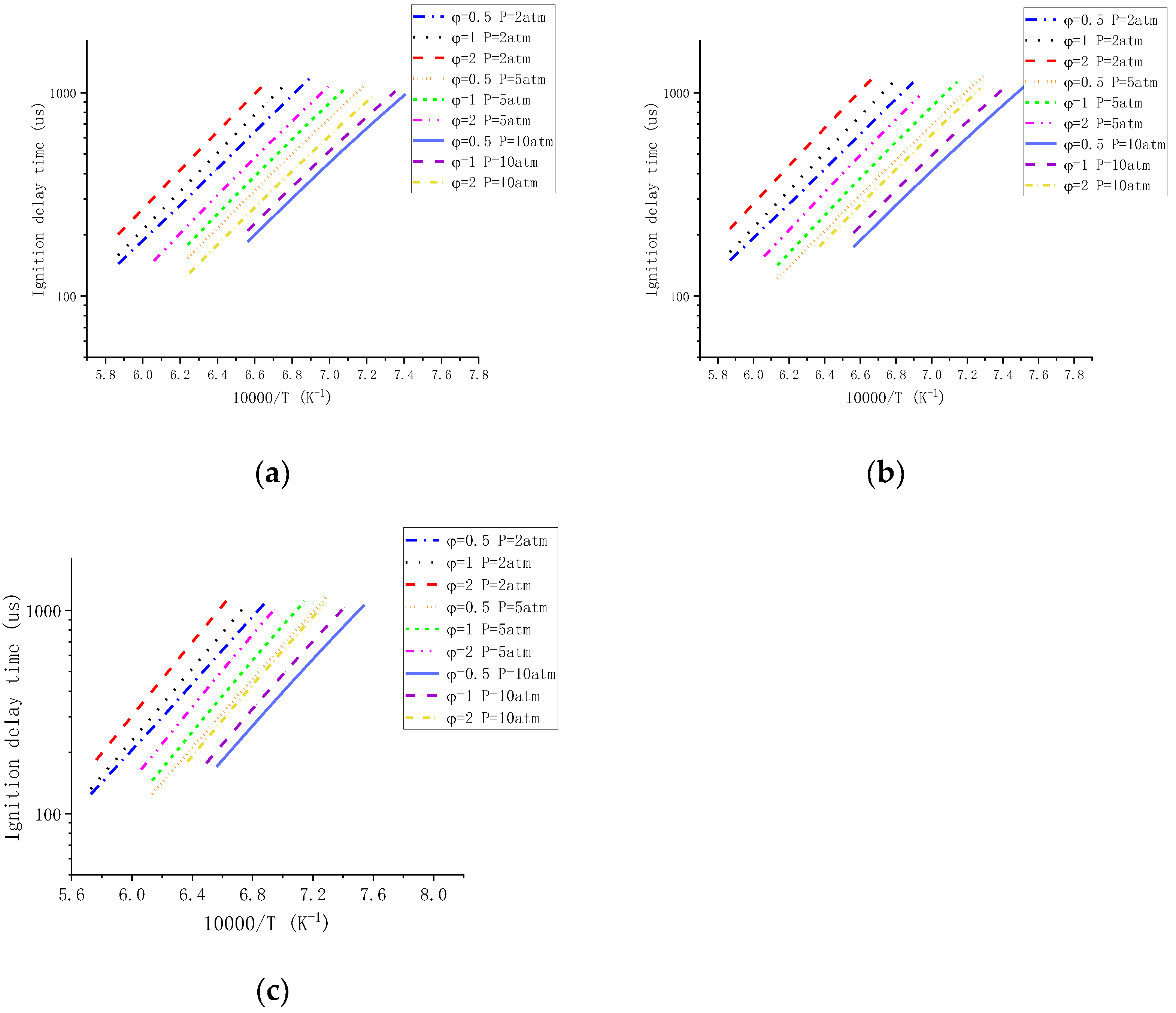
3.2. Effect of Pressure
3.3. Effect of Equivalence Ratio
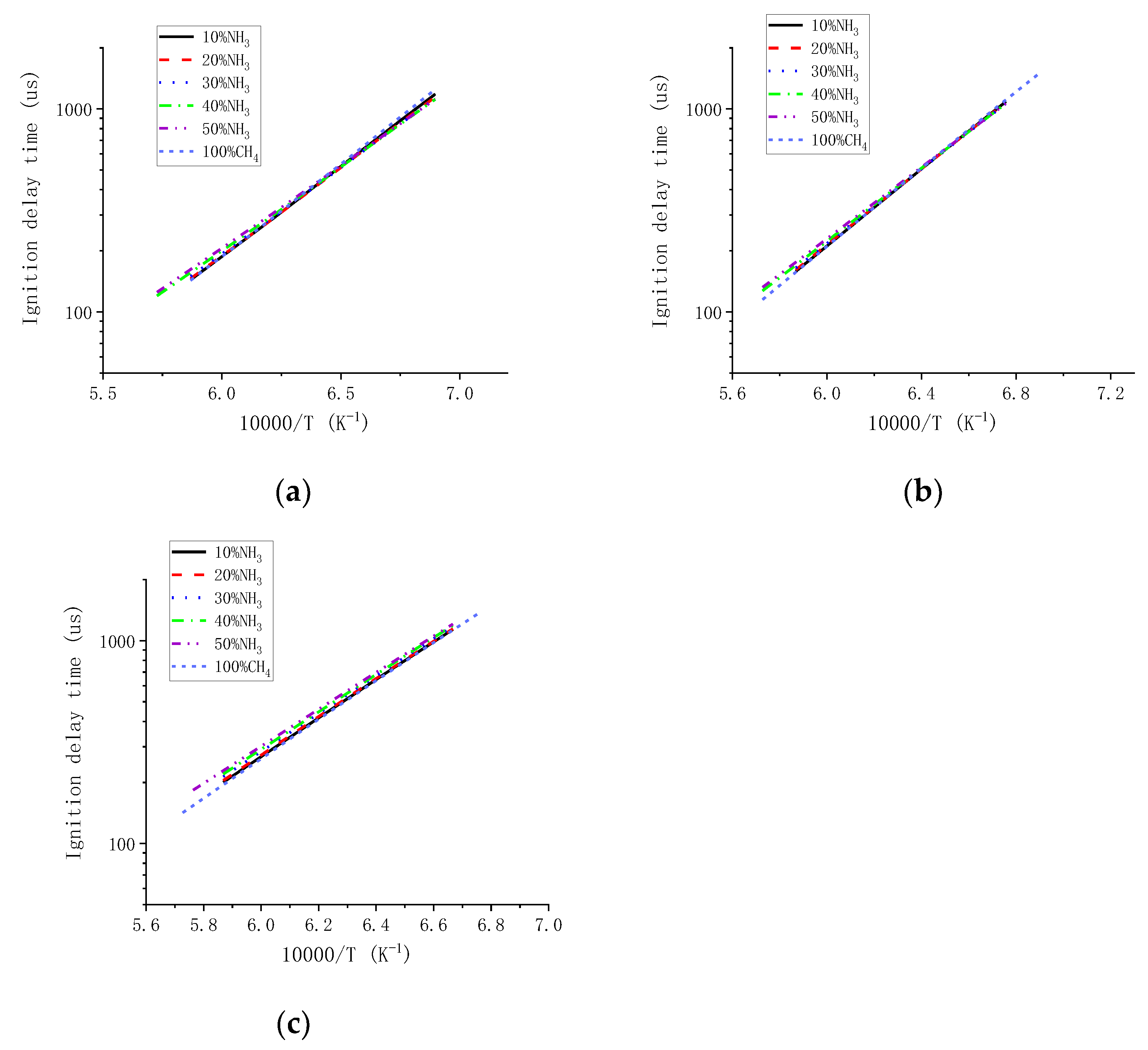
3.4. Effect of Ammonia-Substituted Methane Fuel Blends Composition
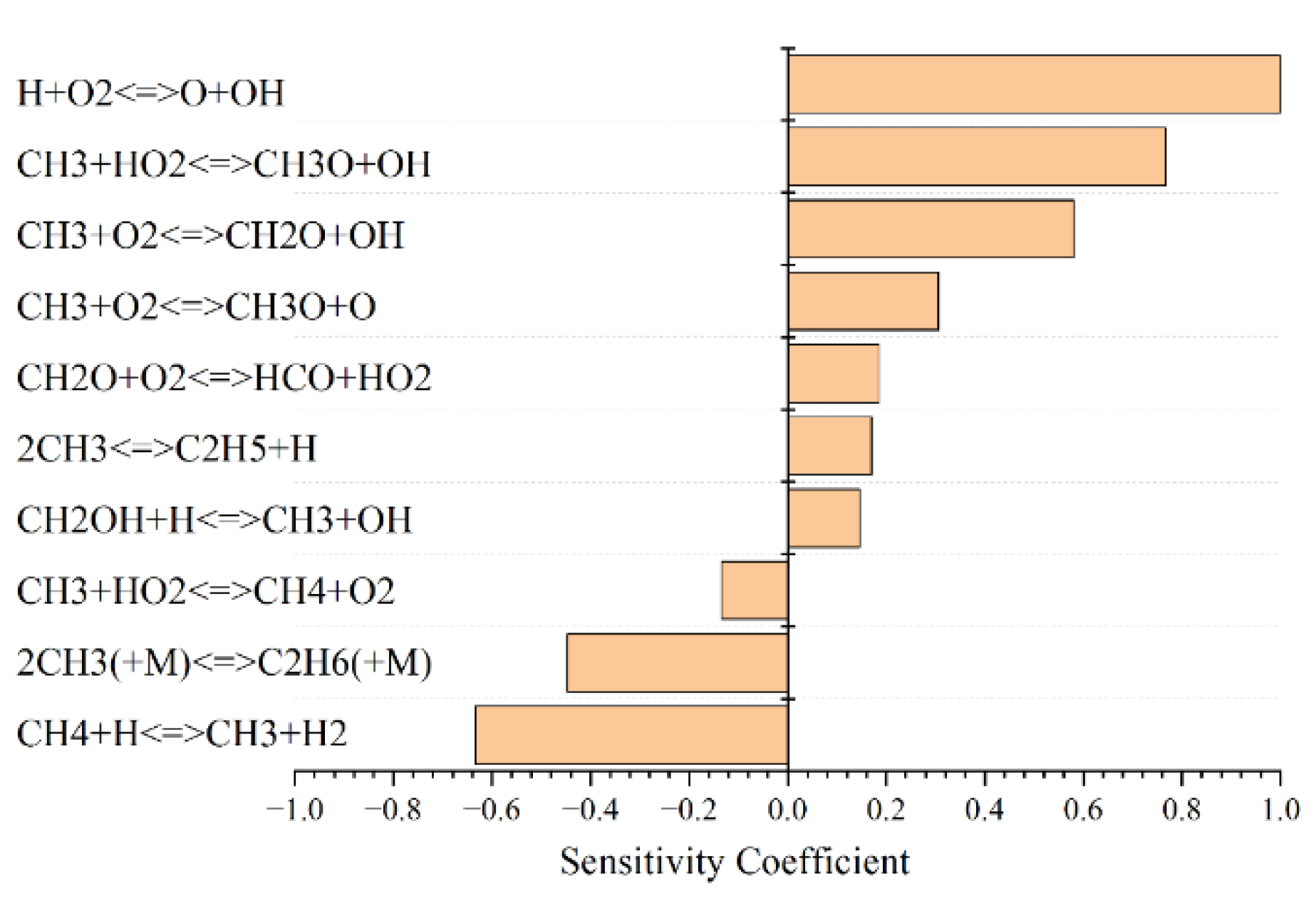
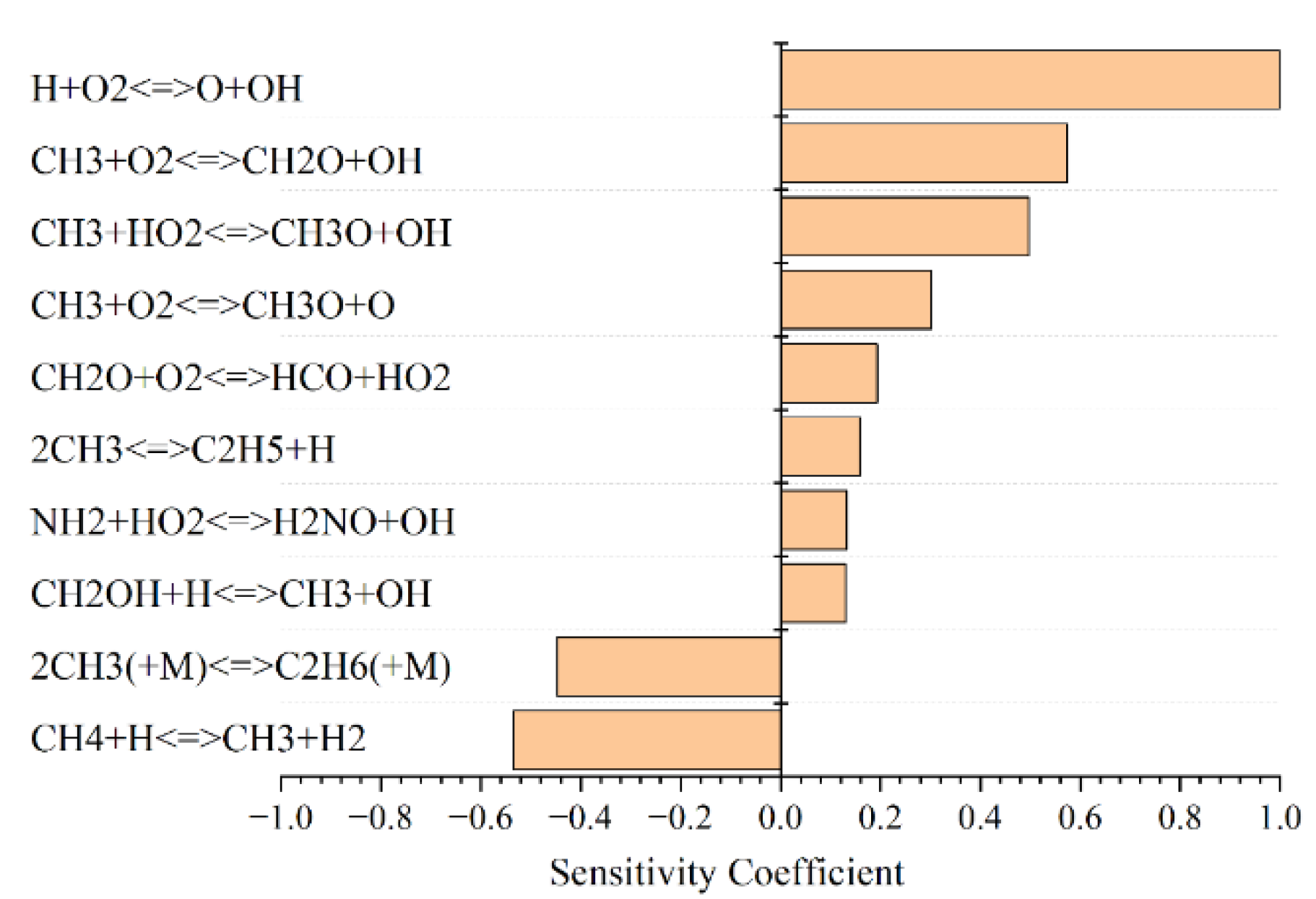
3.5. Sensitivity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Balcombe, P.; Brierley, J.; Lewis, C.; Skatvedt, L.; Speirs, J.; Hawkes, A.; Staffell, I. How to decarbonise international shipping: Options for fuels, technologies and policies. Energy Convers. Manag. 2019, 182, 72–88. [Google Scholar] [CrossRef]
- Yapicioglu, A.; Dincer, I. A review on clean ammonia as a potential fuel for power generators. Renew. Sustain. Energy Rev. 2019, 103, 96–108. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Amer-Hatem, F.; Azad, A.K.; Dedoussi, I.C.; de Joannon, M.; Fernandes, R.X.; Glarborg, P.; Hashemi, H.; He, X.; Mashruk, S.; et al. Review on Ammonia as a Potential Fuel: From Synthesis to Economics. Energy Fuels 2021, 35, 6964–7029. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C. Ammonia-fed solid oxide fuel cells for power generation—A review. Int. J. Energy Res. 2009, 33, 943–959. [Google Scholar] [CrossRef]
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrog. Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Ryu, K.; Zacharakis-Jutz, G.E.; Kong, S.-C. Effects of gaseous ammonia direct injection on performance characteristics of a spark-ignition engine. Appl. Energy 2014, 116, 206–215. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Marsh, R.; Runyon, J.; Pugh, D.; Beasley, P.; Hughes, T.; Bowen, P. Ammonia–methane combustion in tangential swirl burners for gas turbine power generation. Appl. Energy 2017, 185, 1362–1371. [Google Scholar] [CrossRef] [Green Version]
- Okafor, E.C.; Somarathne, K.K.A.; Ratthanan, R.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Tsujimura, T.; Furutani, H.; Kobayashi, H. Control of NOx and other emissions in micro gas turbine combustors fuelled with mixtures of methane and ammonia. Combust. Flame 2020, 211, 406–416. [Google Scholar] [CrossRef]
- Choi, S.; Lee, S.; Kwon, O.C. Extinction limits and structure of counterflow nonpremixed hydrogen-doped ammonia/air flames at elevated temperatures. Energy 2015, 85, 503–510. [Google Scholar] [CrossRef]
- Gross, C.W.; Kong, S.-C. Performance characteristics of a compression-ignition engine using direct-injection ammonia–DME mixtures. Fuel 2013, 103, 1069–1079. [Google Scholar] [CrossRef]
- Lamas, M.I.; Rodriguez, C.G. NOx Reduction in Diesel-Hydrogen Engines Using Different Strategies of Ammonia Injection. Energies 2019, 12, 1255. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, O.; Hargis, J.; Camou, A.; Mulvihill, C.; Petersen, E.L. Ignition delay time measurements behind reflected shock-waves for a representative coal-derived syngas with and without NH3 and H2S impurities. Proc. Combust. Inst. 2015, 35, 3143–3150. [Google Scholar] [CrossRef] [Green Version]
- Desantes, J.M.; Bermúdez, V.; López, J.J.; López-Pintor, D. Correlations for the ignition characteristics of six different fuels and their application to predict ignition delays under transient thermodynamic conditions. Energy Convers. Manag. 2017, 152, 124–135. [Google Scholar] [CrossRef]
- Shu, B.; Vallabhuni, S.; He, X.; Issayev, G.; Moshammer, K.; Farooq, A.; Fernandes, R. A shock tube and modeling study on the autoignition properties of ammonia at intermediate temperatures. Proc. Combust. Inst. 2019, 37, 205–211. [Google Scholar] [CrossRef]
- He, X.; Shu, B.; Nascimento, D.; Moshammer, K.; Costa, M.; Fernandes, R.X. Auto-ignition kinetics of ammonia and ammonia/hydrogen mixtures at intermediate temperatures and high pressures. Combust. Flame 2019, 206, 189–200. [Google Scholar] [CrossRef]
- Dai, L.; Gersen, S.; Glarborg, P.; Mokhov, A.; Levinsky, H. Autoignition studies of NH3/CH4 mixtures at high pressure. Combust. Flame 2020, 218, 19–26. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, W.; Feng, Y.; Wang, W.; Zhu, J.; Qian, Y.; Lu, X. The effect of ammonia addition on the low-temperature autoignition of n-heptane: An experimental and modeling study. Combust. Flame 2020, 217, 4–11. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne KDKunkuma, A.; Okafor Ekenechukwu, C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Mashruk, S.; Xiao, H.; Valera-Medina, A. Rich-Quench-Lean model comparison for the clean use of humidified ammonia/hydrogen combustion systems. Int. J. Hydrog. Energy 2021, 46, 4472–4484. [Google Scholar] [CrossRef]
- Xiao, H.; Lai, S.; Valera-Medina, A.; Li, J.; Liu, J.; Fu, H. Experimental and modeling study on ignition delay of ammonia/methane fuels. Int. J. Energy Res. 2020, 44, 6939–6949. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Measurement and modelling of the laminar burning velocity of methane-ammonia-air flames at high pressures using a reduced reaction mechanism. Combust. Flame 2019, 204, 162–175. [Google Scholar] [CrossRef]
- Du, W.; Ma, Z.; Yin, Z.; Lv, E.; Liu, C.; Hu, E. Auto-ignition and deflagration characteristics of ethanol-gasoline/air at high temperature. Fuel 2019, 255, 115768. [Google Scholar] [CrossRef]
- Reaction Design, I. CHEMKIN-PRO, Release 15101; Reaction Design, Inc.: San Diego, CA, USA, 2010. [Google Scholar]
- Jain, S.; Li, D.; Aggarwal, S.K. Effect of hydrogen and syngas addition on the ignition of iso-octane/air mixtures. Int. J. Hydrog. Energy 2013, 38, 4163–4176. [Google Scholar] [CrossRef]
- Tian, Z.; Li, Y.; Zhang, L.; Glarborg, P.; Qi, F. An experimental and kinetic modeling study of premixed NH3/CH4/O2/Ar flames at low pressure. Combust. Flame 2009, 156, 1413–1426. [Google Scholar] [CrossRef]
- Mitu, M.; Zakel, S.; Brandes, E.; Hirsch, W. Ignition temperature of combustible liquids in mixtures of air with nitrous oxide. Fire Mater. 2021, 46, 544–548. [Google Scholar] [CrossRef]
- Mitu, M.; Brandes, E.; Hirsch, W. Ignition temperatures of combustible liquids with increased oxygen content in the (O2 + N2) mixture. J. Loss Prev. Process Ind. 2019, 62, 103971. [Google Scholar] [CrossRef]
- Prodan, M.; Mitu, M.; Razus, D.; Oancea, D. Spark ignition and propagation properties of methane-air. Rev. Roum. Chim. 2016, 61, 299–305. [Google Scholar]
- Mitu, M.; Razus, D.; Oancea, D. The development of a new optical method to measure the delay time of spark ignition. Studia Univ. Babeș-Bolyai Chem. 2019, 64, 309–322. [Google Scholar] [CrossRef]
- Kumar, P.; Meyer, T.R. Experimental and modeling study of chemical-kinetics mechanisms for H2–NH3–air mixtures in laminar premixed jet flames. Fuel 2013, 108, 166–176. [Google Scholar] [CrossRef]
- Hayakawa, A.; Goto, T.; Mimoto, R.; Arakawa, Y.; Kudo, T.; Kobayashi, H. Laminar burning velocity and Markstein length of ammonia/air premixed flames at various pressures. Fuel 2015, 159, 98–106. [Google Scholar] [CrossRef]




| No. | CH4 | NH3 | Air | Equivalence Ratio (φ) | Temperature | Pressure |
|---|---|---|---|---|---|---|
| 1 | 4.77% | 0.53% | 94.70% | 0.5 | 1415–1813 K | 2 atm |
| 2 | 9.06% | 1.01% | 89.93% | 1.0 | 1665–1877 K | 2 atm |
| 3 | 16.47% | 1.83% | 81.70% | 2.0 | 1479–1559 K | 2 atm |
| 4 | 4.77% | 0.53% | 94.70% | 0.5 | 1390–1712 K | 5 atm |
| 5 | 9.06% | 1.01% | 89.93% | 1.0 | 1375–1643 K | 5 atm |
| 6 | 16.47% | 1.83% | 81.70% | 2.0 | 1355–1709 K | 5 atm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Chen, A.; Guo, Y.; Zhang, L.; Zhang, M.; Deng, X.; Li, J.; Ying, W. Auto-Ignition Delay Characteristics of Ammonia Substitution on Methane. Processes 2022, 10, 2214. https://doi.org/10.3390/pr10112214
Xiao H, Chen A, Guo Y, Zhang L, Zhang M, Deng X, Li J, Ying W. Auto-Ignition Delay Characteristics of Ammonia Substitution on Methane. Processes. 2022; 10(11):2214. https://doi.org/10.3390/pr10112214
Chicago/Turabian StyleXiao, Hua, Aiguo Chen, Yanze Guo, Lifu Zhang, Minghui Zhang, Xi Deng, Jun Li, and Wenxuan Ying. 2022. "Auto-Ignition Delay Characteristics of Ammonia Substitution on Methane" Processes 10, no. 11: 2214. https://doi.org/10.3390/pr10112214
APA StyleXiao, H., Chen, A., Guo, Y., Zhang, L., Zhang, M., Deng, X., Li, J., & Ying, W. (2022). Auto-Ignition Delay Characteristics of Ammonia Substitution on Methane. Processes, 10(11), 2214. https://doi.org/10.3390/pr10112214





