Optimization and Control for Separation of Ethyl Benzene from C8 Aromatic Hydrocarbons with Extractive Distillation
Abstract
:1. Introduction
2. Process Simulation and Optimization Method
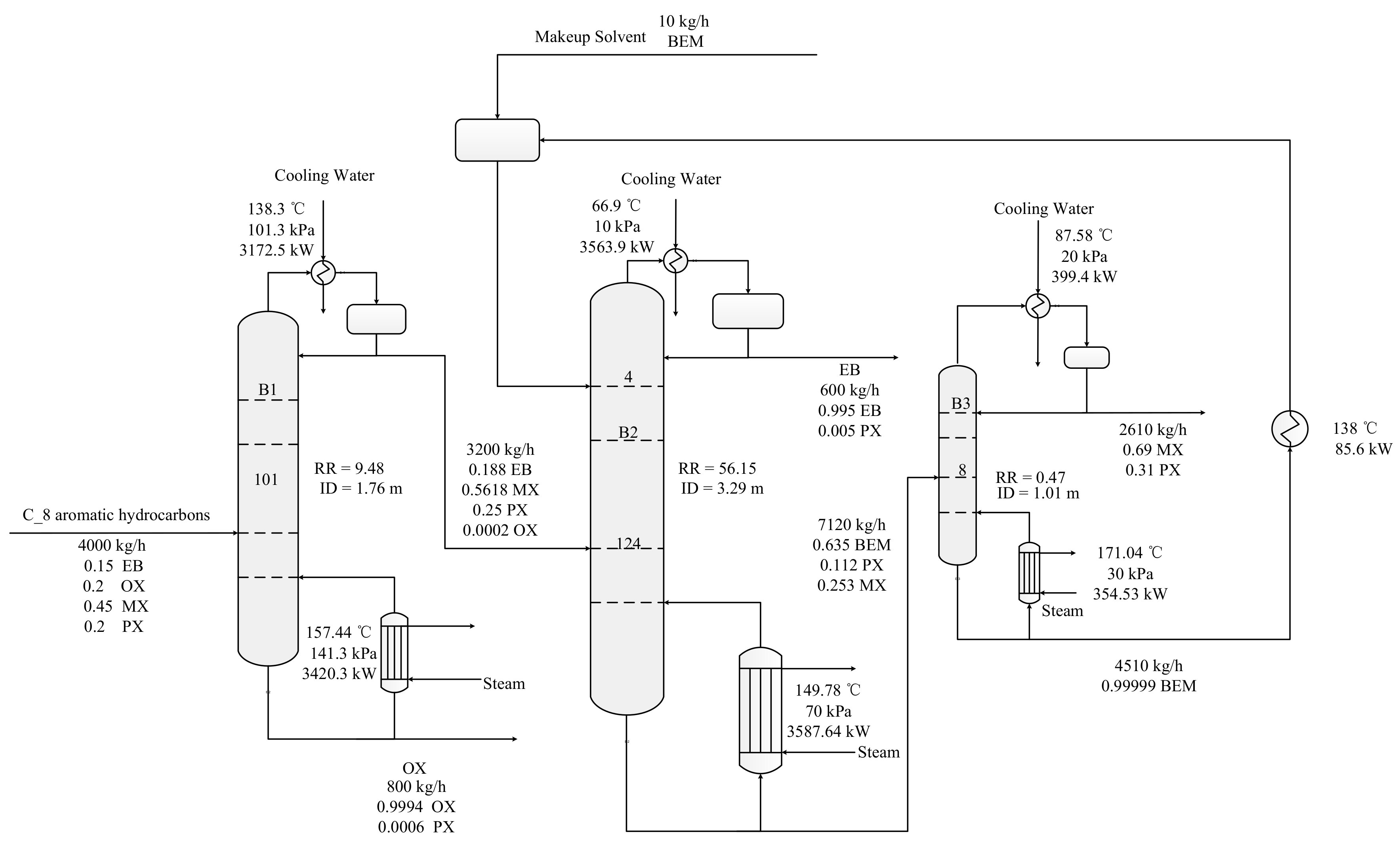
2.1. Steady-State Process
2.2. Optimization Methods
2.2.1. Process Evaluation Indexes
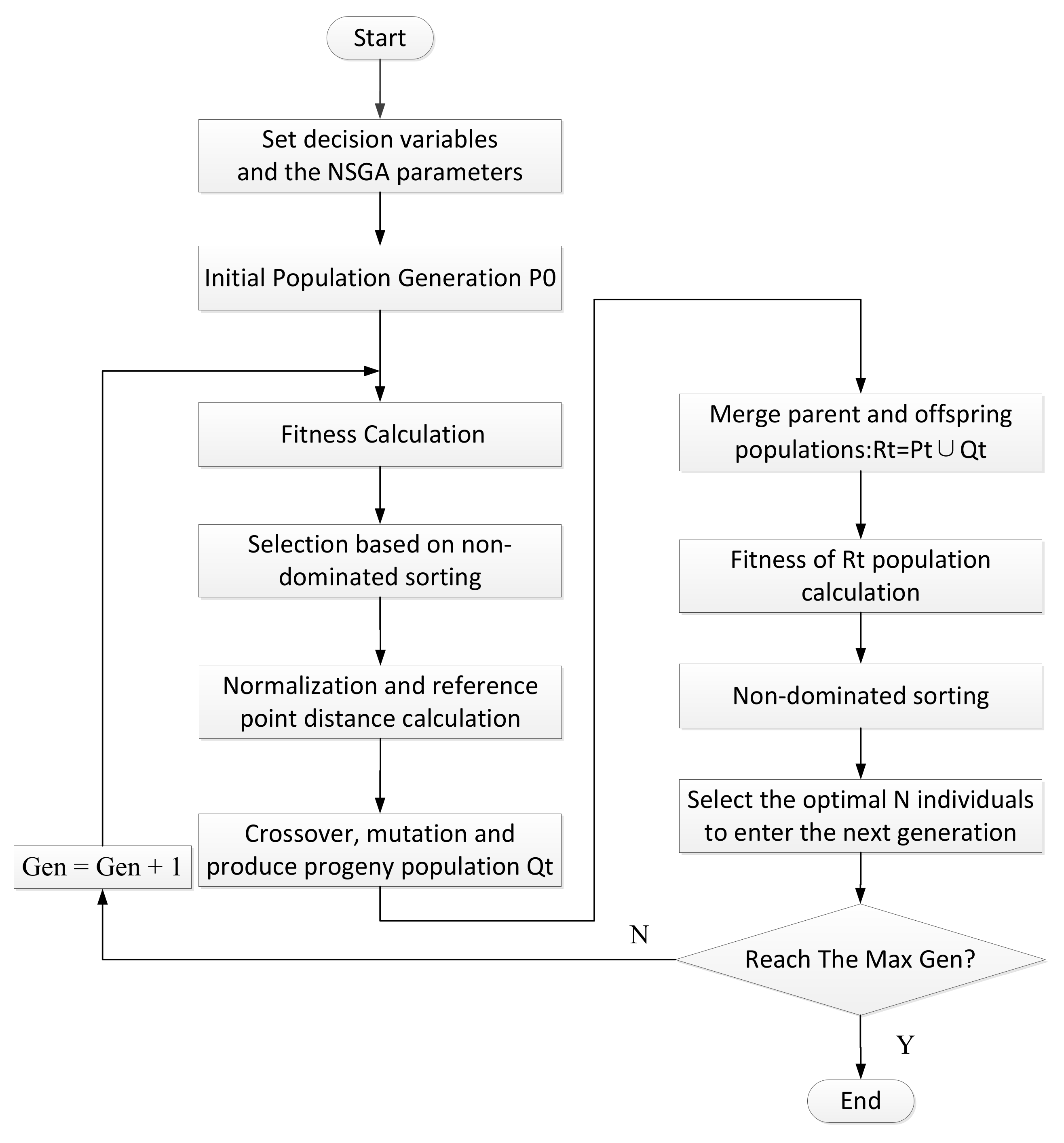
2.2.2. Process Optimization Method
2.3. Dynamic Simulation Method
3. Results and Discussion
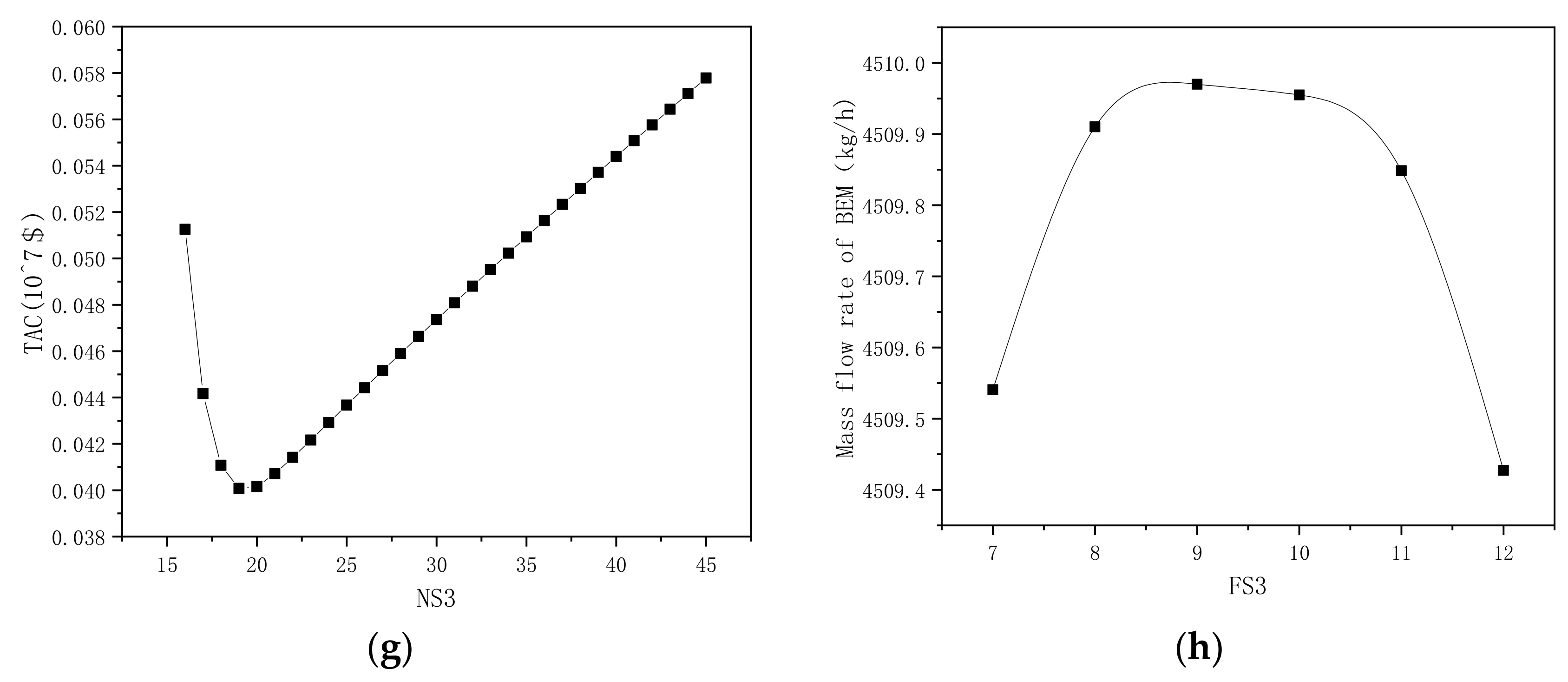
3.1. Sequence Optimization
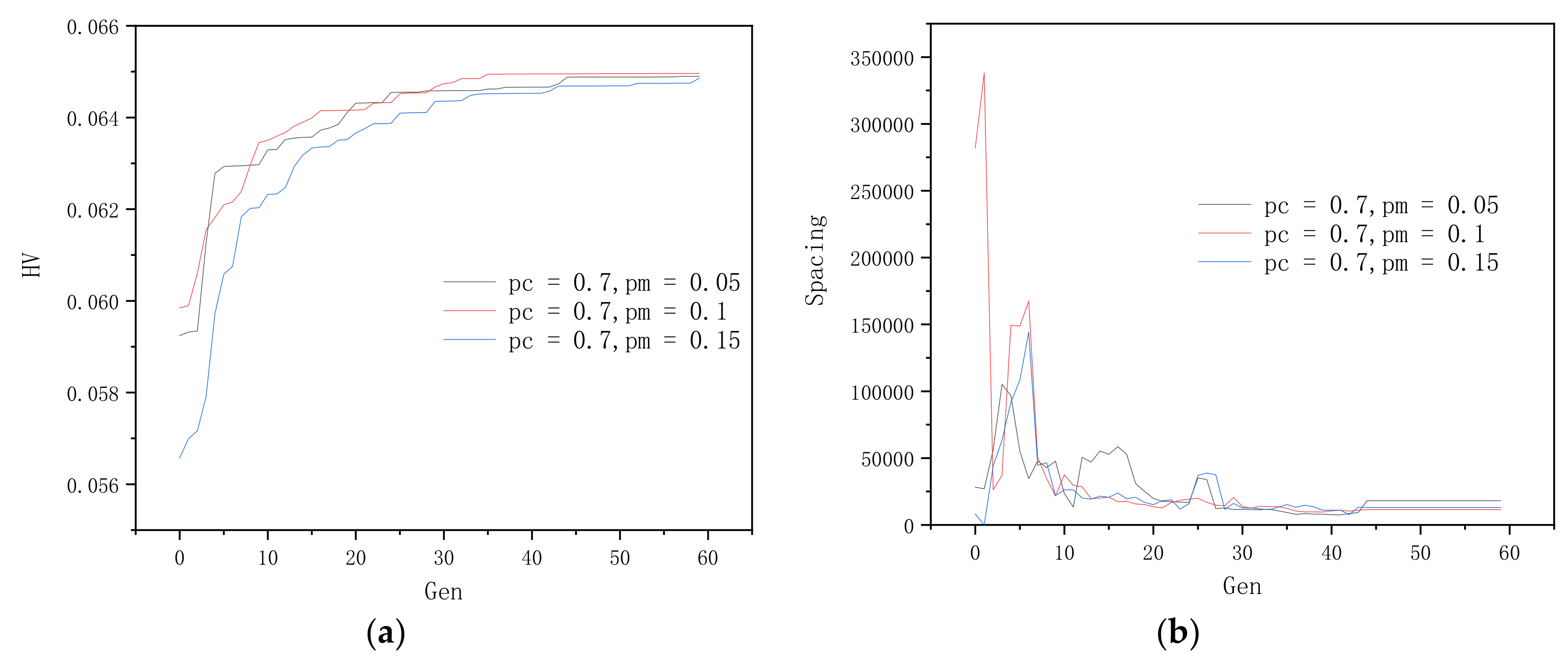
3.2. Optimization of Genetic Algorithm Parameter
3.3. The Global Optimal Solution
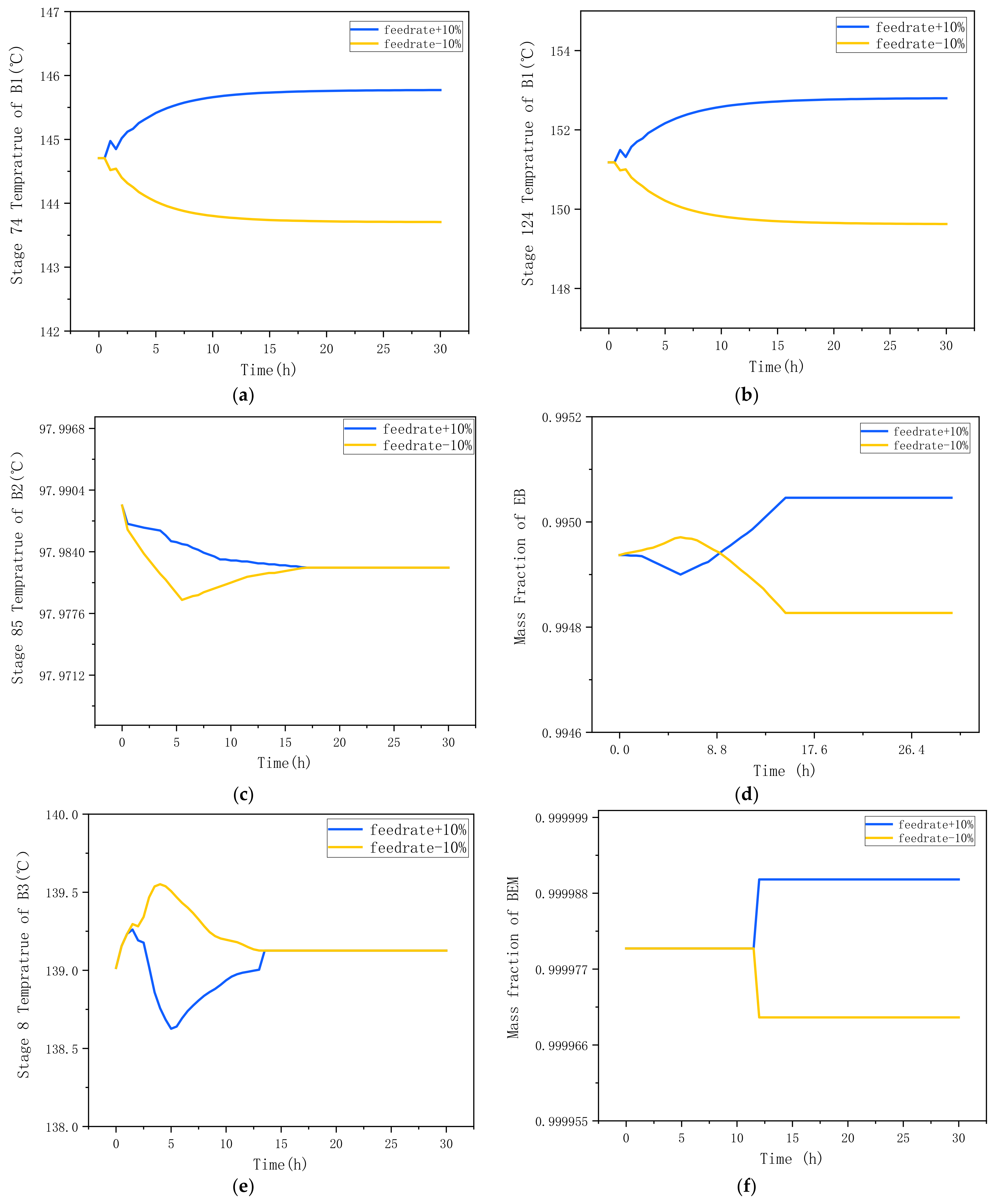
4. Dynamic Control
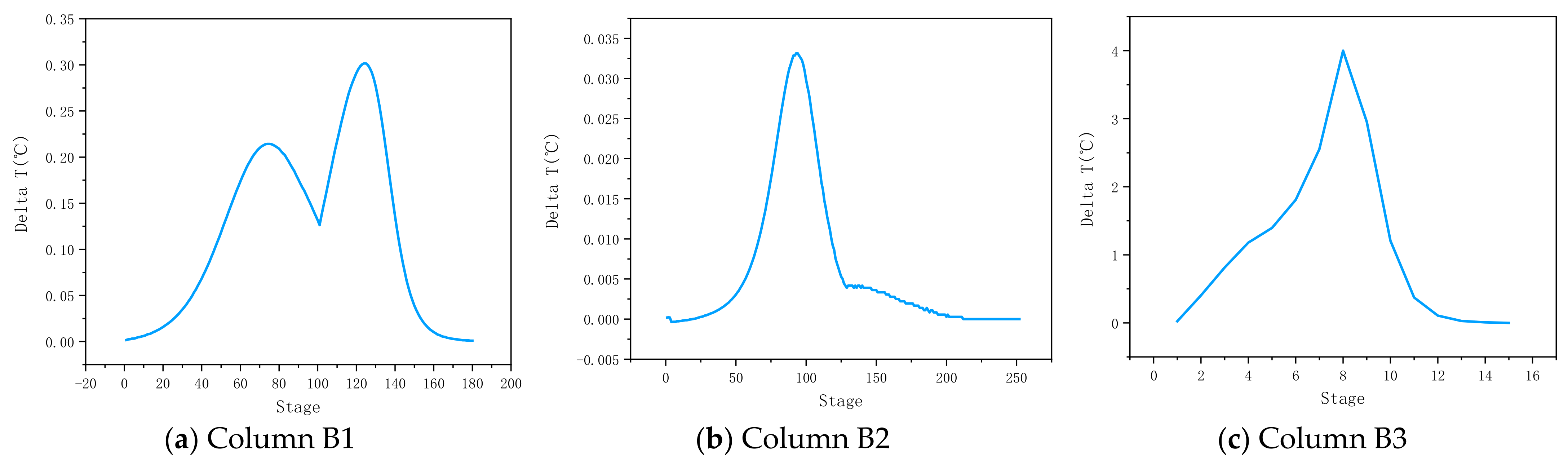
4.1. Determination of Temperature-Sensitive Tray
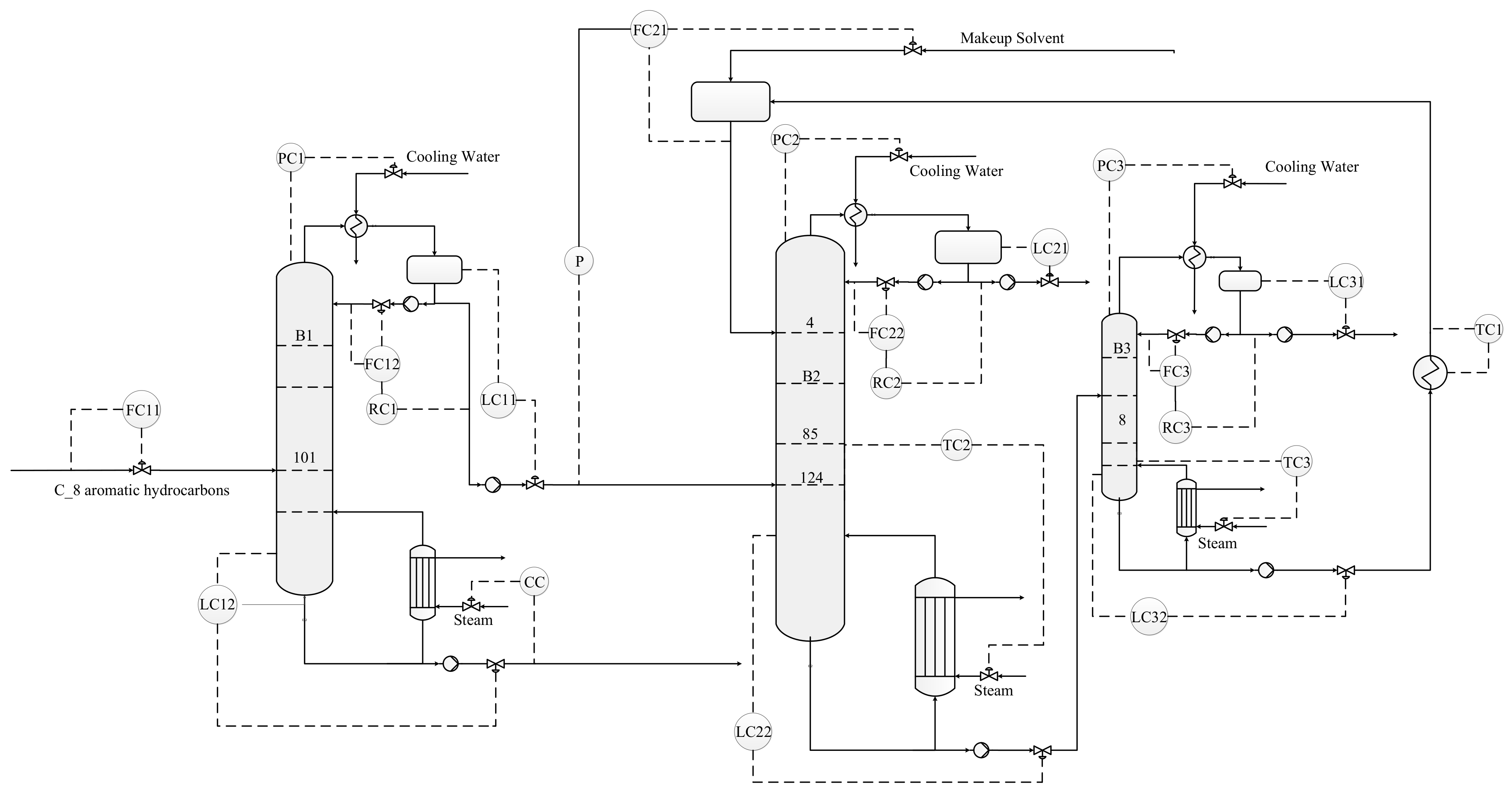
4.2. Basic Control Structure for Circulating Flow Rate of Extractant (CS1)
- The feed flow rate was controlled by a throughput valve (reverse action).
- The liquid level of the reflux tank was manipulated by the distilled flow rates (positive action).
- The sump level of the column of B1 and B2 was manipulated by the bottom flowrate (positive action).
- The sump level of the column of B3 was controlled by the flow of entrainer makeup (reverse action).
- All pressure in the three columns were controlled by manipulating the top condenser duties.
- The reflux ratios in the three columns were fixed.
- The temperature of the recycling solvent was controlled by a heat exchanger (reverse action).
- The recycled solvent flowrate was rationed to the distilled flow rates of column B1 and controlled by a throughput valve (reverse action).
- The temperature of the sensitive tray of the column of B1 was controlled by component controller to manipulate the reboiler duty of column B1 (reverse action).
- The temperatures of the sensitive trays of the columns of B2 and B3 were controlled by changing the reboiler heat duty (reverse action).

4.3. An Improved Control Structure (CS2)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| α | The ratio of the molar molecular mass |
| B1 | The first column |
| B2 | The second column |
| B3 | The third column |
| CC | Capital cost [$/year] |
| [CO2] emissions | The CO2 emissions [kg/h] |
| The average of all distances in the Pareto front | |
| EB | Ethylbenzene |
| ED | Extractive distillation |
| EF | The flowrate of the extraction agent |
| ES | The feed location of extracting agent |
| FS | The feed stage |
| FuelFact | The amount of CO2 emitted per unit of energy |
| GA | Genetic algorithm |
| hproc | The enthalpy of steam gasification [kJ/kg] |
| HV | The hypervolume index |
| IAE | The integral absolute error |
| K | The steady state gain |
| MOO | Multiple objective optimization |
| MX | M-Xylene |
| NHV | The net calorific value |
| NS | The number of stages |
| NSGA | Nondominated sorting genetic algorithm |
| OC | Operating cost [$/year] |
| OX | O-Xylene |
| |P| | The number of points in the Pareto front |
| PX | P-Xylene |
| pc | The crossover probability |
| pm | The mutation probability |
| QFuel | The total heat of fuel combustion [kW] |
| QProc | The heat duty of the rectifying column [kJ/h] |
| ΔR | The heat duty of reboiler [kW] |
| T0 | The ambient temperature [K] |
| TAC | Total annual cost [$/year] |
| TFTB | The flame temperature of boiler [K] |
| Tstack | The chimney temperature [K] |
| ΔTS | The temperature difference of the tray [K] |
| vol(x) | The topological measure value formed by x points |
| λProc | The lantent heat [kJ/kg] |
References
- Pandit, S.R.; Jana, A.K. Transforming conventional distillation sequence to dividing wall column: Minimizing cost, energy usage and environmental impact through genetic algorithm. Sep. Purif. Technol. 2022, 297, 121437. [Google Scholar] [CrossRef]
- You, X.; Gu, J.; Gerbaud, V.; Peng, C.; Liu, H. Optimization of pre-concentration, entrainer recycle and pressure selection for the extractive distillation of acetonitrile-water with ethylene glycol. Chem. Eng. Sci. 2018, 177, 354–368. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; You, X.; Tao, C.; Li, J. Analysis of heat integration, intermediate reboiler and vapor recompression for the extractive distillation of ternary mixture with two binary azeotropes. Chem. Eng. Process. Process Intensif. 2019, 142, 107546. [Google Scholar] [CrossRef]
- Han, D.; Chen, Y.; Shi, D. Different extractive distillation processes for isopropanol dehydration using low transition temperature mixtures as entrainers. Chem. Eng. Process. Process Intensif. 2022, 178, 109049. [Google Scholar] [CrossRef]
- Yang, A.; Yang Kong, Z.; Sunarso, J.; Su, Y.; Wang, Q.; Zhu, S. Insights on sustainable separation of ternary azeotropic mixture tetrahydrofuran/ethyl acetate/water using hybrid vapor recompression assisted side-stream extractive distillation. Sep. Purif. Technol. 2022, 290, 120884. [Google Scholar] [CrossRef]
- Shi, T.; Chun, W.; Yang, A.; Su, Y.; Jin, S.; Ren, J.; Shen, W. Optimization and control of energy saving side-stream extractive distillation with heat integration for separating ethyl acetate-ethanol azeotrope. Chem. Eng. Sci. 2020, 215, 115373. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, N.; Li, Z.; Zhang, Y.; Qu, T.; Zhu, Q. Many-objective optimization for scheduling of crude oil operations based on NSGA-III with consideration of energy efficiency. Swarm Evol. Comput. 2020, 57, 100714. [Google Scholar] [CrossRef]
- Jaime, J.A.; Rodríguez, G.; Gil, I.D. Control of an Optimal Extractive Distillation Process with Mixed-Solvents as Separating Agent. Ind. Eng. Chem. Res. 2018, 57, 9615–9626. [Google Scholar] [CrossRef]
- Qin, J.; Ye, Q.; Xiong, X.; Li, N. Control of Benzene–Cyclohexane Separation System via Extractive Distillation Using Sulfolane as Entrainer. Ind. Eng. Chem. Res. 2013, 52, 10754–10766. [Google Scholar] [CrossRef]
- Ahmadian Behrooz, H. Robust Design and Control of Extractive Distillation Processes under Feed Disturbances. Ind. Eng. Chem. Res. 2017, 56, 4446–4462. [Google Scholar] [CrossRef]
- Yu, B.; Wang, Q.; Xu, C. Design and Control of Distillation System for Methylal/Methanol Separation. Part 2: Pressure Swing Distillation with Full Heat Integration. Ind. Eng. Chem. Res. 2012, 51, 1293–1310. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Q.; Zhang, Z.; Tang, J.; Cui, M.; Chen, X.; Liu, Q.; Fei, Z.; Qiao, X. Surrogate modeling-based multi-objective optimization for the integrated distillation processes. Chem. Eng. Process. Process Intensif. 2021, 159, 108224. [Google Scholar] [CrossRef]
- Yamanee-Nolin, M.; Andersson, N.; Nilsson, B.; Max-Hansen, M.; Pajalic, O. Trajectory optimization of an oscillating industrial two-stage evaporator utilizing a Python-Aspen Plus Dynamics toolchain. Chem. Eng. Res. Des. 2020, 155, 12–17. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Q.; Zhou, M.; Cui, P.; Wang, Y.; Zheng, S.; Zhu, Z.; Gao, J. Economic effect of an efficient and environmentally friendly extractive distillation/pervaporation process on the separation of ternary azeotropes with different compositions. J. Clean. Prod. 2022, 346, 131179. [Google Scholar] [CrossRef]
- Gil, I.D.; Botía, D.C.; Ortiz, P.; Sánchez, O.F. Extractive Distillation of Acetone/Methanol Mixture Using Water as Entrainer. Ind. Eng. Chem. Res. 2009, 48, 4858–4865. [Google Scholar] [CrossRef]
- Chen, J.; Ye, Q.; Liu, T.; Xia, H.; Feng, S. Improving the performance of heterogeneous azeotropic distillation via self-heat recuperation technology. Chem. Eng. Res. Des. 2019, 141, 516–528. [Google Scholar] [CrossRef]
- Ahmed, M.; Abdullah, A.; Laskar, A.; Patle, D.S.; Vo, D.N.; Ahmad, Z. Process simulation and stochastic multiobjective optimisation of homogeneously acid-catalysed microalgal in-situ biodiesel production considering economic and environmental criteria. Fuel 2022, 327, 125165. [Google Scholar] [CrossRef]
- Gutiérrez-Guerra, R.; Segovia-Hernández, J.G.; Hernández, S. Reducing energy consumption and CO2 emissions in extractive distillation. Chem. Eng. Res. Des. 2009, 87, 145–152. [Google Scholar] [CrossRef]
- Di Pretoro, A.; Montastruc, L.; Manenti, F.; Joulia, X. Flexibility assessment of a biorefinery distillation train: Optimal design under uncertain conditions. Comput. Chem. Eng. 2020, 138, 106831. [Google Scholar] [CrossRef]
- Gadalla, M.A.; Olujic, Z.; Jansens, P.J.; Jobson, M.; Smith, R. Reducing CO2 Emissions and Energy Consumption of Heat-Integrated Distillation Systems. Environ. Sci. Technol. 2005, 39, 6860–6870. [Google Scholar] [CrossRef]
- Gu, J.; You, X.; Tao, C.; Li, J.; Gerbaud, V. Energy-Saving Reduced-Pressure Extractive Distillation with Heat Integration for Separating the Biazeotropic Ternary Mixture Tetrahydrofuran–Methanol–Water. Ind. Eng. Chem. Res. 2018, 57, 13498–13510. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Ren, J.; Yang, Y.; Liu, X.; Sun, L. Effective semicontinuous distillation design for separating normal alkanes via multi-objective optimization and control. Chem. Eng. Res. Des. 2021, 168, 340–356. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, X.; Wu, J.; Li, Y. An Improved NSGA-III Algorithm Using Genetic K-Means Clustering Algorithm. IEEE Access 2019, 7, 185239–185249. [Google Scholar] [CrossRef]
- Suggala, S.V.; Bhattacharya, P.K. Real Coded Genetic Algorithm for Optimization of Pervaporation Process Parameters for Removal of Volatile Organics from Water. Ind. Eng. Chem. Res. 2003, 42, 3118–3128. [Google Scholar] [CrossRef]
- Sohani, A.; Delfani, F.; Hosseini, M.; Sayyaadi, H.; Karimi, N.; Li, L.K.B.; Doranehgard, M.H. Dynamic multi-objective optimization applied to a solar-geothermal multi-generation system for hydrogen production, desalination, and energy storage. Int. J. Hydrogen Energy 2022, 47, 31730–31741. [Google Scholar] [CrossRef]
- Li, W.; Shi, L.; Yu, B.; Xia, M.; Luo, J.; Shi, H.; Xu, C. New Pressure-Swing Distillation for Separating Pressure-Insensitive Maximum Boiling Azeotrope via Introducing a Heavy Entrainer: Design and Control. Ind. Eng. Chem. Res. 2013, 52, 7836–7853. [Google Scholar] [CrossRef]
- Kong, Z.Y.; Yang, A.; Segovia Hernández, J.G.; Putranto, A.; Sunarso, J. Towards sustainable separation and recovery of dichloromethane and methanol azeotropic mixture through process design, control, and intensification. J. Chem. Technol. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Li, D.; Zeng, F.; Jin, Q.; Pan, L. Applications of an IMC based PID Controller tuning strategy in atmospheric and vacuum distillation units. Nonlinear Anal. Real World Appl. 2009, 10, 2729–2739. [Google Scholar] [CrossRef]
- Shen, W.; Chien, I. Design and Control of Ethanol/Benzene Separation by Energy-Saving Extraction–Distillation Process Using Glycerol as an Effective Heavy Solvent. Ind. Eng. Chem. Res. 2019, 58, 14295–14311. [Google Scholar] [CrossRef]








| Category | Formula | Unit |
|---|---|---|
| Column diameter | Aspen tray sizing | m |
| Column height | H = 0.125 × (N − 2) | m |
| Column shell cost | shell = 141,320 × D1.066 × H0.802 | $ |
| Column stage cost | packing = 12,000 × 3.14/4 × D2 × H | $ |
| Exchanger area | Area = Q/K/ΔTln | m2 |
| Heat exchanger cost | Exchanger = 6.2 × (8500 + 409 × Area0.85) | $ |
| Capital investment | CC = (shell + packing + Exchanger) × 2 | $ |
| Heat transfer coefficient | KCB1 = 1864.6 | kW/(K × m2) |
| KCB2 = 304.7 | ||
| KCB3 = 789.2 | ||
| KRB1 = 605.58 | ||
| KRB2 = 862.74 | ||
| KRB3 = 613.53 | ||
| Utilities cost | Cooling water: 0.03 | $ |
| HP steam (527 K, 1.5 MPa): 25 | $ |
| B1 | B2 | B3 | Total | |
|---|---|---|---|---|
| NS | 146 | 248 | 19 | \ |
| FS | 83 | 4/128 | 9 | \ |
| Reflux ratio | 15.83 | 64.5 | 0.49 | \ |
| TAC (107 $/year) | 0.553 | 1.015 | 0.040 | 1.608 |
| [CO2]emissions (ton/year) | 10,056.96 | 7082.08 | 676.56 | 17,815.60 |
| B1 | B2 | B3 | Total | |
|---|---|---|---|---|
| NS | 180 | 252 | 15 | \ |
| FS | 101 | 4/128 | 6 | \ |
| Reflux ratio | 9.48 | 56.15 | 0.47 | \ |
| TAC (107 $/year) | 0.432 | 0.950 | 0.038 | 1.420 |
| [CO2]emissions (kg/h) | 6294.4 | 6646.4 | 647.8 | 13,588.6 |
| CS1 | CS2 | |||
|---|---|---|---|---|
| Gain | Integral Time (min) | Gain | Integral Time (min) | |
| CC | 5675 | 28 | 5297 | 30.1 |
| TC1 | 3.1 | 13.2 | 3.7 | 13.2 |
| TC2 | 8.9 | 76.7 | 8.9 | 76 |
| TC3 | 2.1 | 18.5 | 1.1 | 23.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, J.; Ding, J.; Zhang, C.; Wan, H.; Guan, G. Optimization and Control for Separation of Ethyl Benzene from C8 Aromatic Hydrocarbons with Extractive Distillation. Processes 2022, 10, 2237. https://doi.org/10.3390/pr10112237
Pan J, Ding J, Zhang C, Wan H, Guan G. Optimization and Control for Separation of Ethyl Benzene from C8 Aromatic Hydrocarbons with Extractive Distillation. Processes. 2022; 10(11):2237. https://doi.org/10.3390/pr10112237
Chicago/Turabian StylePan, Jincheng, Jiahai Ding, Chundong Zhang, Hui Wan, and Guofeng Guan. 2022. "Optimization and Control for Separation of Ethyl Benzene from C8 Aromatic Hydrocarbons with Extractive Distillation" Processes 10, no. 11: 2237. https://doi.org/10.3390/pr10112237





