Nanofiltration Treatment of Industrial Wastewater Doped with Organic Dye: A Study of Hydrodynamics and Specific Energy
Abstract
:Highlights
- Positive influence of the media through wastewater dilution to permeate quality.
- Reduction in COD removal efficiency with an increase in pressure due to interactions between negative sites on the membrane and ions in solution.
- Optimal energy consumption attained an efficient process for high quality treated wastewater.
- Solution pH and composition changes ion and membrane charge and decreases selectivity.
Abstract
1. Introduction
2. Materials and Methods
2.1. Effluent
2.2. Chemicals
2.3. Nanofiltration Setup and Operations
2.4. Analysis
3. Results and Discussion
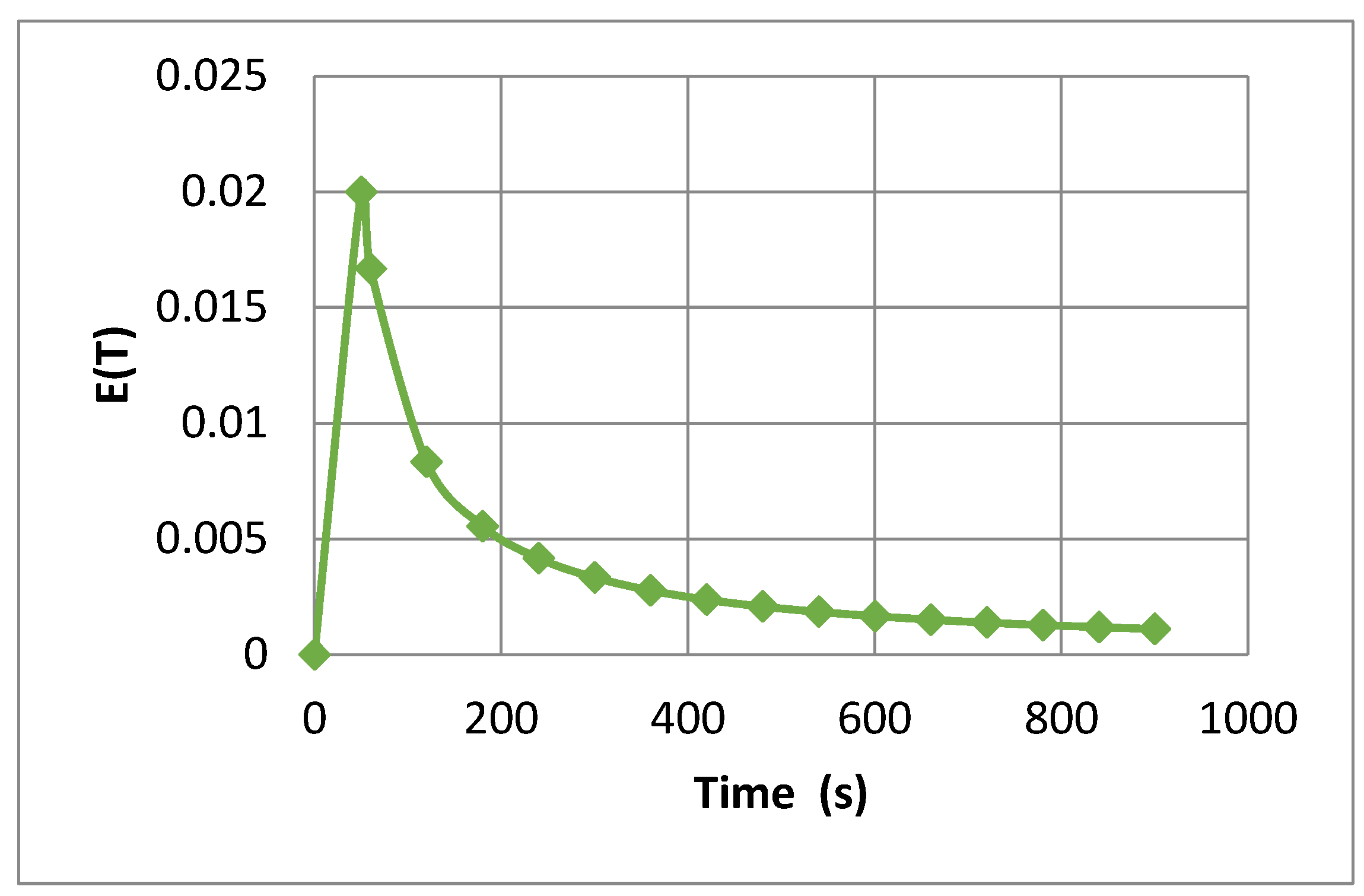
3.1. The Study of the Residence Time Distribution
3.2. Electrical Energy Consumption
3.3. Parametric Study
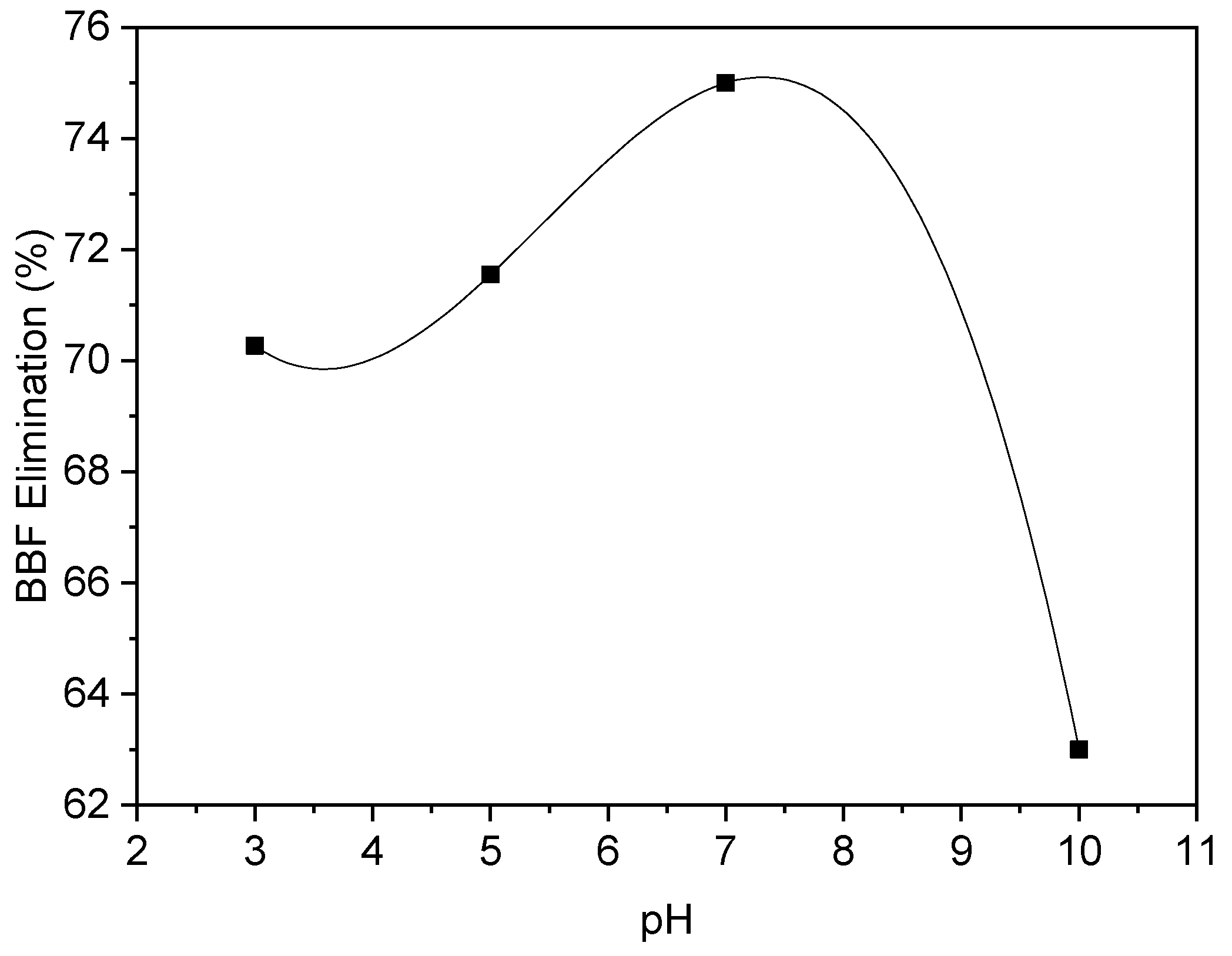
3.3.1. Effect of pH
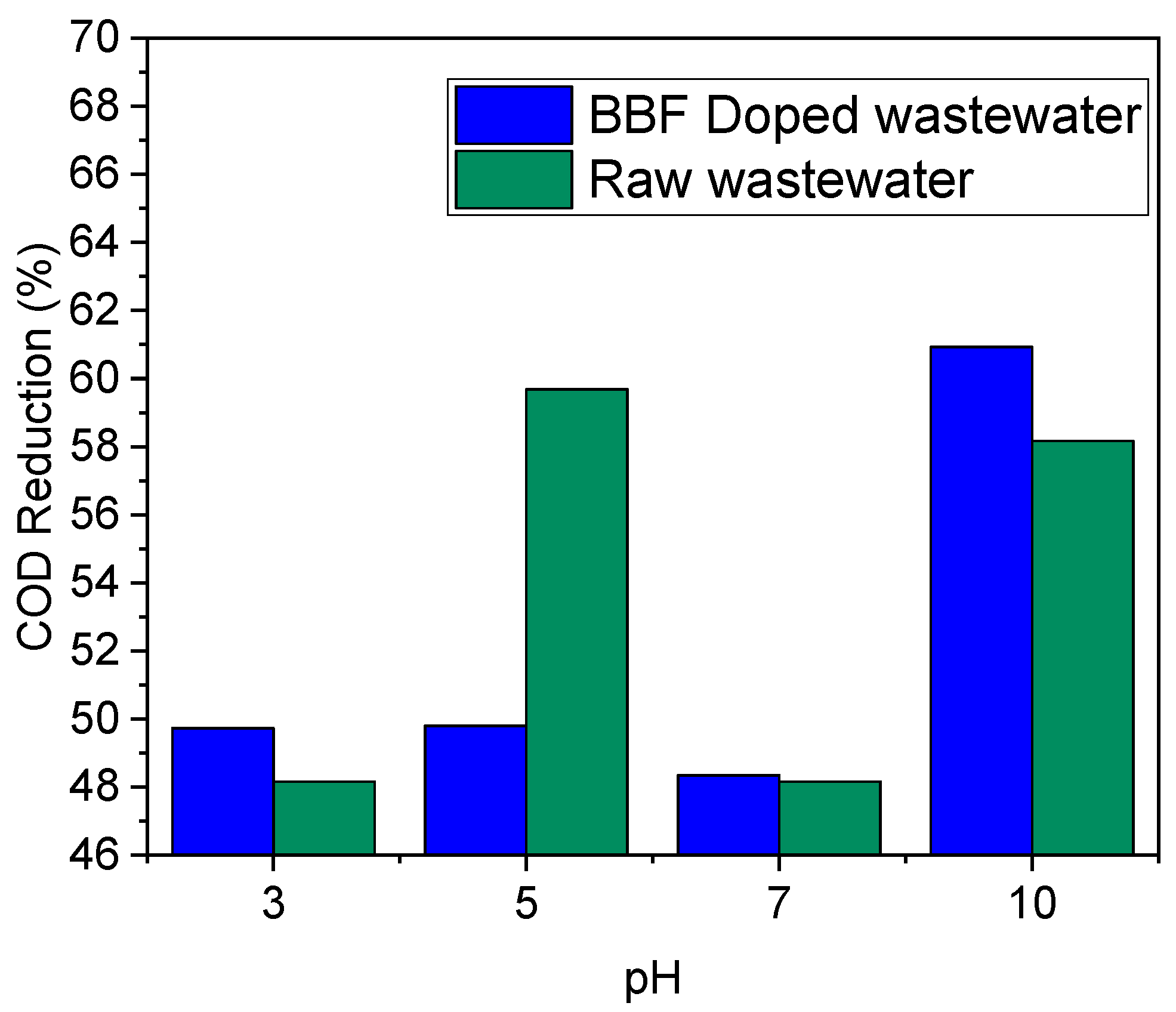
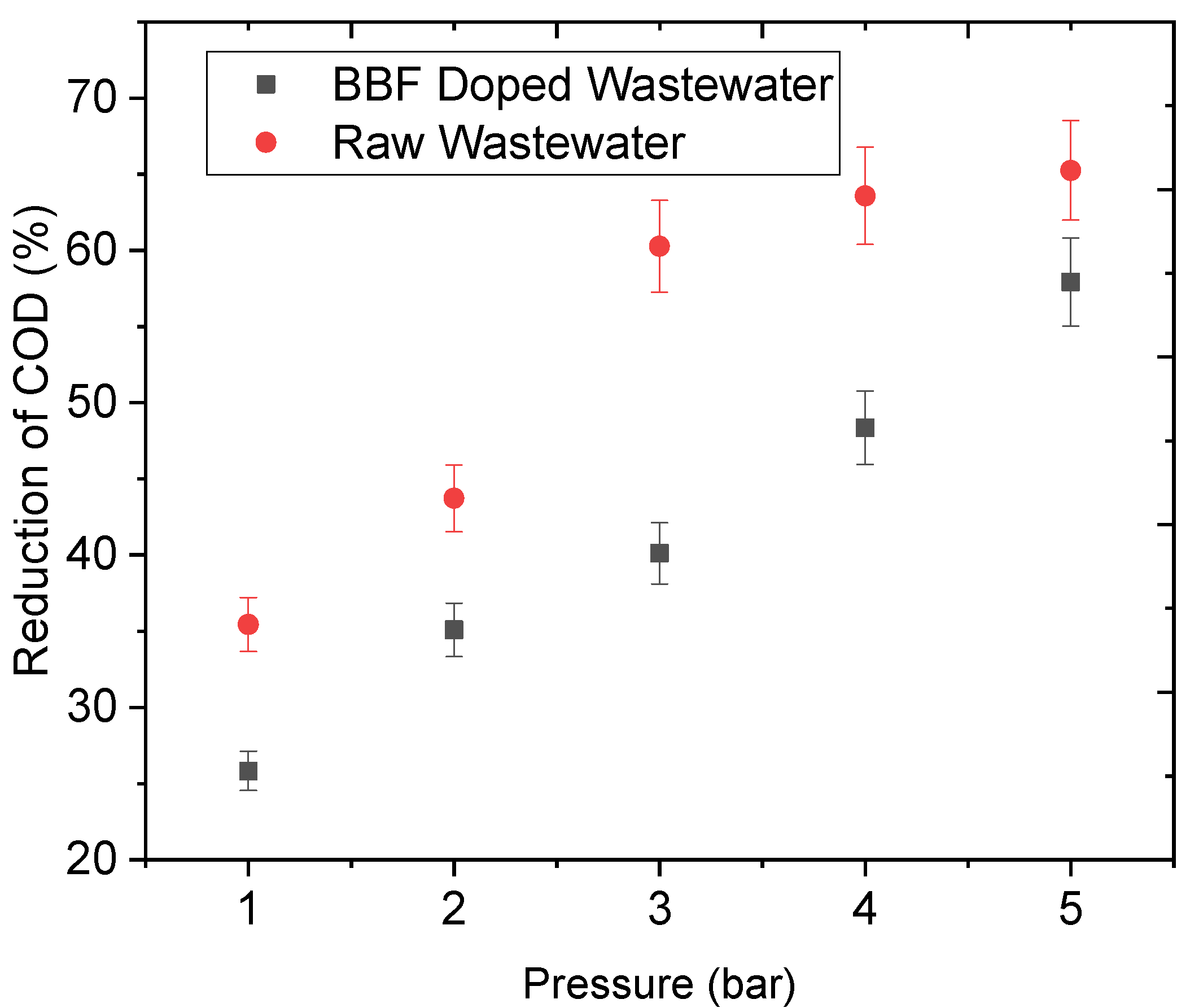
3.3.2. Effect of Pressure
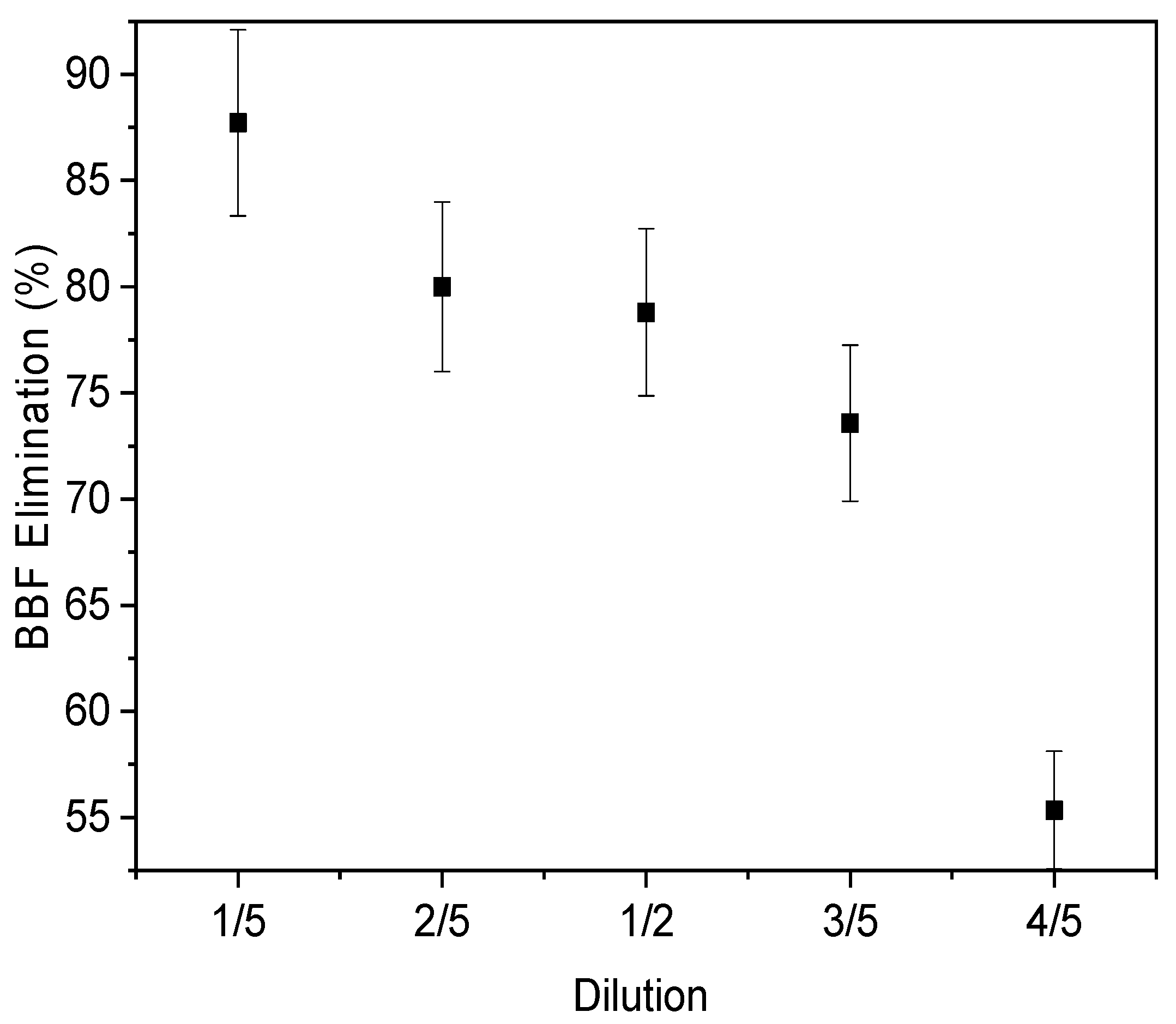
3.3.3. Effect of Matric Dilution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Effective membrane area |
| Ci | Tracer concentration |
| ENF | Specific energy consumption |
| E(t) | Probability density function of the residence times |
| Jw | Water permeate flux |
| Lp | Permeability of the membrane |
| P | Pump power |
| QA | Alimentation flow |
| Qp | Permeate flow |
| t | Permeate collection time |
| tm | Mean residence time |
| V | Volume of permeated water |
| Vr | Reactor volume |
| Work of the hight pressure pump | |
| ∆p | Transmembrane pressure |
| ∆π | Osmotic pressure |
| η | Pump efficiency |
| θ | Normalized time |
| σ2 | Variance of the residence time |
| σ2D | Dimensions variance |
| τ | Residence time |
References
- Hube, S.; Eskafi, M.; Hrafnkelsdóttir, K.F.; Bjarnadóttir, B.; Bjarnadóttir, M.; Axelsdóttir, S.; Wu, B. Direct membrane filtration for wastewater treatment and resource recovery: A review. Sci. Total Environ. 2019, 710, 136375. [Google Scholar] [CrossRef]
- Ilia, M. Impact of the Precoagulation Performance of the Ultrafiltration Process in the Tertiary Treatment for Recycling of Urban Sewage. Curr. Environ. Manag. 2020, 6, 188–195. [Google Scholar] [CrossRef]
- Ahmed, M.T.; Chaabane, T.; Maachi, R.; Darchen, A. Efficiency of a Pretreatment by Electrocoagulation with Aluminum Electrodes in a Nanofiltration Treatment of Polluted Water. Procedia Eng. 2012, 33, 465–474. [Google Scholar] [CrossRef]
- Ahriz, S.; Nedjraoui, D.; Sadki, N. The impact of industrial pollution on the ecosystem of Réghaia Lake (Algeria). Desalination Water Treat. 2010, 24, 1–6. [Google Scholar] [CrossRef]
- Bouchelouche, D.; Saal, I.; Arab, A. Study of the impact of metal and organic pollution on benthic macrofauna using multivariate analyses in coastal wetland of Reghaïa, Algeria. Environ. Sci. Pollut. Res. 2021, 28, 46816–46826. [Google Scholar] [CrossRef]
- Liu, Z.; Lei, M.; Chen, G.; Yuan, J. Treatment of Chromium Removal Wastewater from Tanning by a New Coupling Technology. Processes 2022, 10, 1134. [Google Scholar] [CrossRef]
- Fernández-Medrano, V.; Cuartas-Uribe, B.; Bes-Piá, M.-A.; Mendoza-Roca, J.-A. Application of Nanofiltration and Reverse Osmosis Membranes for Tannery Wastewater Reuse. Water 2022, 14, 2035. [Google Scholar] [CrossRef]
- Altowayti, W.A.H.; Shahir, S.; Othman, N.; Eisa, T.A.E.; Yafooz, W.M.S.; Al-Dhaqm, A.; Soon, C.Y.; Yahya, I.B.; Rahim, N.A.N.B.C.; Abaker, M.; et al. The Role of Conventional Methods and Artificial Intelligence in the Wastewater Treatment: A Comprehensive Review. Processes 2022, 10, 1832. [Google Scholar] [CrossRef]
- Boutilier, L.; Jamieson, R.; Gordon, R.; Lake, C.; Hart, W. Adsorption, sedimentation, and inactivation of E. coli within wastewater treatment wetlands. Water Res. 2009, 43, 4370–4380. [Google Scholar] [CrossRef]
- Marszałek, J.; Żyłła, R. Recovery of Water from Textile Dyeing Using Membrane Filtration Processes. Processes 2021, 9, 1833. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, Z.; Ma, H.; Venkateswaran, S.; Hsiao, B.S. Ultra-fine electrospun nanofibrous membranes for multicomponent wastewater treatment: Filtration and adsorption. Sep. Purif. Technol. 2020, 242, 116794. [Google Scholar] [CrossRef]
- Nelabhotla, A.; Savva, I.; Jensen, J.T.; Wang, S. High Salinity Wastewater Treatment Study Using an Automated Pilot Combining Anaerobic and Aerobic Biofilm Processes. Processes 2022, 10, 766. [Google Scholar] [CrossRef]
- Drioli, E.; Romano, M. Progress and New Perspectives on Integrated Membrane Operations for Sustainable Industrial Growth. Ind. Eng. Chem. Res. 2001, 40, 1277–1300. [Google Scholar] [CrossRef]
- Li, P.; Yang, C.; Sun, F.; Li, X.-Y. Fabrication of conductive ceramic membranes for electrically assisted fouling control during membrane filtration for wastewater treatment. Chemosphere 2021, 280, 130794. [Google Scholar] [CrossRef]
- Shi, Y.-T.; Meng, X.; Yao, L.; Tian, M. A full-scale study of nanofiltration: Separation and recovery of NaCl and Na2SO4 from coal chemical industry wastewater. Desalination 2021, 517, 115239. [Google Scholar] [CrossRef]
- Ivić, I.; Kopjar, M.; Jakobek, L.; Jukić, V.; Korbar, S.; Marić, B.; Mesić, J.; Pichler, A. Influence of Processing Parameters on Phenolic Compounds and Color of Cabernet Sauvignon Red Wine Concentrates Obtained by Reverse Osmosis and Nanofiltration. Processes 2021, 9, 89. [Google Scholar] [CrossRef]
- Sharif, A.; Merdaw, A.; Al-Bahadili, H.; Al-Taee, A.; Al-Aibi, S.; Rahal, Z.; Derwish, G. A new theoretical approach to estimate the specific energy consumption of reverse osmosis and other pressure-driven liquid-phase membrane processes. Desalination Water Treat. 2009, 3, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Adda, A.; Naceur, W.M.; Abbas, M. Modélisation et optimisation de la consommation d’énergie d’une station de dessalement par procédé d’osmose inverse en Algérie. Rev. Energ. Renouvelables 2016, 19, 157–164. [Google Scholar] [CrossRef]
- Ejraei, A.; Aroon, M.A.; Saravani, A.Z. Wastewater treatment using a hybrid system combining adsorption, photocatalytic degradation and membrane filtration processes. J. Water Process Eng. 2019, 28, 45–53. [Google Scholar] [CrossRef]
- El Bahja, H.; Vega, P.; Tadeo, F. Integrating Dynamic Economic Optimization and Nonlinear Closed-Loop GPC: Application to a WWTP. Processes 2022, 10, 1821. [Google Scholar] [CrossRef]
- Grzechulska-Damszel, J.; Mozia, S.; Morawski, A.W. Integration of photocatalysis with membrane processes for purification of water contaminated with organic dyes. Catal. Today 2010, 156, 295–300. [Google Scholar] [CrossRef]
- Ganzenko, O.; Sistat, P.; Trellu, C.; Bonniol, V.; Rivallin, M.; Cretin, M. Reactive electrochemical membrane for the elimination of carbamazepine in secondary effluent from wastewater treatment plant. Chem. Eng. J. 2021, 419, 129467. [Google Scholar] [CrossRef]
- Waqas, S.; Harun, N.Y.; Bilad, M.R.; Samsuri, T.; Nordin, N.A.H.; Shamsuddin, N.; Nandiyanto, A.B.D.; Huda, N.; Roslan, J. Response Surface Methodology for Optimization of Rotating Biological Contactor Combined with External Membrane Filtration for Wastewater Treatment. Membranes 2022, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Giwa, A.; Jung, S.M.; Kong, J.; Hasan, S.W. Combined process of electrically-membrane bioreactor and TiO2 aerogel filtration for efficient wastewater treatment. J. Water Process Eng. 2019, 28, 107–114. [Google Scholar] [CrossRef]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef] [Green Version]
- Taleb-Ahmed, M.; Taha, S.; Maachi, R.; Dorange, G. The influence of physico-chemistry on the retention of chromium ions during nanofiltration. Desalination 2002, 145, 103–108. [Google Scholar] [CrossRef]
- Djahida, Z.; Amel, B.; Mourad, T.A.; Hayet, D.; Rachida, M. Treatment of a dye solophenyle 4GE by coupling electrocoagulation/nanofiltration. Membr. Water Treat. 2014, 5, 251–263. [Google Scholar] [CrossRef]
- Ounnar, A.; Bouzaza, A.; Favier, L.; Bentahar, F. Macrolide antibiotics removal using a circulating TiO2-coated paper photoreactor: Parametric study and hydrodynamic flow characterization. Water Sci. Technol. 2016, 73, 2627–2637. [Google Scholar] [CrossRef]
- Lali, F. A hydrodynamic study of cylindrical metal foam packings: Residence time distribution and two phase pressure drop. Chem. Eng. Process. Process Intensif. 2017, 115, 1–10. [Google Scholar] [CrossRef]
- Teng, J.; Shen, L.; He, Y.; Liao, B.-Q.; Wu, G.; Lin, H. Novel insights into membrane fouling in a membrane bioreactor: Elucidating interfacial interactions with real membrane surface. Chemosphere 2018, 210, 769–778. [Google Scholar] [CrossRef]
- Okamoto, Y.; Lienhard, J.H. How RO membrane permeability and other performance factors affect process cost and energy use: A review. Desalination 2019, 470, 114064. [Google Scholar] [CrossRef]
- Meriläinen, A.; Seppälä, A.; Kauranen, P. Minimizing specific energy consumption of oxygen enrichment in polymeric hollow fiber membrane modules. Appl. Energy 2012, 94, 285–294. [Google Scholar] [CrossRef]
- Hafiz, M.; Hawari, A.; Alfahel, R.; Hassan, M.; Altaee, A. Comparison of Nanofiltration with Reverse Osmosis in Reclaiming Tertiary Treated Municipal Wastewater for Irrigation Purposes. Membranes 2021, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Hakami, M.W.; Alkhudhiri, A.; Al-Batty, S.; Zacharof, M.-P.; Maddy, J.; Hilal, N. Ceramic Microfiltration Membranes in Wastewater Treatment: Filtration Behavior, Fouling and Prevention. Membranes 2020, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Mulyanti, R.; Susanto, H. Wastewater treatment by nanofiltration membranes. IOP Conf. Ser. Earth Environ. Sci. 2018, 142, 012017. [Google Scholar] [CrossRef]
- Bellona, C.; Drewes, J.E.; Xu, P.; Amy, G. Factors affecting the rejection of organic solutes during NF/RO treatment—A literature review. Water Res. 2004, 38, 2795–2809. [Google Scholar] [CrossRef]
- Zhang, X. Selective separation membranes for fractionating organics and salts for industrial wastewater treatment: Design strategies and process assessment. J. Membr. Sci. 2021, 643, 120052. [Google Scholar] [CrossRef]
- Ringkjøb, H.-K.; Haugan, P.M.; Solbrekke, I.M. A review of modelling tools for energy and electricity systems with large shares of variable renewables. Renew. Sustain. Energy Rev. 2018, 96, 440–459. [Google Scholar] [CrossRef]
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- Dreier, L.B.; Nagata, Y.; Lutz, H.; Gonella, G.; Hunger, J.; Backus, E.H.; Bonn, M. Saturation of charge-induced water alignment at model membrane surfaces. Sci. Adv. 2018, 4, eaap7415. [Google Scholar] [CrossRef]
- Yaroshchuk, A.; Bruening, M.L.; Zholkovskiy, E. Modelling nanofiltration of electrolyte solutions. Adv. Colloid Interface Sci. 2019, 268, 39–63. [Google Scholar] [CrossRef] [PubMed]
- Meschke, K.; Daus, B.; Haseneder, R.; Repke, J.-U. Strategic elements from leaching solutions by nanofiltration—Influence of pH on separation performance. Sep. Purif. Technol. 2017, 184, 264–274. [Google Scholar] [CrossRef]
- Kaya, C.; Jarma, Y.A.; Guler, E.; Kabay, N.; Arda, M.; Yükse, M. Seawater Desalination by using Nanofiltration (NF) and Brackish Water Reverse Osmosis (BWRO) Membranes in Sequential Mode of Operation. J. Membr. Sci. Res. 2020, 6, 40–46. [Google Scholar] [CrossRef]
- Shon, H.K.; Phuntsho, S.; Chaudhary, D.S.; Vigneswaran, S.; Cho, J. Nanofiltration for water and wastewater treatment—A mini review. Drink. Water Eng. Sci. 2013, 6, 47–53. [Google Scholar] [CrossRef] [Green Version]
- AL Mashrafi, S.; Diaz-Elsayed, N.; Benjamin, J.; Arias, M.E.; Zhang, Q. An environmental and economic sustainability assessment of a pressure retarded osmosis system. Desalination 2022, 537, 115869. [Google Scholar] [CrossRef]
- Tang, C.Y.; Chong, T.; Fane, A.G. Colloidal interactions and fouling of NF and RO membranes: A review. Adv. Colloid Interface Sci. 2011, 164, 126–143. [Google Scholar] [CrossRef]
- Damtie, M.M.; Woo, Y.C.; Kim, B.; Hailemariam, R.H.; Park, K.-D.; Shon, H.K.; Park, C.; Choi, J.-S. Removal of fluoride in membrane-based water and wastewater treatment technologies: Performance review. J. Environ. Manag. 2019, 251, 109524. [Google Scholar] [CrossRef]
- Tu, S.; Ravindran, V.; Pirbazari, M. A pore diffusion transport model for forecasting the performance of membrane processes. J. Membr. Sci. 2005, 265, 29–50. [Google Scholar] [CrossRef]
- Ng, M.; Dalhatou, S.; Wilson, J.; Kamdem, B.P.; Temitope, M.B.; Paumo, H.K.; Djelal, H.; Assadi, A.A.; Nguyen-Tri, P.; Kane, A. Characterization of Slaughterhouse Wastewater and Development of Treatment Techniques: A Review. Processes 2022, 10, 1300. [Google Scholar] [CrossRef]
- Larrañaga, A.; Lomora, M.; Sarasua, J.; Palivan, C.; Pandit, A. Polymer capsules as micro-/nanoreactors for therapeutic applications: Current strategies to control membrane permeability. Prog. Mater. Sci. 2017, 90, 325–357. [Google Scholar] [CrossRef]
- Abdelhamid, A.E.; El-Sayed, A.A.; Khalil, A.M. Polysulfone nanofiltration membranes enriched with functionalized graphene oxide for dye removal from wastewater. J. Polym. Eng. 2020, 40, 833–841. [Google Scholar] [CrossRef]




| Wastewater | Raw Wastewater | Mixed Wastewater with BBF | Discharge Standards (WHO) | |
|---|---|---|---|---|
| Parameters | ||||
| T (°C) | 22 | 22 | ˂30 °C | |
| Turbidity (FNU) | 91.7 | 95.2 | 5 NTU | |
| pH | 7.27 | 7.06 | 6.5-8.5 | |
| Conductivity (mS/cm) | 1.71 | 1.70 | - | |
| TDS (mg/L) | 205 | 210 | ˂20 mg/L | |
| COD (mg O2/L) | 620 | 660 | ˂90 mg O2/L | |
| BOD5 (mg O2/L) | 218 | 209 | ˂30 mg O2/L | |
| COD/BOD5 | 2.84 | 3.16 | ˂3 | |
| NO3− (mg/L) | 0.58 | 0.66 | ˂1 mg/L | |
| NO2−(mg/L) | 2.26 | 2.20 | 1 mg/L | |
| SO4− (mg/L) | 0.31 | 0.30 | - | |
| PO4− (mg/L) | 12.29 | 12.19 | - | |
| Absorbance at 254 nm | 1.092 | 1.106 | - | |
| Membrane Name | Nanomax-50 |
|---|---|
| Membrane material | polysulfon |
| Molecular weight cutoff | 300 Da |
| pH operating range | 2–10 |
| Maximum temperature | 40 °C |
| Maximum pressure | 5 bar |
| Water permeability | 8.97 µm/s bar |
| t (min) | θ | tm (min) | τ (min−1) | σ2 | σ2D |
|---|---|---|---|---|---|
| 1 | 0.4658 | 211.16 | 2.13 | 1.35 × 10+16 | 4.12 × 10+27 |
| 5 | 2.3292 | ||||
| 10 | 4.6564 | ||||
| 15 | 6.9876 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youcef, R.; Sabba, N.; Benhadji, A.; Djelal, H.; Fakhfakh, N.; Taleb Ahmed, M. Nanofiltration Treatment of Industrial Wastewater Doped with Organic Dye: A Study of Hydrodynamics and Specific Energy. Processes 2022, 10, 2277. https://doi.org/10.3390/pr10112277
Youcef R, Sabba N, Benhadji A, Djelal H, Fakhfakh N, Taleb Ahmed M. Nanofiltration Treatment of Industrial Wastewater Doped with Organic Dye: A Study of Hydrodynamics and Specific Energy. Processes. 2022; 10(11):2277. https://doi.org/10.3390/pr10112277
Chicago/Turabian StyleYoucef, Rokia, Nassila Sabba, Amel Benhadji, Hayet Djelal, Nadim Fakhfakh, and Mourad Taleb Ahmed. 2022. "Nanofiltration Treatment of Industrial Wastewater Doped with Organic Dye: A Study of Hydrodynamics and Specific Energy" Processes 10, no. 11: 2277. https://doi.org/10.3390/pr10112277
APA StyleYoucef, R., Sabba, N., Benhadji, A., Djelal, H., Fakhfakh, N., & Taleb Ahmed, M. (2022). Nanofiltration Treatment of Industrial Wastewater Doped with Organic Dye: A Study of Hydrodynamics and Specific Energy. Processes, 10(11), 2277. https://doi.org/10.3390/pr10112277









