Exploration of Super-Gravity Rapid Dissolution Method of Polymer for Offshore Oil Repellent
Abstract
:1. Introduction
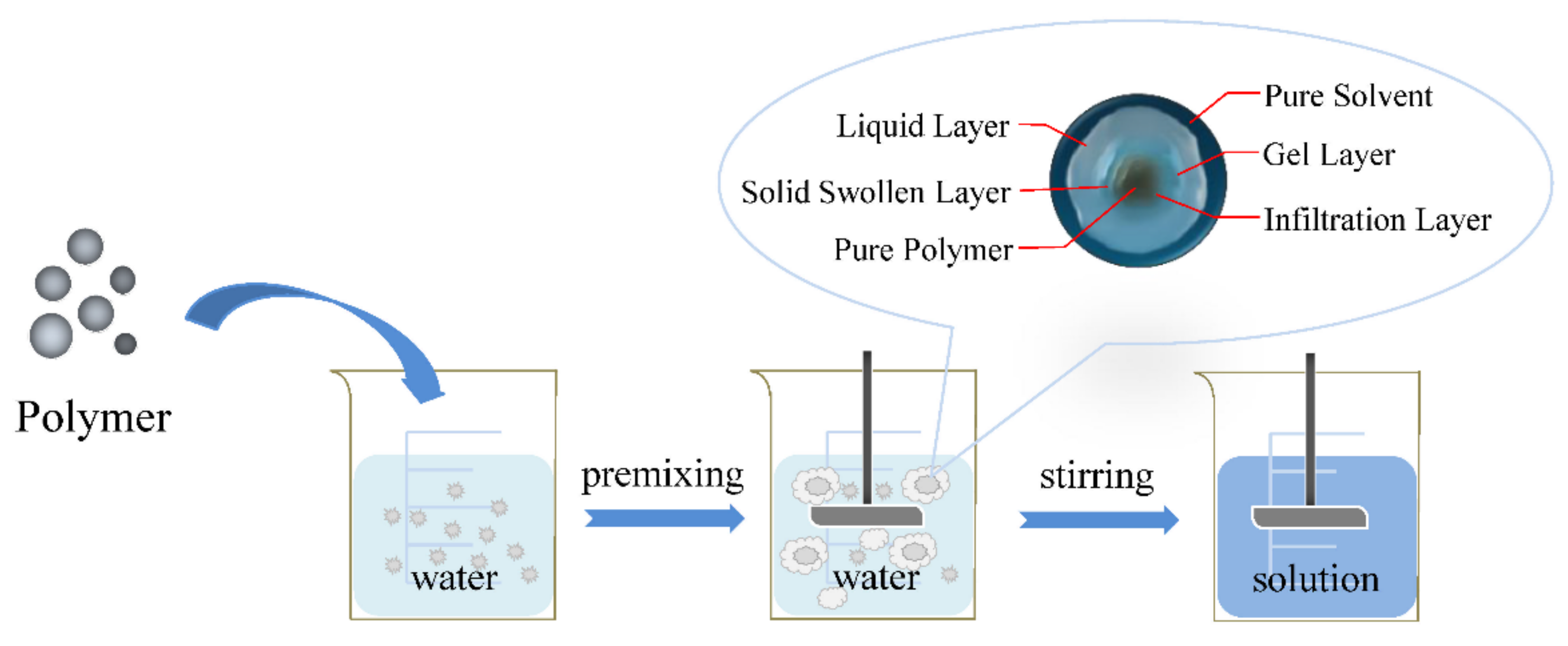
2. Polymer Dissolution Processes
3. Super-Gravity Rapid Dissolution Method
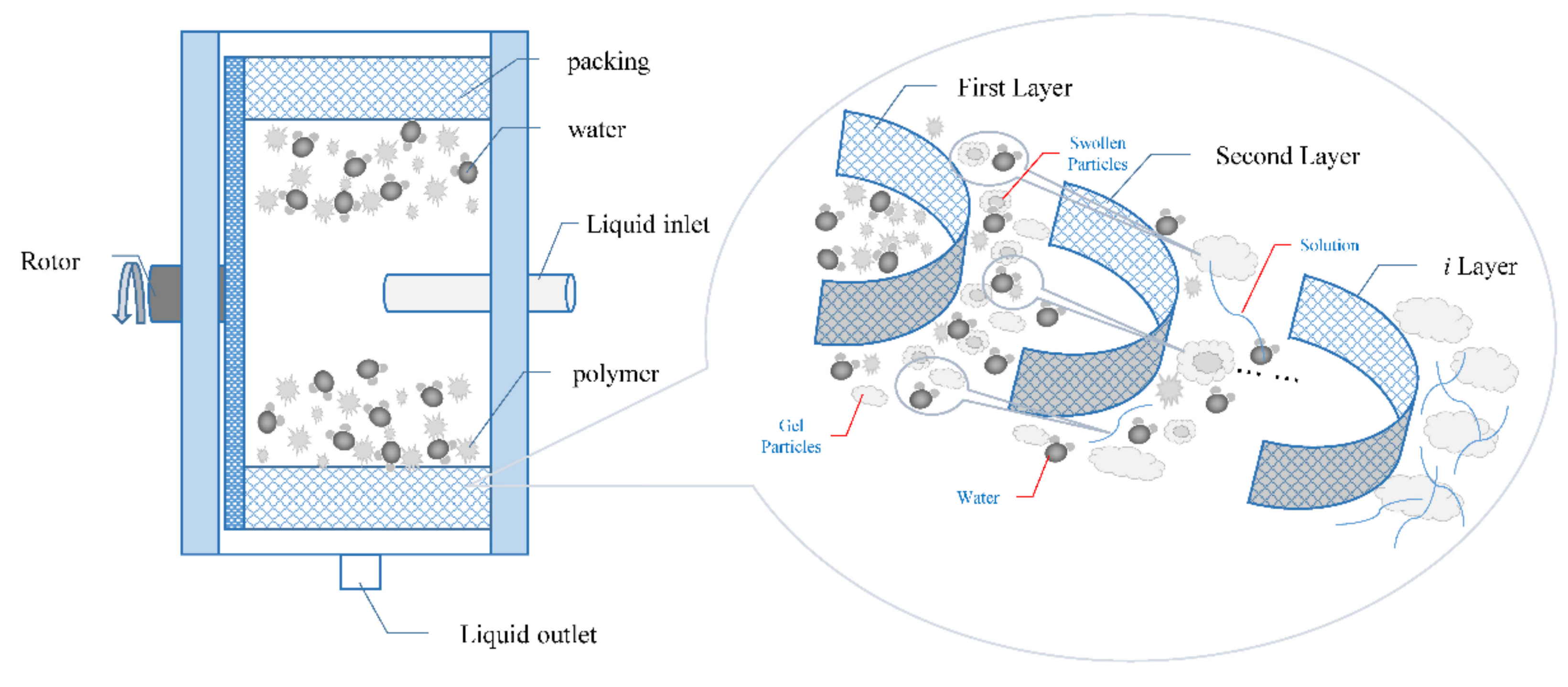
3.1. Super-Gravity Rapid Dissolution Mechanism
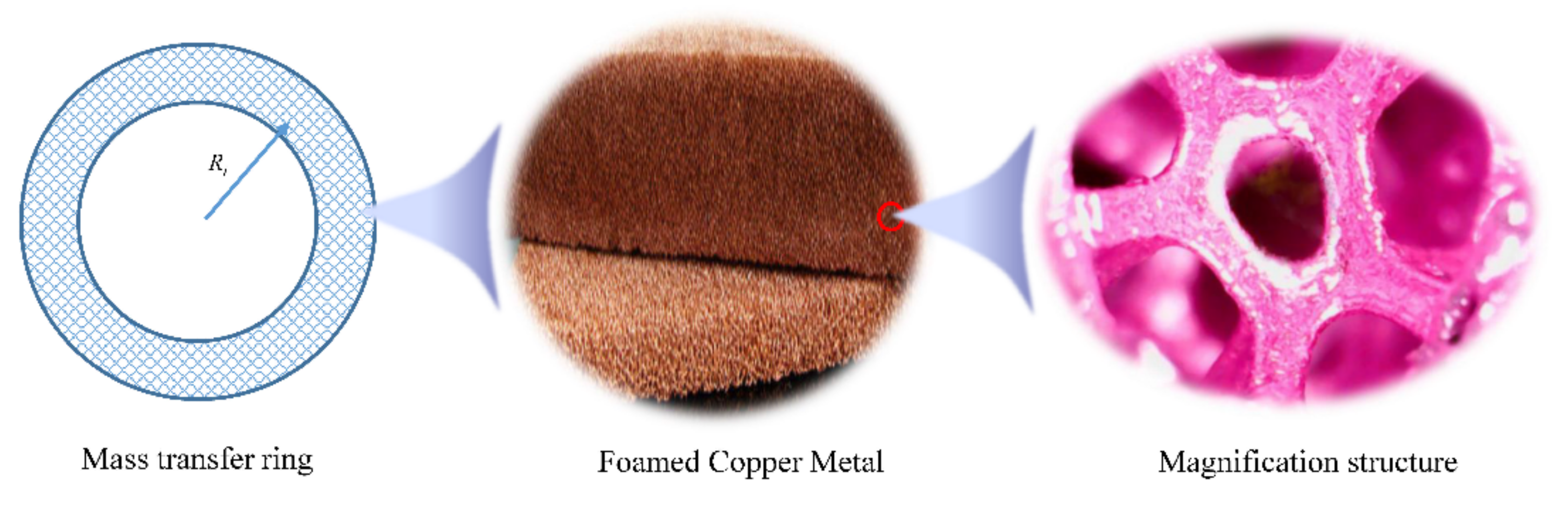
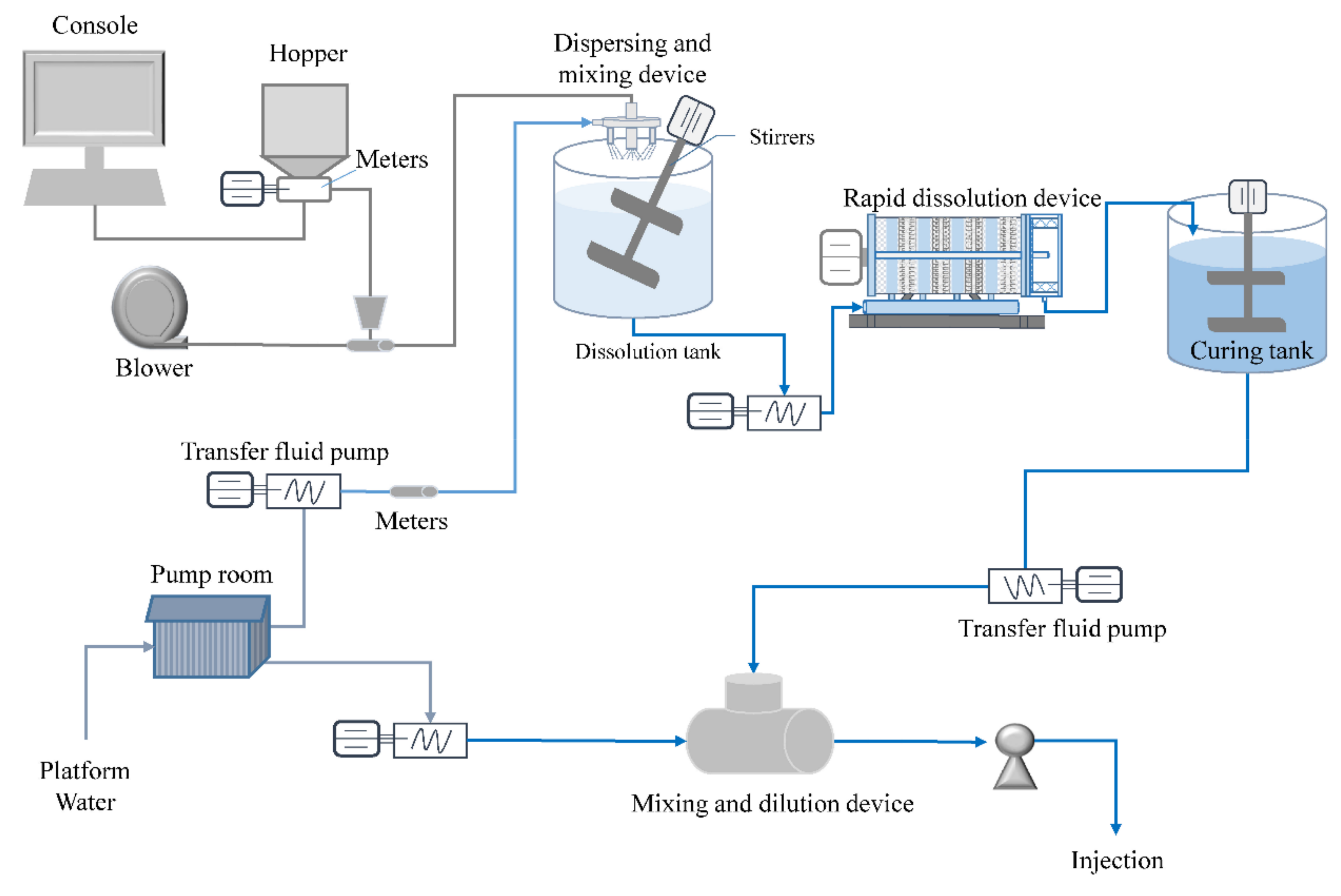
3.2. Super-Gravity Rapid Dissolution Realization Method
4. Experimental Section
4.1. Materials
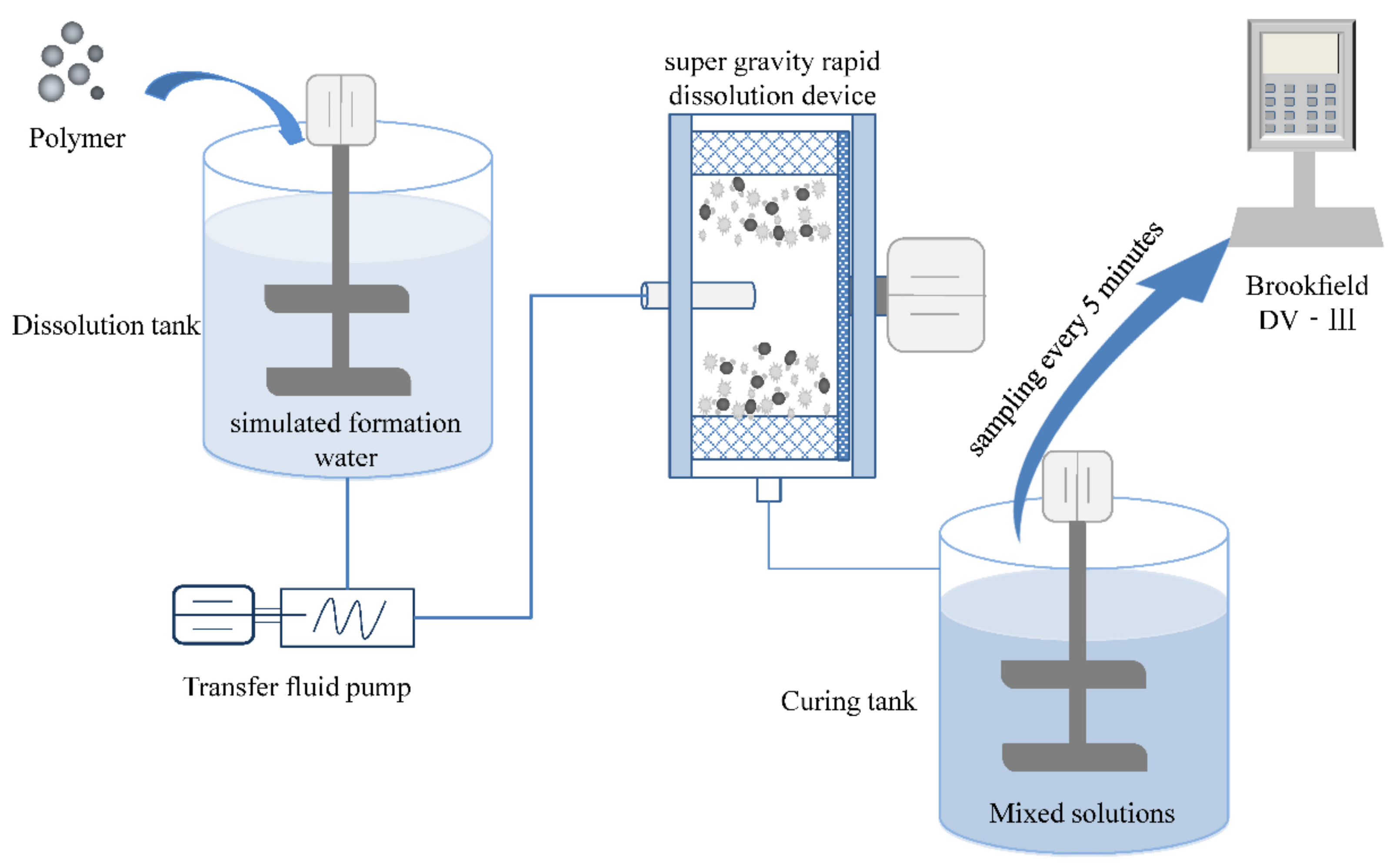
4.2. Methodology
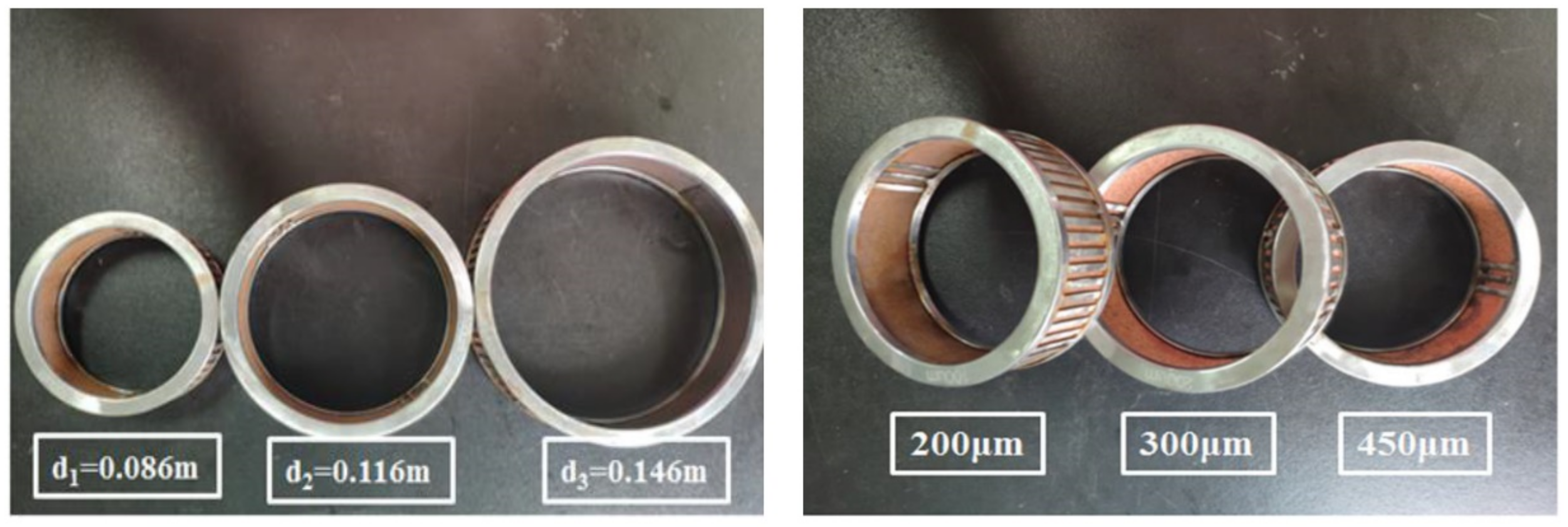
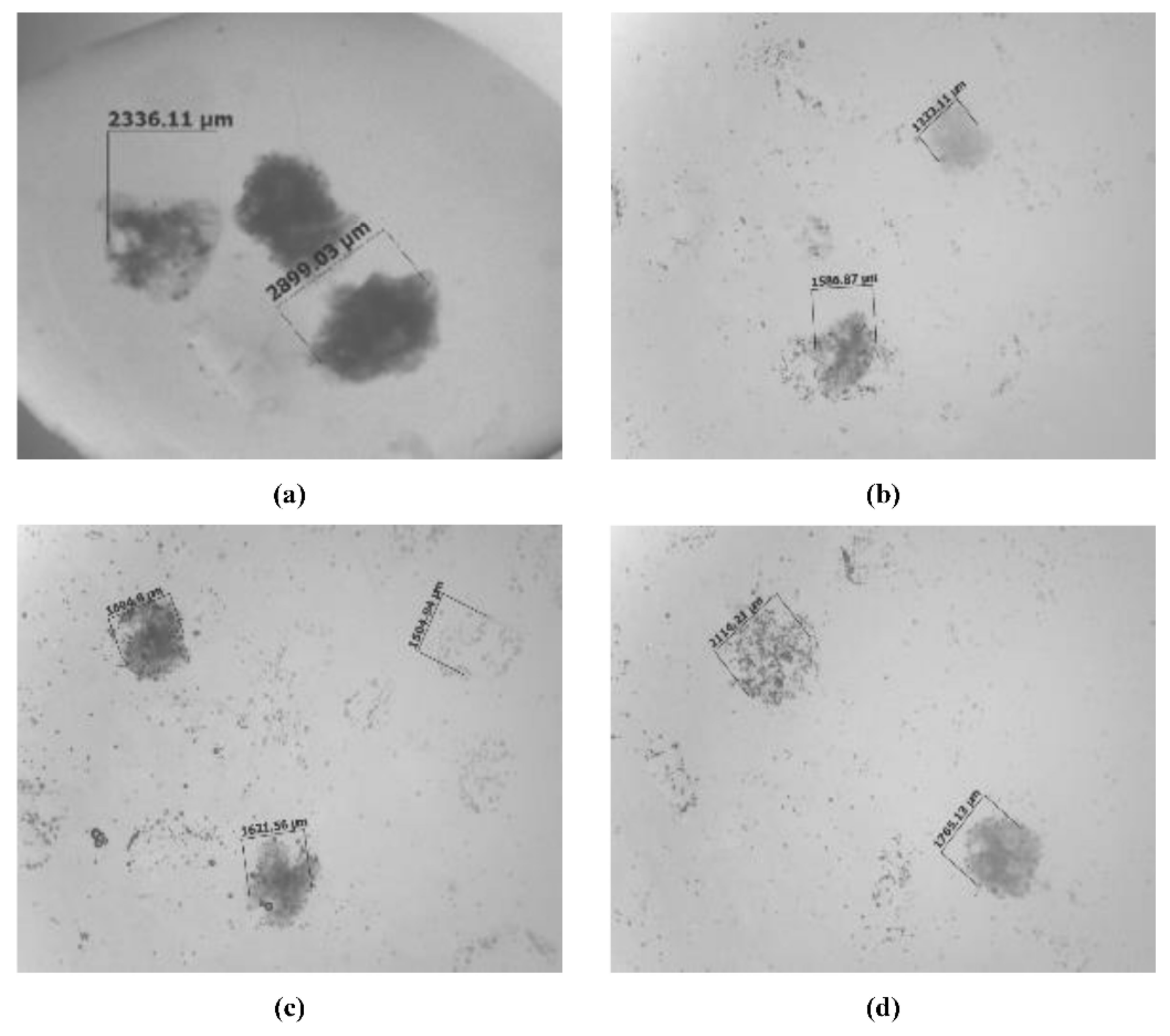
4.2.1. Micro-Dimensional Measurement
4.2.2. Dissolution Time Measurement
4.2.3. Solution Viscosity Measurement
5. Results and Discussion
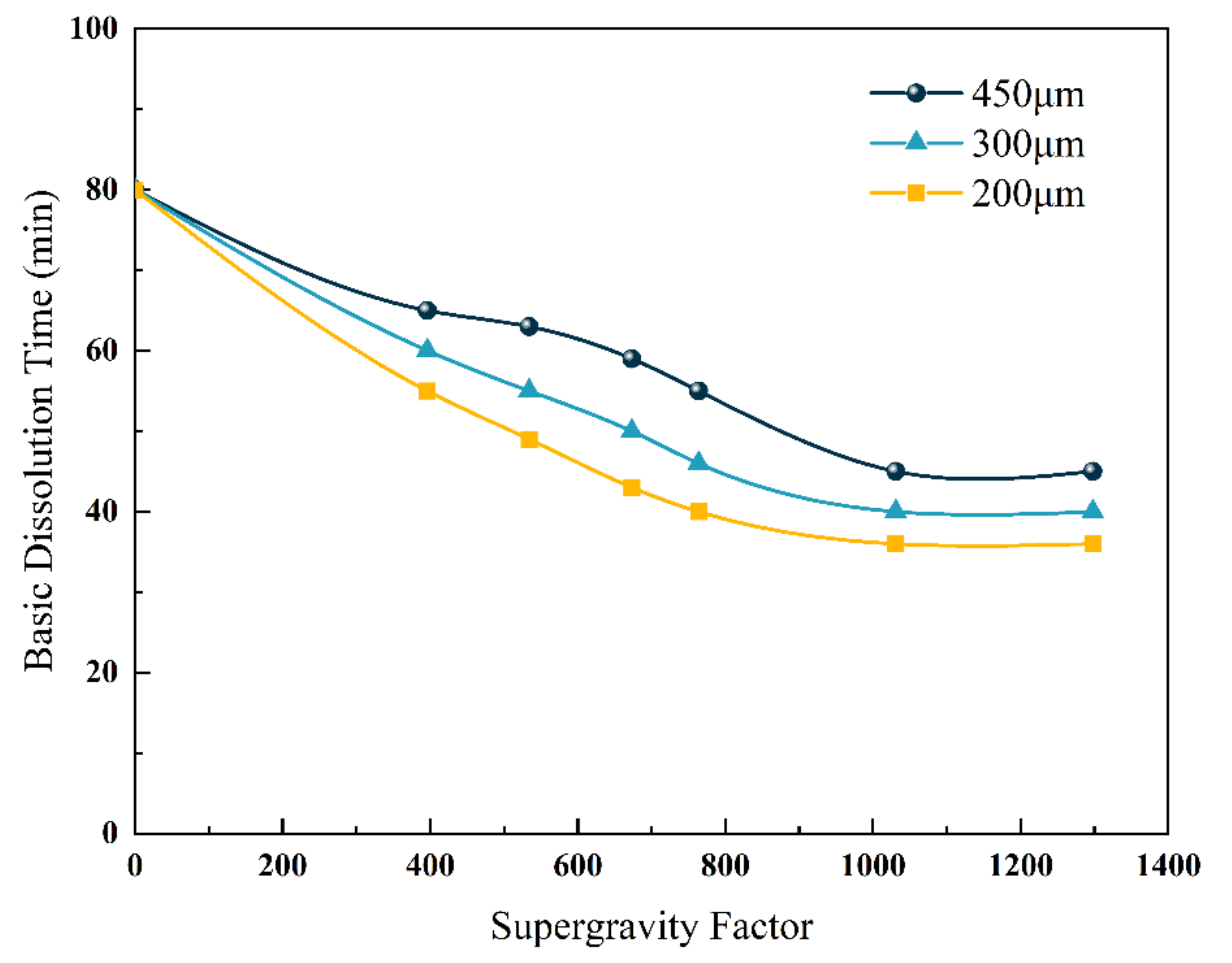
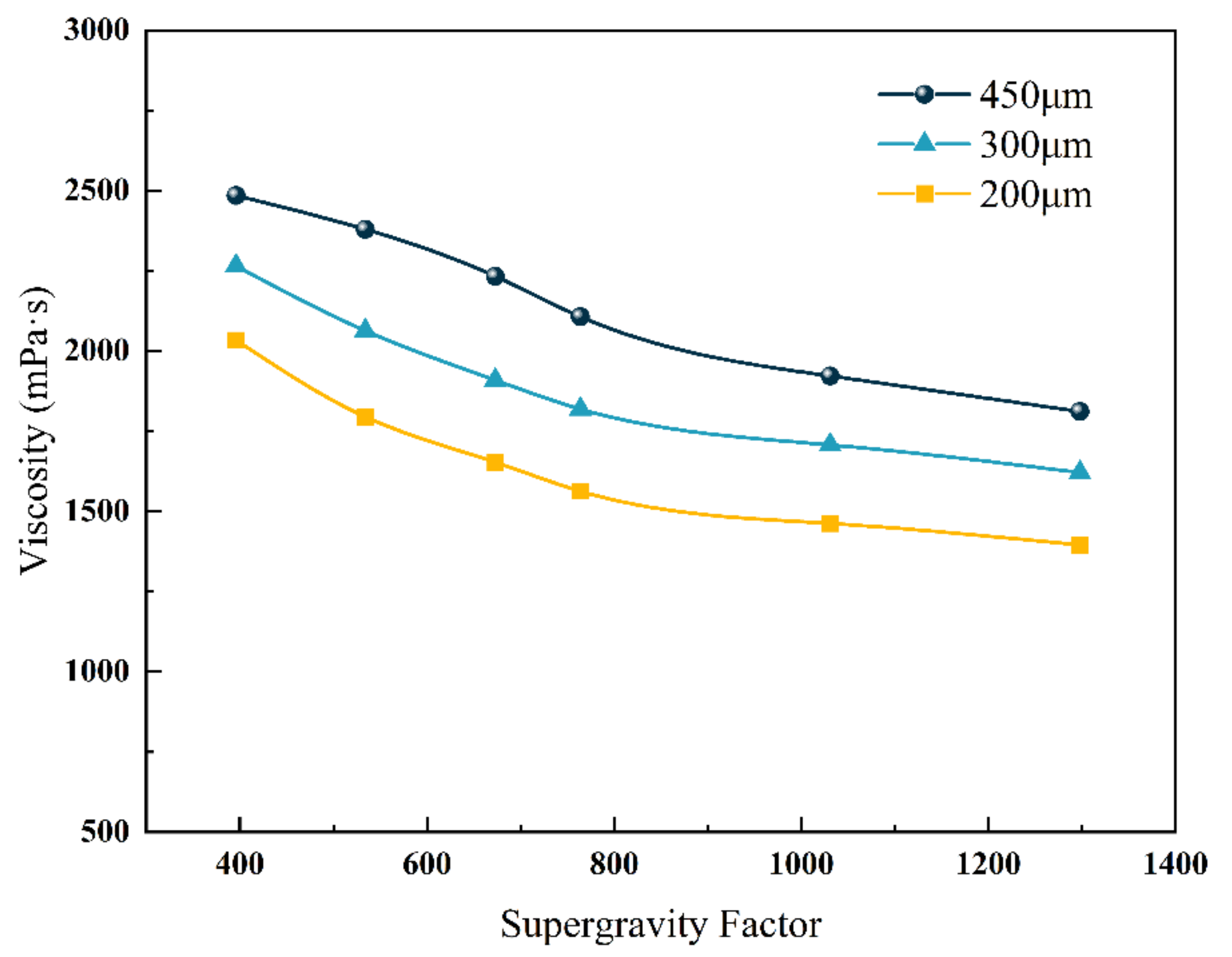
5.1. Effects of Micro-Dimensional Polymer Size
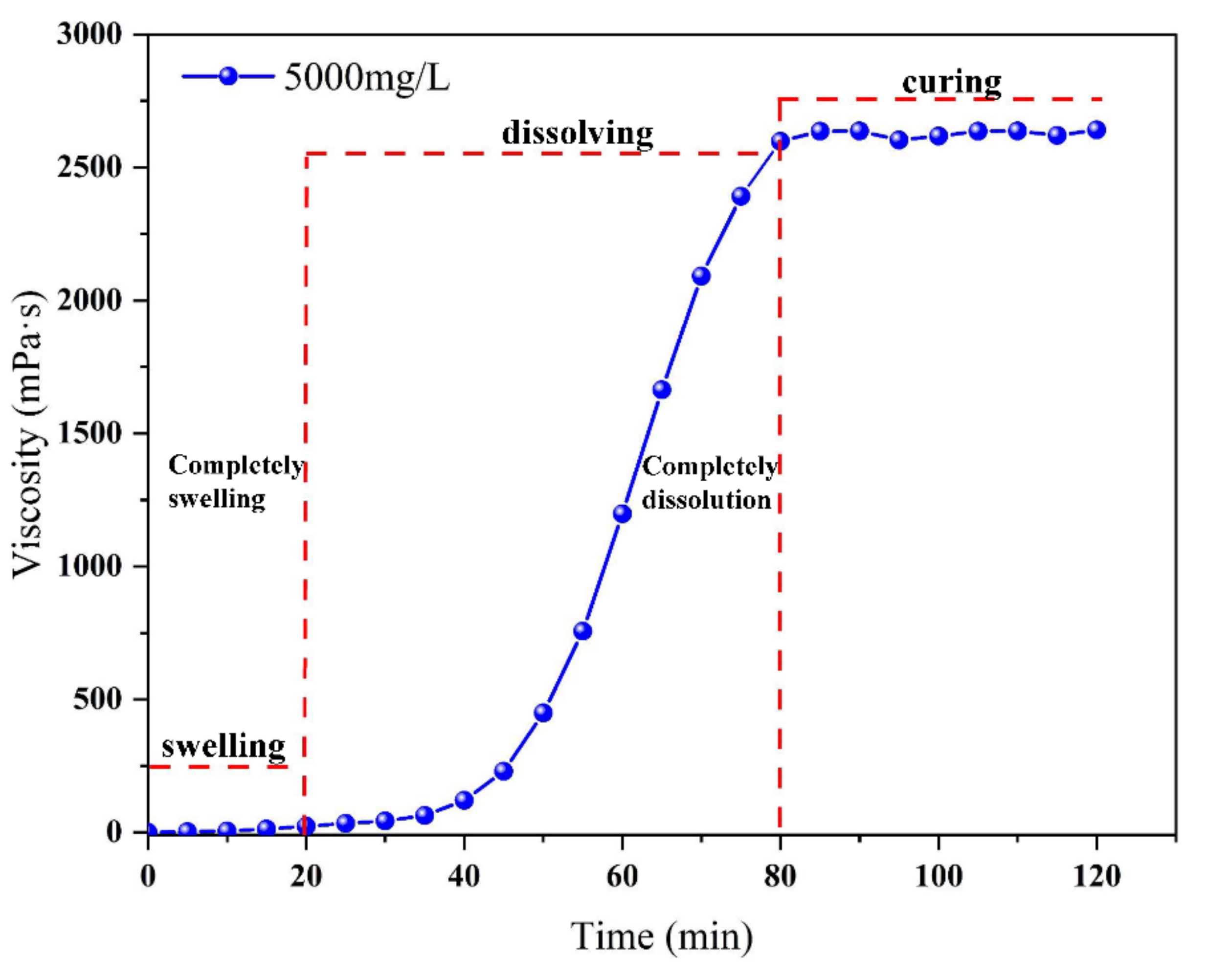
5.2. Effects of Dissolution Time
5.3. Effects of Solution Viscosity
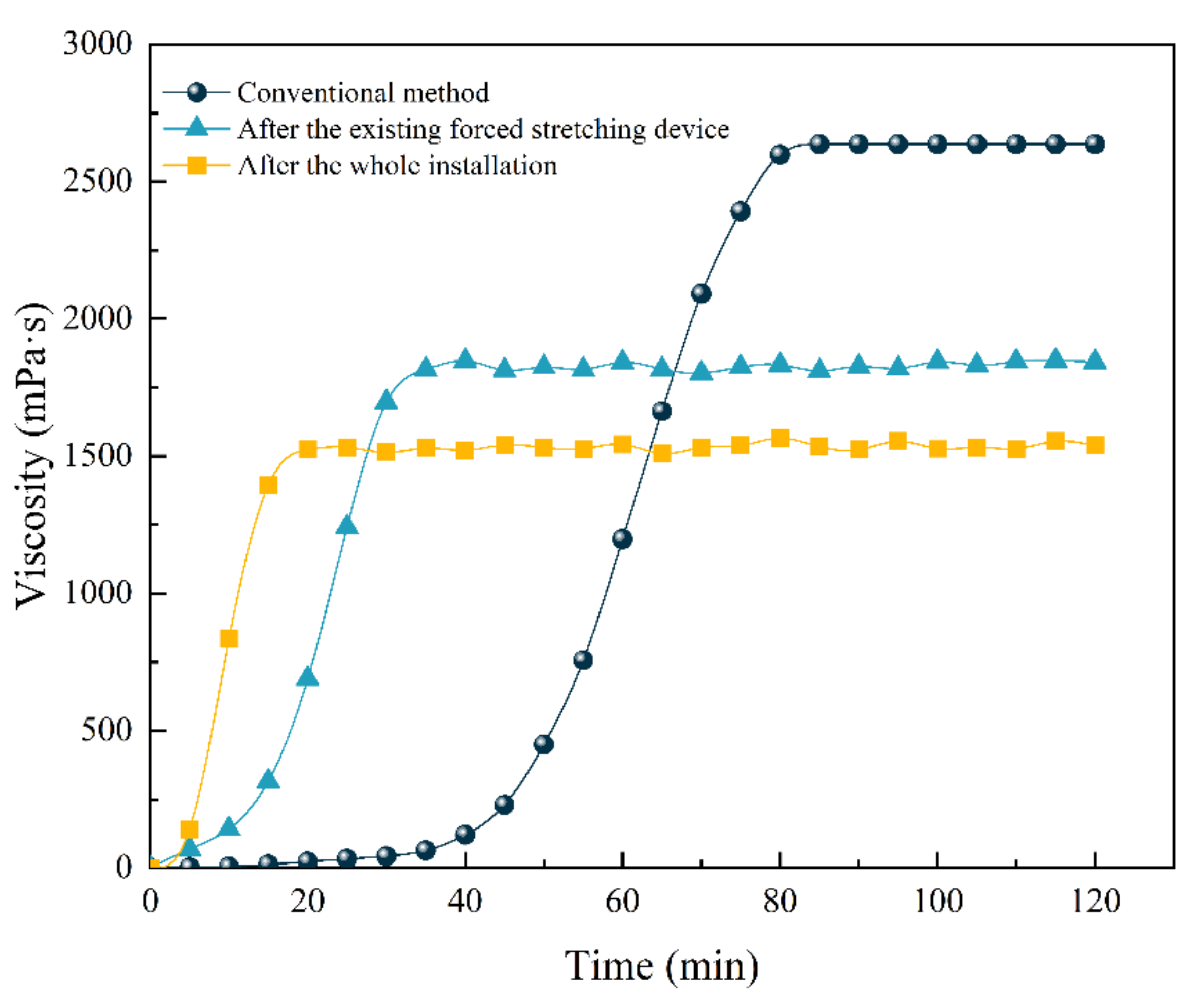
5.4. Magnification Experiments
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| ω | Angular velocity of mass transfer ring rotation, s−1; |
| r | Radius of mass transfer ring, m; |
| β | Super-gravity factor; |
| Axial velocity of the fluid on the packing wire, m/s; | |
| Radius of filler in layer i, m; | |
| Circumferential velocity of the liquid on the packing wire, m/s; | |
| Motion Viscosity, m2/s; | |
| Liquid film thickness, m; | |
| Radius of liquid film, m; | |
| Inner radius of liquid film, m; | |
| Inner radius of liquid film, m; | |
| Instantaneous axial velocity of polymer swelling particles after being trapped by packing, m/s; | |
| Instantaneous circumferential velocity of polymer-swollen particles after being trapped by packing, m/s. |
References
- Xie, K.; Lu, X.; Pan, H.; Han, D.; Hu, G.; Zhang, J.; Zhang, B.; Cao, B. Analysis of dynamic imbibition effect of surfactant in microcracks of reservoir at high temperature and low permeability. SPE Prod. Oper. 2018, 33, 596–606. [Google Scholar] [CrossRef]
- LU, X.; CAO, B.; XIE, K.; CAO, W.; LIU, Y.; ZHANG, Y.; WANG, X.; ZHANG, J. Enhanced oil recovery mechanisms of polymer flooding in a heterogeneous oil reservoir. Pet. Explor. Dev. 2021, 48, 169–178. [Google Scholar] [CrossRef]
- Hatzignatiou, D.G.; Giske, N.H. Sodium silicate gelants for water management in naturally fractured hydrocarbon carbonate formations. Chem. Eng. Res. Des. 2018, 132, 40–56. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, D.; Bai, B. Experimental study of degradable preformed particle gel (DPPG) as temporary plugging agent for carbonate reservoir matrix acidizing to improve oil recovery. J. Pet. Sci. Eng. 2021, 205, 108760. [Google Scholar] [CrossRef]
- Morel, D.; Vert, M.; Jouenne, S.; Gauchet, R.; Bouger, Y. First polymer injection in deep offshore field Angola: Recent advances on dalia/camelia field case. In Proceedings of the SPE Annual Technical Conference and Exhibition, Florence, Italy, 20–22 September 2010; Volume 7, pp. 5699–5713. [Google Scholar] [CrossRef]
- Taylor, K.C.; Nasr-El-Din, H.A. Water-Soluble Hydrophobically Associating Polymers for Improved Oil Recovery: A Literature Review. In Proceedings of the SPE International Symposium on Oilfield Chemistry, San Antonio, TX, USA, 14–17 February 1995; pp. 265–280. [Google Scholar]
- Cao, B.; Xie, K.; Lu, X.; Cao, W.; He, X.; Xiao, Z.; Zhang, Y.; Wang, X.; Su, C. Effect and mechanism of combined operation of profile modification and water shutoff with in-depth displacement in high-heterogeneity oil reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127673. [Google Scholar] [CrossRef]
- Zareie, C.; Bahramian, A.R.; Sefti, M.V.; Salehi, M.B. Network-gel strength relationship and performance improvement of polyacrylamide hydrogel using nano-silica; with regards to application in oil wells conditions. J. Mol. Liq. 2019, 278, 512–520. [Google Scholar] [CrossRef]
- Pal, N.; Saxena, N.; Mandal, A. Characterization of alkali-surfactant-polymer slugs using synthesized gemini surfactant for potential application in enhanced oil recovery. J. Pet. Sci. Eng. 2018, 168, 283–300. [Google Scholar] [CrossRef]
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Rukavishnikov, V.S.; Minaev, K.M. Experimental and field applications of nanotechnology for enhanced oil recovery purposes: A review. Fuel 2022, 324, 124669. [Google Scholar] [CrossRef]
- Lakatos, I.J.; Lakatos-Szabo, J.; Szentes, G.; Vago, A.; Karaffa, Z.; Bodi, T. New alternatives in conformance control: Nanosilica and liquid polymer aided silicate technology. In Proceedings of the SPE European Formation Damage Conference and Exhibition, Budapest, Hungary, 3–5 June 2015; pp. 881–904. [Google Scholar] [CrossRef]
- Alvarado, V.; Manrique, E. Enhanced oil recovery: An update review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Standnes, D.C.; Skjevrak, I. Literature review of implemented polymer field projects. J. Pet. Sci. Eng. 2014, 122, 761–775. [Google Scholar] [CrossRef]
- Gao, C.; Shi, J.; Zhao, F. Successful polymer flooding and surfactant-polymer flooding projects at Shengli Oilfield from 1992 to 2012. J. Pet. Explor. Prod. Technol. 2014, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Niu, C.C.; Yang, X.Y. Improved understanding nanocomposite gel working mechanisms: From laboratory investigation to wellbore plugging application. J. Pet. Sci. Eng. 2020, 191, 107214. [Google Scholar] [CrossRef]
- Yang, X.; Ni, L. Synthesis of hybrid hydrogel of poly(AM co DADMAC)/silica sol and removal of methyl orange from aqueous solutions. Chem. Eng. J. 2012, 209, 194–200. [Google Scholar] [CrossRef]
- Shi, L.T.; Li, C.; Zhu, S.S.; Xu, J.; Sun, B.Z.; Ye, Z.B. Study on properties of branched hydrophobically modified polyacrylamide for polymer flooding. J. Chem. 2013, 2013, 675826. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Meng, R.; Xing, Q.; Lou, M.; Chao, H.; Bing, L. Assessing oil spill risk in the Chinese Bohai Sea: A case study for both ship and platform related oil spills. Ocean Coast. Manag. 2015, 108, 140–146. [Google Scholar] [CrossRef]
- Guo, W.; Wu, G.; Liang, B.; Xu, T.; Chen, X.; Yang, Z.; Xie, M.; Jiang, M. The influence of surface wave on water exchange in the Bohai Sea. Cont. Shelf Res. 2016, 118, 128–142. [Google Scholar] [CrossRef]
- Zhou, G.; Xie, M.; Liu, M.; Wu, H.; Long, X.; Yu, P. Dissolution Characteristics of Hydrophobically Associating Polyacrylamide in Stirred Tanks. Chin. J. Chem. Eng. 2010, 18, 170–174. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Zhang, J.; Zhao, W.S.; Wang, S.S.; Jing, B.; Meng, F.X.; Zhang, H. Research on efficient polymer dissolving technology for hydrophobically associating polymer flooding on offshore platform. Appl. Mech. Mater. 2014, 670–671, 290–294. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, S.; Wu, G. Quantitative oil spill risk from offshore fields in the Bohai Sea, China. Sci. Total Environ. 2019, 688, 494–504. [Google Scholar] [CrossRef]
- Cao, W.; Xie, K.; Lu, X.; Liu, Y.; Zhang, Y. Effect of profile-control oil-displacement agent on increasing oil recovery and its mechanism. Fuel 2019, 237, 1151–1160. [Google Scholar] [CrossRef]
- Kamcev, J.; Galizia, M.; Benedetti, F.M.; Jang, E.S.; Paul, D.R.; Freeman, B.D.; Manning, G.S. Partitioning of mobile ions between ion exchange polymers and aqueous salt solutions: Importance of counter-ion condensation. Phys. Chem. Chem. Phys. 2016, 18, 6021–6031. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, Q. Synthesis, characterization, and solution properties of a surface-active hydrophobically associating polymer. J. Appl. Polym. Sci. 2018, 135, 46569. [Google Scholar] [CrossRef]
- Liu, J.; Almakimi, A.; Wei, M.; Bai, B.; Ali Hussein, I. A comprehensive review of experimental evaluation methods and results of polymer micro/nanogels for enhanced oil recovery and reduced water production. Fuel 2022, 324, 124664. [Google Scholar] [CrossRef]
- Kang, W.; Kang, X.; Lashari, Z.A.; Li, Z.; Zhou, B.; Yang, H.; Sarsenbekuly, B.; Aidarova, S. Progress of polymer gels for conformance control in oilfield. Adv. Colloid Interface Sci. 2021, 289, 102363. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wu, G.; Xu, T.; Li, X.; Ren, X.; Hao, Y. Numerical modelling of temporal and spatial patterns of petroleum hydrocarbons concentration in the Bohai Sea. Mar. Pollut. Bull. 2018, 127, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, X.; Du, K.; Liu, H. Development of a new high-temperature and high-strength polymer gel for plugging fractured reservoirs. Upstream Oil Gas Technol. 2020, 5, 100014. [Google Scholar] [CrossRef]
- Solyman, S.M.; Elsharaky, E.A.; El-Tabey, A.E. Synthesize and characterization of new polymeric surfactants based on nonionic maleate surfmer by catalytic bulk copolymerization with methyl methacrylate. Egypt. J. Pet. 2018, 27, 257–264. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, B.; Mao, J.; Zhang, Y.; Yang, X.; Zhang, Z.; Shao, Y. A Novel Hydrophobic Associative Polymer by RAFT-MADIX Copolymerization for Fracturing Fluids with High Thermal Stability. Energy Fuels 2018, 32, 3039–3051. [Google Scholar] [CrossRef]
- Zheng, A.; Feng, Q.; Wei, Q.; Liu, D. Research on profile inversion pattern of polymer flooding. Geosyst. Eng. 2018, 21, 135–141. [Google Scholar] [CrossRef]
- Sheng, J.J.; Leonhardt, B.; Gmbh, W.H. Status of Polymer-Flooding Technology. J. Can. Pet. Technol. 2015, 54, 116–126. [Google Scholar] [CrossRef]
- Dongqi, W.; Daiyin, Y.; Yazhou, Z. Fine classification of ultra-low permeability reservoirs around the Placanticline of Daqing oilfield (PR of China). J. Pet. Sci. Eng. 2019, 174, 1042–1052. [Google Scholar] [CrossRef]
- Unsal, E.; ten Berge, A.B.G.M.; Wever, D.A.Z. Low salinity polymer flooding: Lower polymer retention and improved injectivity. J. Pet. Sci. Eng. 2018, 163, 671–682. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, W.; Xu, H.; Xiao, C.; Jiang, S. Experimental study of hydraulic fracture initiation and propagation in unconsolidated sand with the injection of temporary plugging agent. J. Pet. Sci. Eng. 2020, 190, 106813. [Google Scholar] [CrossRef]
- Höök, M.; Xu, T.; Xiongqi, P.; Aleklett, K. Development journey and outlook of Chinese giant oilfields. Pet. Explor. Dev. 2010, 37, 237–249. [Google Scholar] [CrossRef]
- Xie, K.; Cao, B.; Lu, X.; Jiang, W.; Zhang, Y.; Li, Q.; Song, K.; Liu, J.; Wang, W.; Lv, J.; et al. Matching between the diameter of the aggregates of hydrophobically associating polymers and reservoir pore-throat size during polymer flooding in an offshore oilfield. J. Pet. Sci. Eng. 2019, 177, 558–569. [Google Scholar] [CrossRef]
- Feng, J.; Li, D.; Li, Y.; Liu, Q.; Wang, A. Storm surge variation along the coast of the Bohai Sea. Sci. Rep. 2018, 8, 11309. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Pan, B.Z.; Yang, Q.S. Characteristics of resistivity log response of oil layers under polymer flooding. Appl. Geophys. 2012, 9, 187–194. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, X.; Xu, G.; Kang, X.; Li, Q.; Jiang, W. Effects of core structure and clay mineral on gel-forming performance of chromium polymer. Colloids Surf. A Physicochem. Eng. Asp. 2018, 540, 256–264. [Google Scholar] [CrossRef]
- Gou, S.; Yin, T.; Yan, L.; Guo, Q. Water-soluble complexes of hydrophobically modified polymer and surface active imidazolium-based ionic liquids for enhancing oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2015, 471, 45–53. [Google Scholar] [CrossRef]
- Saboorian-Jooybari, H.; Dejam, M.; Chen, Z. Heavy oil polymer flooding from laboratory core floods to pilot tests and field applications: Half-century studies. J. Pet. Sci. Eng. 2016, 142, 85–100. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, X.; Wang, R.; Liu, Y. Numerical Simulation for Optimizing Injection—Production Parameters When Using Cyclic Steam Injection Plus Polymer Gel Flooding in an Offshore Heavy-Oil Field. Chem. Technol. Fuels Oils 2017, 53, 621–631. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.M.; Ye, Z.B.; Han, L.J. The solution behavior of hydrophobically associating zwitterionic polymer in salt water. J. Appl. Polym. Sci. 2014, 131, 1–7. [Google Scholar] [CrossRef]
- Amir-Heidari, P.; Raie, M. Probabilistic risk assessment of oil spill from offshore oil wells in Persian Gulf. Mar. Pollut. Bull. 2018, 136, 291–299. [Google Scholar] [CrossRef]
- Fan, Y.W.; Liu, K.X.; Zhang, L.L.; Zhao, H.; Sun, B.C.; Chu, G.W.; Chen, J.F. Rapid and continuous polymer dissolution by rotating packed bed for enhanced oil recovery. Chem. Eng. Process.-Process Intensif. 2020, 153, 107952. [Google Scholar] [CrossRef]
- Wang, M.Y.; Wang, Z.; Guo, Z.C. Regularity and mechanism of Pb electrodeposition intensified by super gravity field. Wuli Huaxue Xuebao/Acta Phys.-Chim. Sin. 2009, 25, 883–889. [Google Scholar] [CrossRef]
- Leon, M.A.; Maas, R.J.; Bieberle, A.; Schubert, M.; Nijhuis, T.A.; van der Schaaf, J.; Hampel, U.; Schouten, J.C. Hydrodynamics and gas-liquid mass transfer in a horizontal rotating foam stirrer reactor. Chem. Eng. J. 2013, 217, 10–21. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.H.; Nirdosh, I.; Sedahmed, G.H. Liquid—Solid mass transfer behaviour of heterogeneous reactor made of a rotating tubular packed bed of spheres. Int. J. Heat Mass Transf. 2018, 126, 1129–1137. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Chu, G.; Shao, L.; Luo, Y.; Chen, J. Liquid-solid mass transfer in a rotating packed bed reactor with structured foam packing. Chin. J. Chem. Eng. 2020, 28, 2507–2512. [Google Scholar] [CrossRef]
- Sedahmed, G.H.; Al-Abd, M.Z.; El-Taweel, Y.A.; Darwish, M.A. Liquid-solid mass transfer behaviour of rotating screen discs. Chem. Eng. J. 2000, 76, 247–252. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Li, Q.; Gao, C.; Cheng, Z. Effect of pressure fluctuation in oil-gas multiphase pump on cavitation and performance of sealing liquid film. J. Pet. Sci. Eng. 2022, 210, 110074. [Google Scholar] [CrossRef]
- Ouyang, Y.; Xiang, Y.; Gao, X.Y.; Li, W.L.; Zou, H.K.; Chu, G.W.; Chen, J.F. Micromixing efficiency in a rotating packed bed with non-Newtonian fluid. Chem. Eng. J. 2018, 354, 162–171. [Google Scholar] [CrossRef]
- Yang, Y.; Xiang, Y.; Chu, G.; Zou, H.; Luo, Y.; Arowo, M.; Chen, J.F. A noninvasive X-ray technique for determination of liquid holdup in a rotating packed bed. Chem. Eng. Sci. 2015, 138, 244–255. [Google Scholar] [CrossRef]
- Burns, J.R.; Ramshaw, C. Process intensification: Visual study of liquid maldistribution in rotating packed beds. Chem. Eng. Sci. 1996, 51, 1347–1352. [Google Scholar] [CrossRef]
- Swift, M.R.; Orlandini, E.; Osborn, W.R.; Yeomans, J.M. Lattice Boltzmann simulations of liquid-gas and binary fluid systems. Phys. Rev. E-Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 1996, 54, 5041–5052. [Google Scholar] [CrossRef] [PubMed]
- Turner, L. Driven-dissipative Euler’s equations for a rigid body: A chaotic system relevant to fluid dynamics. Phys. Rev. E-Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 1996, 54, 5822–5825. [Google Scholar] [CrossRef] [PubMed]
- Rosenau, P. Extending hydrodynamics via the regularization of the Chapman-Enskog expansion. Phys. Rev. A 1989, 40, 7193–7196. [Google Scholar] [CrossRef]
- Frisch, U.; Hasslacher, B.; Pomeau, Y. Lattice-gas automata for the Navier-Stokes equation. Phys. Rev. Lett. 1986, 56, 1505–1508. [Google Scholar] [CrossRef] [Green Version]
- Kraichnan, R.H. Physical review. Nature 1965, 207, 1238. [Google Scholar] [CrossRef]
- Becker, R.J. Lagrangian/Hamiltonian formalism for description of Navier-Stokes fluids. Phys. Rev. Lett. 1987, 58, 1419–1422. [Google Scholar] [CrossRef]
- Ghia, U.; Ghia, K.N.; Shin, C.T. High-Re solutions for incompressible flow using the Navier-Stokes equations and a multigrid method. J. Comput. Phys. 1982, 48, 387–411. [Google Scholar] [CrossRef]
- Buick, J.M.; Greated, C.A. Gravity in a lattice Boltzmann model. Phys. Rev. E-Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 2000, 61, 5307–5320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalantarov, V.K.; Titi, E.S. Global attractors and determining modes for the 3D Navier-Stokes-Voight equations. Chin. Ann. Math. Ser. B 2009, 30, 697–714. [Google Scholar] [CrossRef] [Green Version]
- She, Z.S.; Chen, S.; Doolen, G.; Kraichnan, R.H.; Orszag, S.A. Reynolds number dependence of isotropic Navier-Stokes turbulence. Phys. Rev. Lett. 1993, 70, 3251–3254. [Google Scholar] [CrossRef]
- Eggers, J. Universal pinching of 3D axisymmetric free-surface flow. Phys. Rev. Lett. 1993, 71, 3458–3460. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shu, Z.; Ye, Z.; Zhu, S.; Zhang, L. Simulation and optimization of working parameters of the rapid-dissolution device of hydrophobically associating polymer. AIP Adv. 2021, 11, 015003. [Google Scholar] [CrossRef]



| Particle Size (mm) | Quality (g) | Percent Content (%) |
|---|---|---|
| >0.850 | 0.35 | 3.52 |
| 0.425~0.850 | 4.57 | 45.74 |
| 0.250~0.425 | 3.29 | 32.93 |
| 0.178~0.250 | 1.12 | 11.25 |
| 0.150~0.178 | 0.66 | 6.60 |
| Ions | Na+ + K+ | Ca2+ | Mg2+ | CO32− | HCO3− | SO42− | Cl− | TDS |
|---|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | 3091.96 | 276.17 | 158.68 | 14.21 | 311.48 | 85.29 | 5436.34 | 9374.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, Z.; Qi, Y.; Luo, P.; Wang, T. Exploration of Super-Gravity Rapid Dissolution Method of Polymer for Offshore Oil Repellent. Processes 2022, 10, 2332. https://doi.org/10.3390/pr10112332
Shu Z, Qi Y, Luo P, Wang T. Exploration of Super-Gravity Rapid Dissolution Method of Polymer for Offshore Oil Repellent. Processes. 2022; 10(11):2332. https://doi.org/10.3390/pr10112332
Chicago/Turabian StyleShu, Zheng, Yong Qi, Pingya Luo, and Tongwang Wang. 2022. "Exploration of Super-Gravity Rapid Dissolution Method of Polymer for Offshore Oil Repellent" Processes 10, no. 11: 2332. https://doi.org/10.3390/pr10112332




