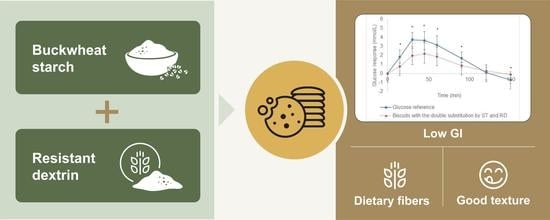
The Roles of a Native Starch and a Resistant Dextrin in Texture Improvement and Low Glycemic Index of Biscuits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biscuit Preparation
2.3. Textural Properties
2.4. In Vitro Starch Digestibility
2.5. Glycemic Index
2.6. Statistical Analysis
3. Results and Discussion
3.1. Textural Properties
3.2. In Vitro Starch Digestibility
3.3. Glycemic Index
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [Green Version]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://www.diabetesatlas.org (accessed on 24 November 2021).
- Thomas, D.E.; Elliott, E.J. The use of low-glycaemic index diets in diabetes control. Br. J. Nutr. 2010, 104, 797–802. [Google Scholar] [CrossRef] [Green Version]
- Brand, J.C.; Colagiuri, S.; Crossman, S.; Allen, A.; Roberts, D.C.; Truswell, A.S. Low-glycemic index foods improve long-term glycemic control in NIDDM. Diabetes Care 1991, 14, 95–101. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Buckley, G.; Lam, K.Y.; Giudici, S.; Kalmusky, J.; Jenkins, A.L.; Patten, R.L.; Bird, J.; Wong, G.S. Low-glycemic-index starchy foods in the diabetic diet. Am. J. Clin. Nutr. 1988, 48, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Teng, A.; Witt, T.; Wang, K.; Li, M.; Hasjim, J. Molecular rearrangement of waxy and normal maize starch granules during in vitro digestion. Carbohydr. Polym. 2016, 139, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, R.G.; Wu, A.C.; Sullivan, M.A.; Sumarriva, G.E.; Ersch, N.; Hasjim, J. Improving human health through understanding the complex structure of glucose polymers. Anal. Bioanal. Chem. 2013, 405, 8969–8980. [Google Scholar] [CrossRef]
- Holm, J.; Lundquist, I.; Björck, I.; Eliasson, A.C.; Asp, N.G. Degree of starch gelatinization, digestion rate of starch in vitro, and metabolic response in rats. Am. J. Clin. Nutr. 1988, 47, 1010–1016. [Google Scholar] [CrossRef]
- Zhang, G.; Ao, Z.; Hamaker, R.B. Slow digestion property of native cereal starches. Biomacromolecules 2006, 7, 3252–3258. [Google Scholar] [CrossRef]
- Yu, S.; Prakash, A.; Pora, B.L.R.; Hasjim, J. Using buckwheat starch to produce slowly digestible biscuits with good palatability. Cereal Chem. 2022, 99, 1166–1177. [Google Scholar] [CrossRef]
- Lefranc-Millot, C. NUTRIOSE® 06: A useful soluble dietary fibre for added nutritional value. Nutr. Bull. 2008, 33, 234–239. [Google Scholar] [CrossRef]
- Hobden, M.R.; Martin-Morales, A.; Guerin-Deremaux, L.; Wils, D.; Costabile, A.; Walton, G.E.; Rowland, I.; Kennedy, O.B.; Gibson, G.R. In vitro fermentation of NUTRIOSE® FB06, a wheat dextrin soluble fibre, in a continuous culture human colonic model system. PLoS ONE 2013, 8, e77128. [Google Scholar] [CrossRef] [Green Version]
- Lefranc-Millot, C.; Guérin-Deremaux, L.; Wils, D.; Neut, C.; Miller, L.E.; Saniez-Degrave, M.H. Impact of a resistant dextrin on intestinal ecology: How altering the digestive ecosystem with NUTRIOSE®, a soluble fibre with prebiotic properties, may be beneficial for health. J. Int. Med. Res. 2012, 40, 211–224. [Google Scholar] [CrossRef]
- Scientific Opinion on the substantiation of a health claim related to Nutriose® 06 and a reduction of post-prandial glycaemic responses pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2014, 12, 3839. [CrossRef] [Green Version]
- Hobden, M.R.; Commane, D.M.; Guérin-Deremaux, L.; Wils, D.; Thabuis, C.; Martin-Morales, A.; Wolfram, S.; Dìaz, A.; Collins, S.; Morais, I.; et al. Impact of dietary supplementation with resistant dextrin (NUTRIOSE®) on satiety, glycaemia, and related endpoints, in healthy adults. Eur. J. Nutr. 2021, 60, 4635–4643. [Google Scholar] [CrossRef]
- Ferrer-Mairal, A.; Peñalva-Lapuente, C.; Iglesia, I.; Urtasun, L.; De Miguel-Etayo, P.; Remón, S.; Cortés, E.; Moreno, L.A. In vitro and in vivo assessment of the glycemic index of bakery products: Influence of the reformulation of ingredients. Eur. J. Nutr. 2012, 51, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Vujić, L.; Čepo, D.V.; Dragojević, I.V. Impact of dietetic tea biscuit formulation on starch digestibility and selected nutritional and sensory characteristics. LWT—Food Sci. Technol. 2015, 62, 647–653. [Google Scholar] [CrossRef]
- Bernhardt, D.C.; Castelli, M.V.; Arqueros, V.; Gerschenson, L.N.; Fissore, E.N.; Rojas, A.M. Effect of fibers from bracts of maize (Zea mays) as natural additives in wheat bread-making: A technological approach. J. Food Meas. Charact. 2022, 16, 4036–4049. [Google Scholar] [CrossRef]
- Bhavya, S.N.; Prakash, J. Nutritional and sensory quality of buns enriched with soy fiber (Okara). J. Eng. Process. Manage. 2019, 10, 23–31. [Google Scholar] [CrossRef]
- Barbhai, M.D.; Hymavathi, T.V.; Kuna, A.; Mulinti, S.; Voliveru, S.R. Quality assessment of nutri-cereal bran rich fraction enriched buns and muffins. J. Food Sci. Technol. 2022, 59, 2231–2242. [Google Scholar] [CrossRef]
- Hu, H.; Lin, H.; Xiao, L.; Guo, M.; Yan, X.; Su, X.; Liu, L.; Sang, S. Impact of native form oat β-glucan on the physical and starch digestive properties of whole oat bread. Foods 2022, 11, 2622. [Google Scholar] [CrossRef]
- Madar, Z.; Thorne, R. Dietary fiber. Prog. Food Nutr. Sci. 1987, 11, 153–174. Available online: http://europepmc.org/abstract/MED/2819947 (accessed on 21 November 2021). [PubMed]
- El-Salhy, M.; Ystad, S.O.; Mazzawi, T.; Gundersen, D. Dietary fiber in irritable bowel syndrome (Review). Int. J. Mol. Med. 2017, 40, 607–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, K.R.; Lewis, J.; Wyatt, G.M.; Fenwick, G.R. Review article Flatulence—Causes, relation to diet and remedies. Food/Nahrung 1988, 32, 609–626. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Du, D.; Wu, A.C.; Bai, Y.; Wu, P.; Li, C.; Gilbert, R.G. Effects of Nonstarch Genetic Modifications on Starch Structure and Properties. Foods 2020, 9, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull. World Health Organ. 2001, 79, 373–374. Available online: https://apps.who.int/iris/handle/10665/268312 (accessed on 21 November 2021).
- Gonçalves, C.; Moreira, S.M.; Carvalho, V.; Silva, D.M.; Gama, M. Dextrin. In Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; Taylor & Francis: Abingdon-on-Thames, UK, 2016; pp. 2634–2649. [Google Scholar] [CrossRef] [Green Version]
- Sherman, P. A texture profile of foodstuffs based upon well-defined rheological properties. J. Food Sci. 1969, 34, 458–462. [Google Scholar] [CrossRef]
- Curti, E.; Carini, E.; Tribuzio, G.; Vittadini, E. Bread staling: Effect of gluten on physico-chemical properties and molecular mobility. LWT—Food Sci. Technol. 2014, 59, 418–425. [Google Scholar] [CrossRef]
- Li, E.; Dhital, S.; Hasjim, J. Effects of grain milling on starch structures and flour/starch properties. Starch—Stärke 2014, 66, 15–27. [Google Scholar] [CrossRef]
- Hasjim, J.; Srichuwong, S.; Scott, M.P.; Jane, J. Kernel composition, starch structure, and enzyme digestibility of opaque-2 maize and quality protein maize. J. Agric. Food Chem. 2009, 57, 2049–2055. [Google Scholar] [CrossRef]
- Zhu, F. Buckwheat starch: Structures, properties, and applications. Trends Food Sci. Technol. 2016, 49, 121–135. [Google Scholar] [CrossRef]
- Takahama, U.; Hirota, S. Fatty acids, epicatechin-dimethylgallate, and rutin interact with buckwheat starch inhibiting its digestion by amylase: Implications for the decrease in glycemic index by buckwheat flour. J. Agric. Food Chem. 2010, 58, 12431–12439. [Google Scholar] [CrossRef]
- Yang, J.; Gu, Z.; Zhu, L.; Cheng, L.; Li, Z.; Li, C.; Hong, Y. Buckwheat digestibility affected by the chemical and structural features of its main components. Food Hydrocoll. 2019, 96, 596–603. [Google Scholar] [CrossRef]
- Wolf, B.W.; Wolever, T.M.; Bolognesi, C.; Zinker, B.A.; Garleb, K.A. Glycemic response to a rapidly digested starch is not affected by the addition of an indigestible dextrin in humans. Nutr. Res. 2001, 21, 1099–1106. [Google Scholar] [CrossRef]
- ISO 26642:2010; Food products—Determination of the Glycaemic Index (GI) and Recommendation for Food Classification. International Organisation of Standardisation (ISO): Geneva, Switzerland, 2010. Available online: https://www.iso.org/standard/43633.html (accessed on 25 November 2011).
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [Green Version]
- Foster-Powell, K.; Miller, J.B. International tables of glycemic index. Am. J. Clin. Nutr. 1995, 62, 871S–890S. [Google Scholar] [CrossRef]
- Lee, J.J.L.; Chan, B.; Chun, C.; Bhaskaran, K.; Chen, W.N. A preparation of β-glucans and anthocyanins (LoGiCarb™) lowers the: In vitro digestibility and in vivo glycemic index of white rice. RSC Adv. 2020, 10, 5129–5133. [Google Scholar] [CrossRef]
- Mateo-Gallego, R.; Pérez-Calahorra, S.; Lamiquiz-Moneo, I.; Marco-Benedí, V.; Bea, A.M.; Fumanal, A.J.; Prieto-Martín, A.; Laclaustra, M.; Cenarro, A.; Civeira, F. Effect of an alcohol-free beer enriched with isomaltulose and a resistant dextrin on insulin resistance in diabetic patients with overweight or obesity. Clin. Nutr. 2020, 39, 475–483. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Yu, H.; Liu, L.; Lu, T.; Li, J.; Ji, Y.; Le, Z.; Bao, L.; Ma, W.; Xiao, R.; et al. Milk powder co-supplemented with inulin and resistant dextrin improves glycemic control and insulin resistance in elderly type 2 diabetes mellitus: A 12-week randomized, double-blind, placebo-controlled trial. Mol. Nutr. Food Res. 2018, 62, 1800865. [Google Scholar] [CrossRef]


| Formula | Unit | Control | Single Substitution by ST | Single Substitution by RD | Double Substitution by ST and RD |
|---|---|---|---|---|---|
| Ingredient | |||||
| Wheat flour (WF) | g | 180 | 62 | 152 | 37 |
| Starch (ST) | g | - | 109 | - | 109 |
| Resistant dextrin (RD) | g | - | - | 25 | 25 |
| Wheat gluten | g | - | 9 | 3 | 9 |
| Egg | g | 40 | 40 | 40 | 40 |
| Sugar | g | 18 | 18 | 18 | 18 |
| Maltitol | g | 18 | 18 | 18 | 18 |
| Salt | g | 1.5 | 1.5 | 1.5 | 1.5 |
| Butter | g | 10 | 10 | 10 | 10 |
| Milk powder | g | 10 | 10 | 10 | 10 |
| Baking powder | g | 3 | 3 | 3 | 3 |
| Palm oil | g | 40 | 40 | 40 | 40 |
| Water | g | 22 | 22 | 13 | 21 |
| Total excluding water | g | 310.5 | 310.5 | 310.5 | 310.5 |
| Chemical composition | |||||
| Moisture | g/100 g | 7.8 | 4.2 | 3.7 | 4.6 |
| Ash | g/100 g dry | 1.8 | 2.0 | 1.9 | 1.8 |
| Protein | g/100 g dry | 8.6 | 9.2 | 9.0 | 7.5 |
| Fat | g/100 g dry | 17.3 | 17.2 | 15.1 | 16.3 |
| Total dietary fibers | g/100 g dry | 1.3 | 2.1 | 5.7 | 5.3 |
| Starch | g/100 g dry | 52.0 | 52.7 | 49.6 | 51.7 |
| Total carbohydrates * | g/100 g dry | 72.3 | 71.7 | 74.1 | 74.4 |
| Available carbohydrates * | g/100 g dry | 71.0 | 69.6 | 68.4 | 69.2 |
| Number of subjects | 13 | |
| Gender | Female | 30.8% |
| Male | 69.2% | |
| Age * | 40.8 ± 13.9 | |
| Height * | 1.69 ± 0.09 m | |
| Weight * | 61.3 ± 10.8 kg | |
| Body mass index (BMI) * | 21.2 ± 2.3 | |
| Ethnicity | Chinese (100%) | |
| Biscuits Substituted with ST and RD | |
|---|---|
| Serving size | 75.8 g |
| Total carbohydrates | 53.8 g per serving size |
| Available carbohydrates | 50.0 g per serving size |
| Sugars | 5.7 g per serving size |
| Dietary fibers | 3.8 g per serving size |
| Proteins | 5.4 g per serving size |
| Lipids | 11.7 g per serving size |
| Energy | 342 kcal per serving size |
| Biscuits | Firmness (g) | Distance to Break (mm) |
|---|---|---|
| Control | 1058 ± 115 b | 0.12 ± 0.6 a |
| With ST | 800 ± 177 ab | 0.21 ± 0.5 b |
| With RD | 1609 ± 388 c | 0.20 ± 1.6 b |
| With ST and RD | 697 ± 121 a | 0.23 ± 0.5 c |
| Biscuits | Digestion Rate Coefficient, k (1/min) | Total Undigested Starch, C∞ (%) |
|---|---|---|
| Control | 0.0283 ± 0.0001 a | −1.44 ± 0.13 a |
| Single substitution by ST | 0.0200 ± 0.0002 b | −1.09 ± 0.46 a |
| Single substitution by RD | 0.0123 ± 0.0001 c | 7.29 ± 0.76 b |
| Double substitution by ST and RD | 0.0119 ± 0.0019 c | 9.91 ± 0.11 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Dong, K.; Pora, B.L.R.; Hasjim, J. The Roles of a Native Starch and a Resistant Dextrin in Texture Improvement and Low Glycemic Index of Biscuits. Processes 2022, 10, 2404. https://doi.org/10.3390/pr10112404
Yu S, Dong K, Pora BLR, Hasjim J. The Roles of a Native Starch and a Resistant Dextrin in Texture Improvement and Low Glycemic Index of Biscuits. Processes. 2022; 10(11):2404. https://doi.org/10.3390/pr10112404
Chicago/Turabian StyleYu, Shiyao, Ke Dong, Bernard L. R. Pora, and Jovin Hasjim. 2022. "The Roles of a Native Starch and a Resistant Dextrin in Texture Improvement and Low Glycemic Index of Biscuits" Processes 10, no. 11: 2404. https://doi.org/10.3390/pr10112404
APA StyleYu, S., Dong, K., Pora, B. L. R., & Hasjim, J. (2022). The Roles of a Native Starch and a Resistant Dextrin in Texture Improvement and Low Glycemic Index of Biscuits. Processes, 10(11), 2404. https://doi.org/10.3390/pr10112404