Revealing Impact Characteristics of the Cassava Dust Explosion Process: Experimental and Numerical Research
Abstract
:1. Introduction
2. Experiment on Explosion Characteristics
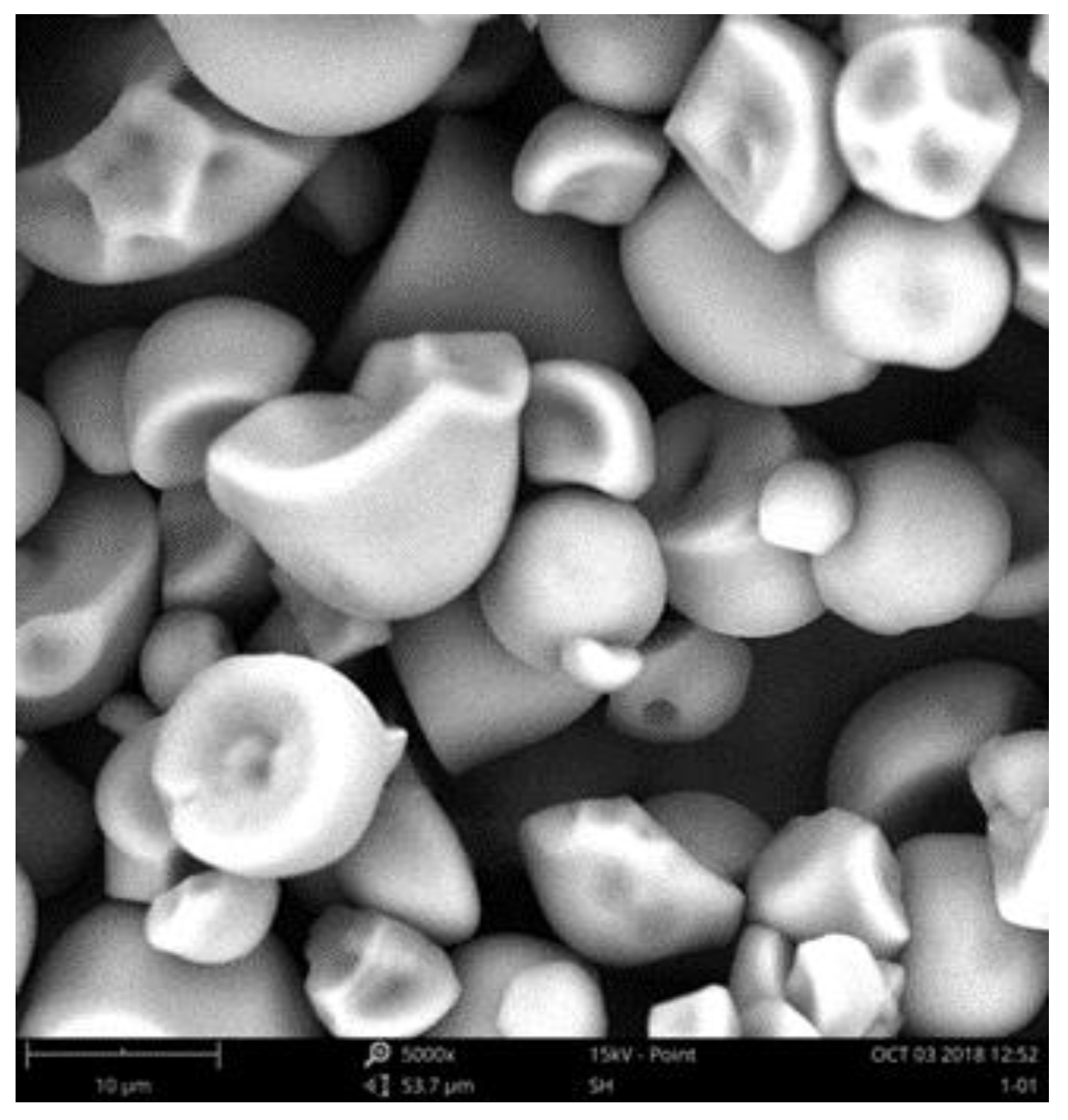
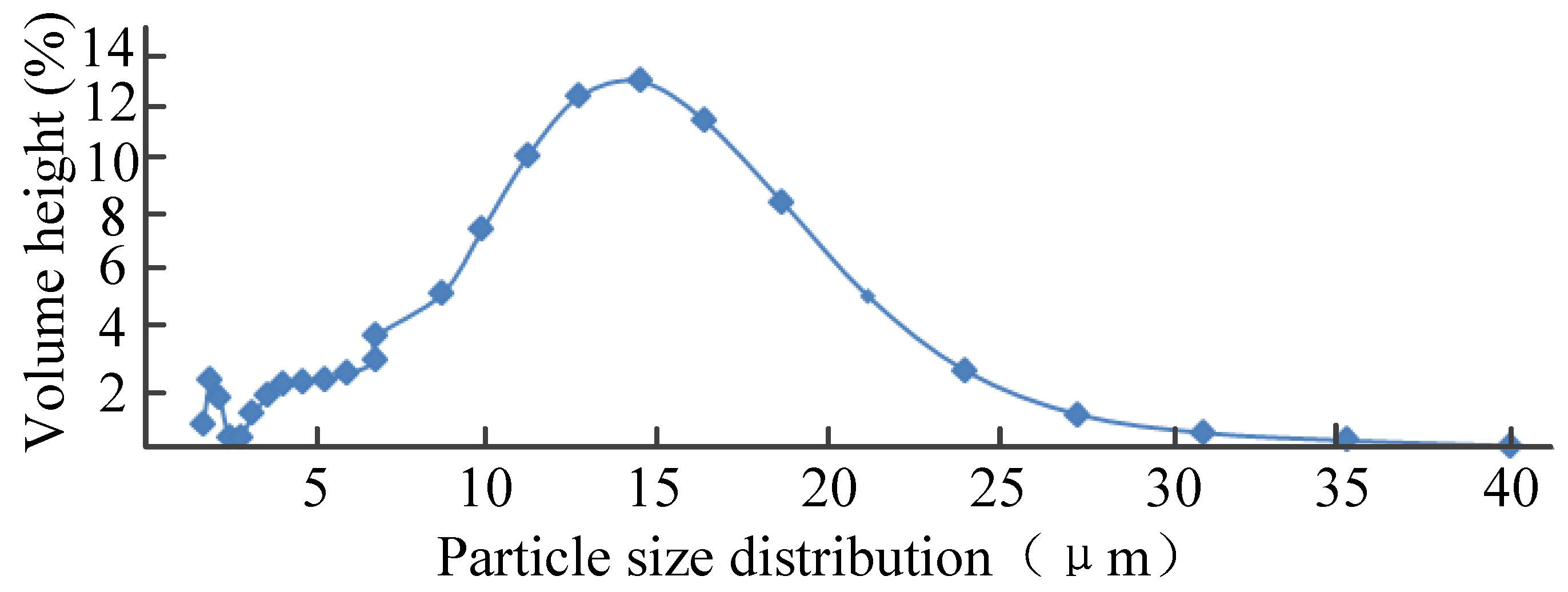
2.1. Experimental Samples and Conditions
2.2. Experimental Device
2.3. Experimental Procedure
2.4. Analysis of Results
- (1)
- The effect of ignition delay time on the explosion of cassavas
- (2)
- Effect of Dust Concentration on Cassava Starch Explosion
- (3)
- Influence of dusting pressure on explosion pressure of cassava dust
3. Numerical Simulation of Cassava Starch Explosion
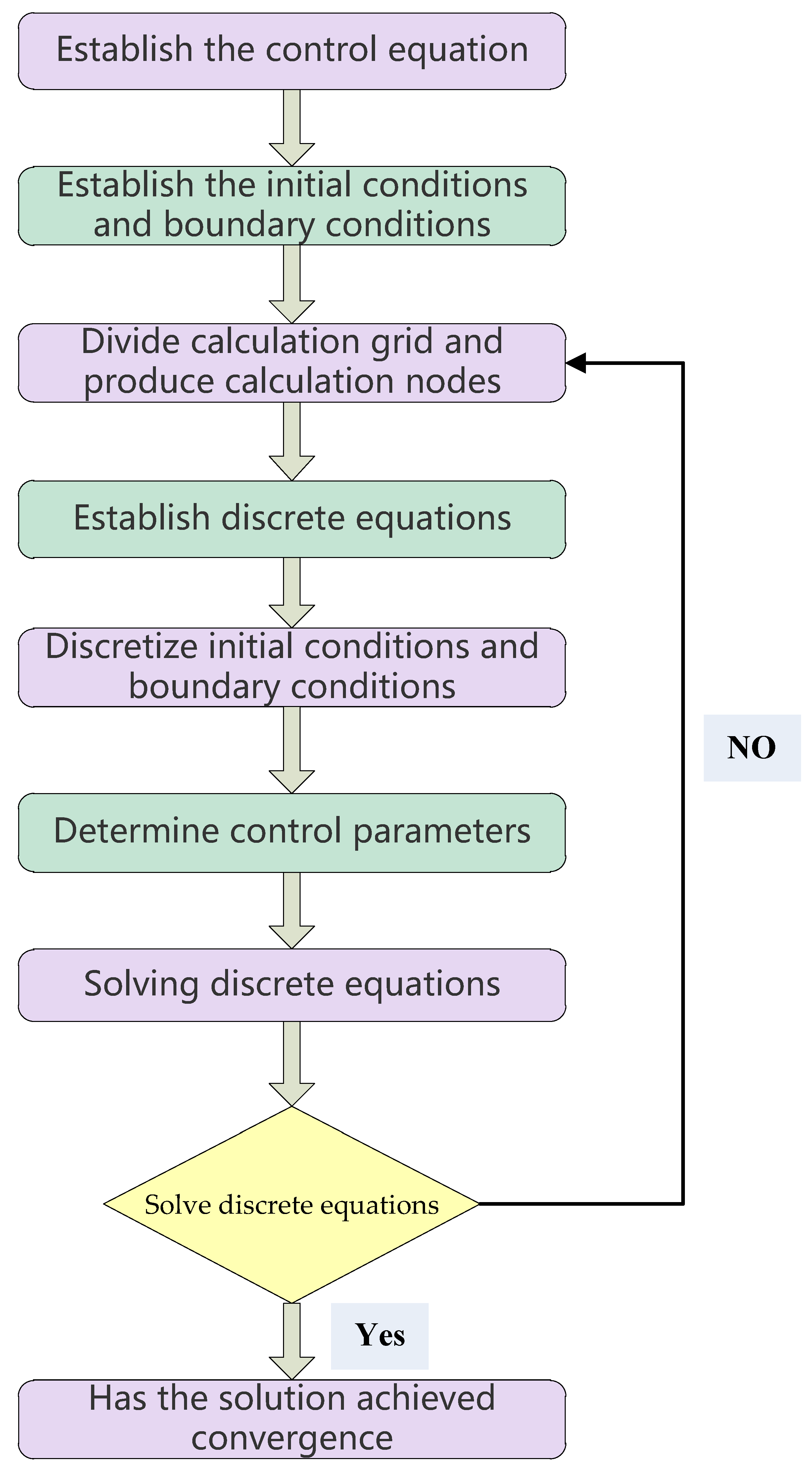
3.1. Simulation Procedure
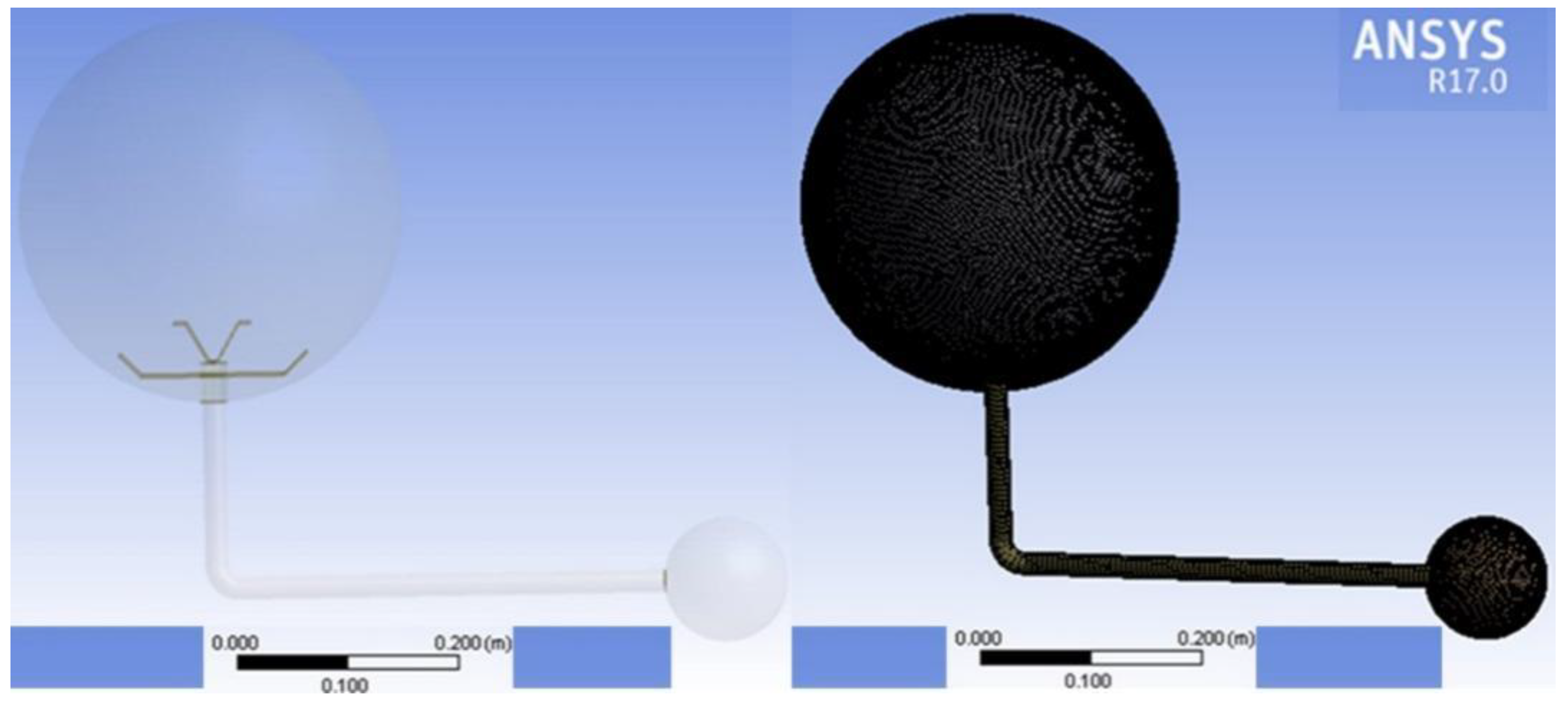
3.2. Partitioning Computational Grids and Models
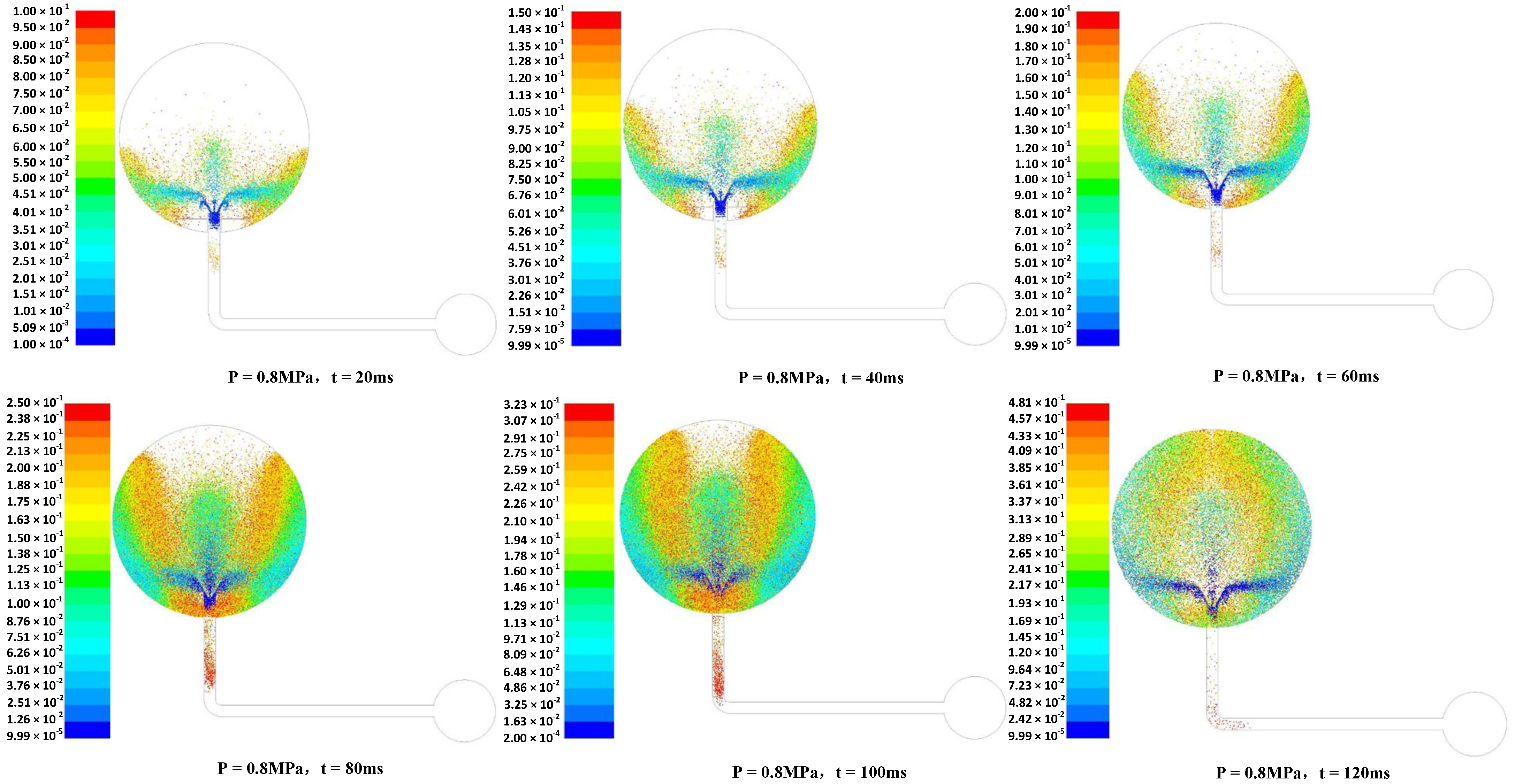
3.3. Numerical Simulation Analysis
- (1)
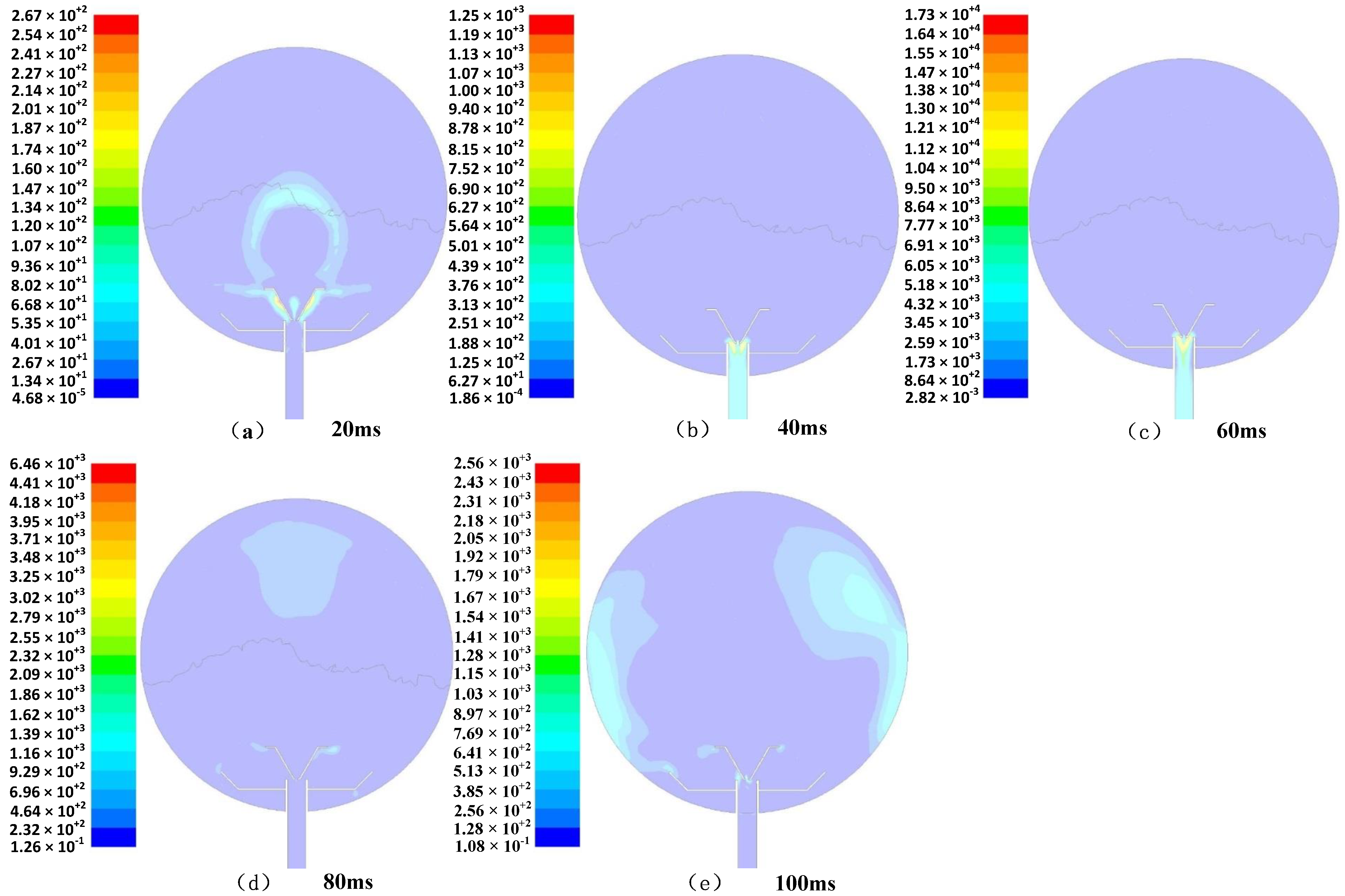
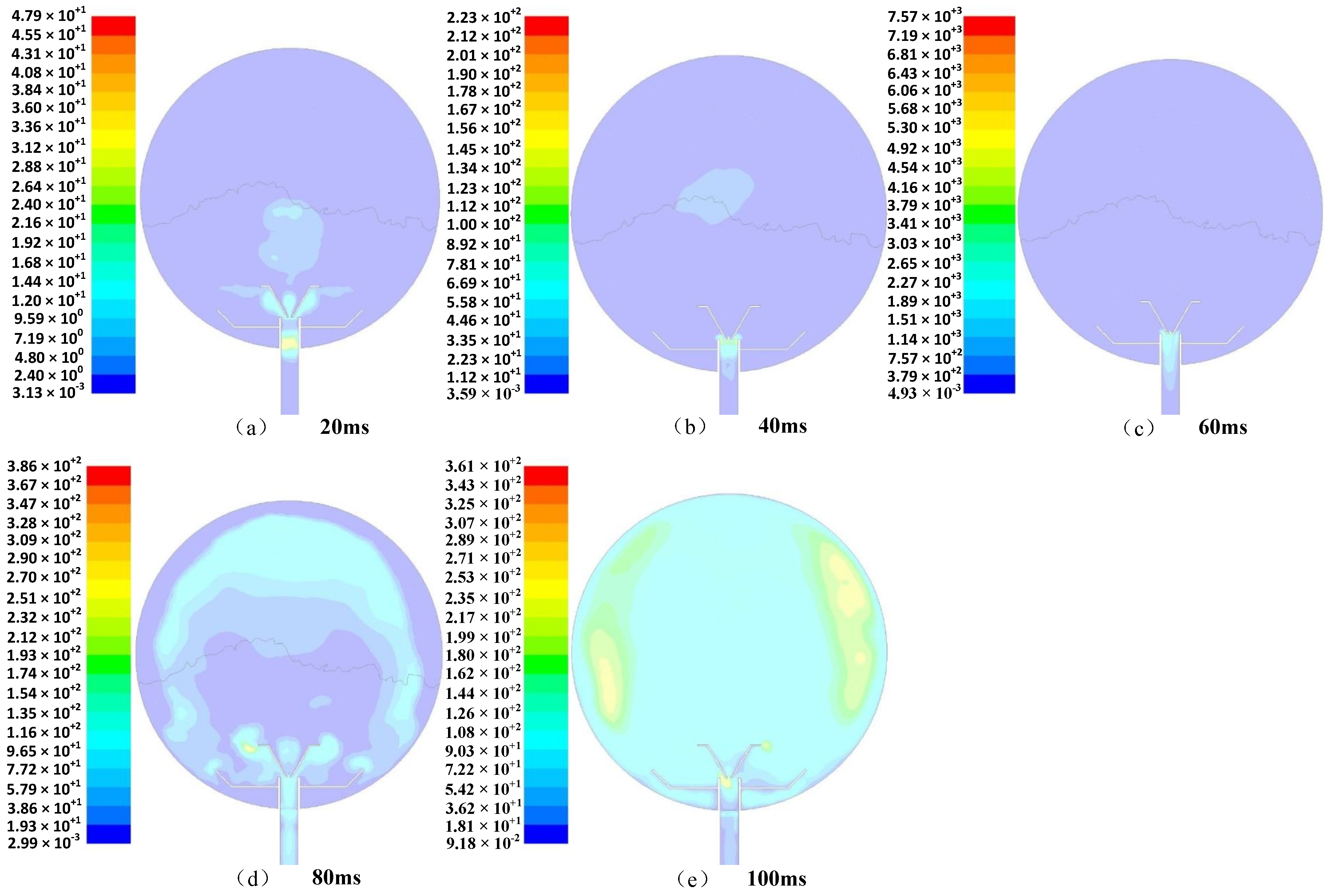
- When the dust was ejected from the nozzle at a certain pressure, the dust had a large initial velocity under the action of the powder spraying pressure, and mainly scattered as a result of the two horizontal changes in the diffusion nozzle and the downward diffusion. With increasing time, the dust spread upward along the wall of the sphere and toward the center of the sphere. Due to the conversion of kinetic energy into potential energy, and also due to the friction between the particles, there was a decrease in the movement speed of the overall upward and center diffusion of the dust. The entrainment effect of airflow on particles decreased, and the time and quantity of the dust particles remaining at the center of the sphere increased.
- (2)
- After the dust was ejected from the nozzle, the dust mass first moved upward in a straight line for a certain period of time, and during this time, a small vortex could have formed in the side of the aerosol. When the clump of dust continued to move, the outer part of the dust group spread out and moved continuously in the inner space of the explosion container at a certain speed. When the dust reached the top, most of the dust was blocked by the top of the container and moved downward, and the gas dust spread into the space of the explosion container and continued to move in a disordered manner at a certain speed within the sphere.
- (3)
- With the passage of time, gravity gradually came to play a major role, and the dust particles began to settle freely under the action of mutual collision. The amount of dust floating in the sphere gradually decreased, and the descending trajectory of the particle group remained in a disordered state until the energy had been exhausted, and ultimately all of the dust settled.
- (4)
- The distribution of dust in the sphere was the most uniform at a dusting diffusion time of 120 ms, which is consistent with the actual experiment, in which the explosion pressure was very high with an ignition delay time of 130 ms, which was the best delay time.
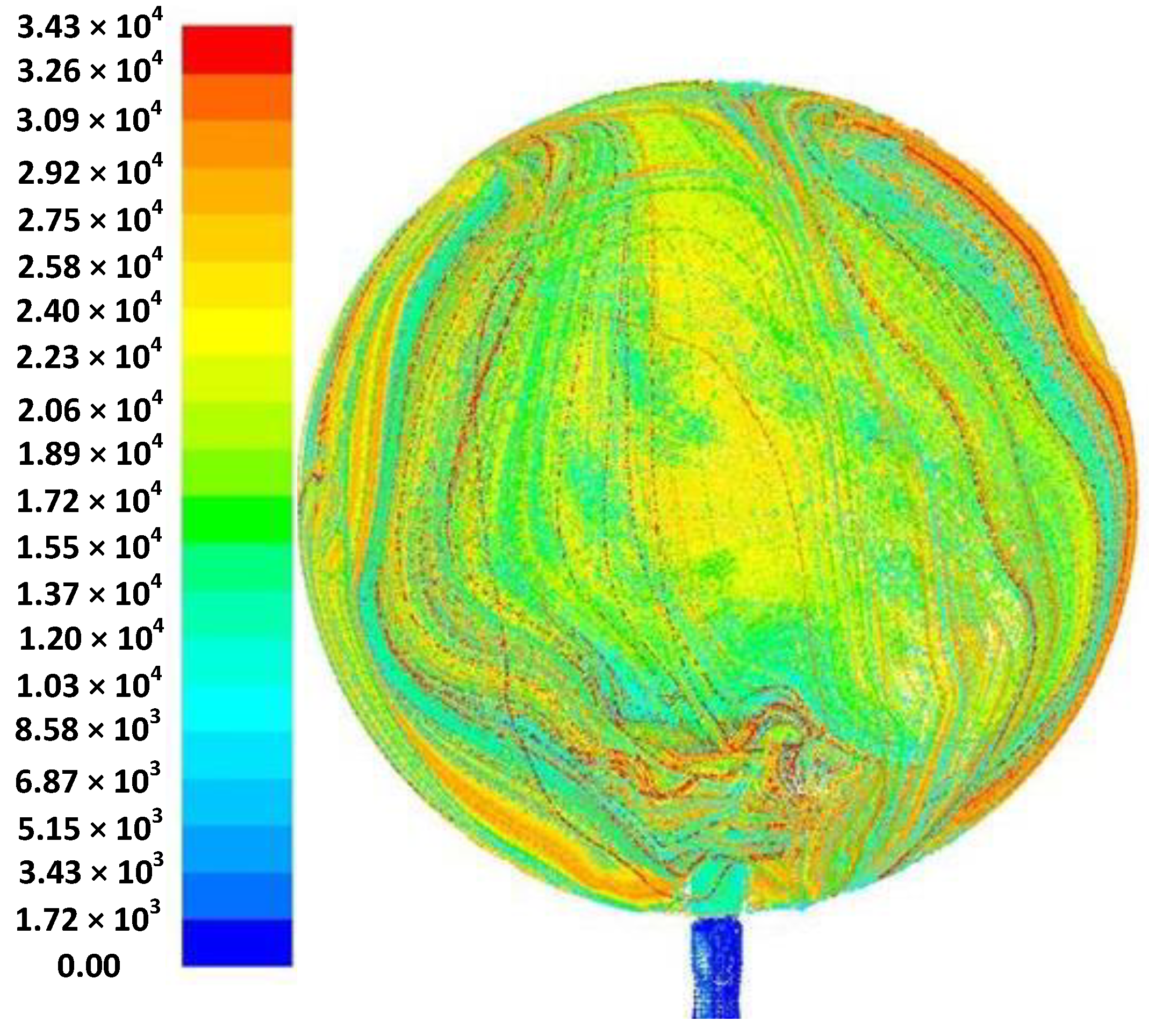
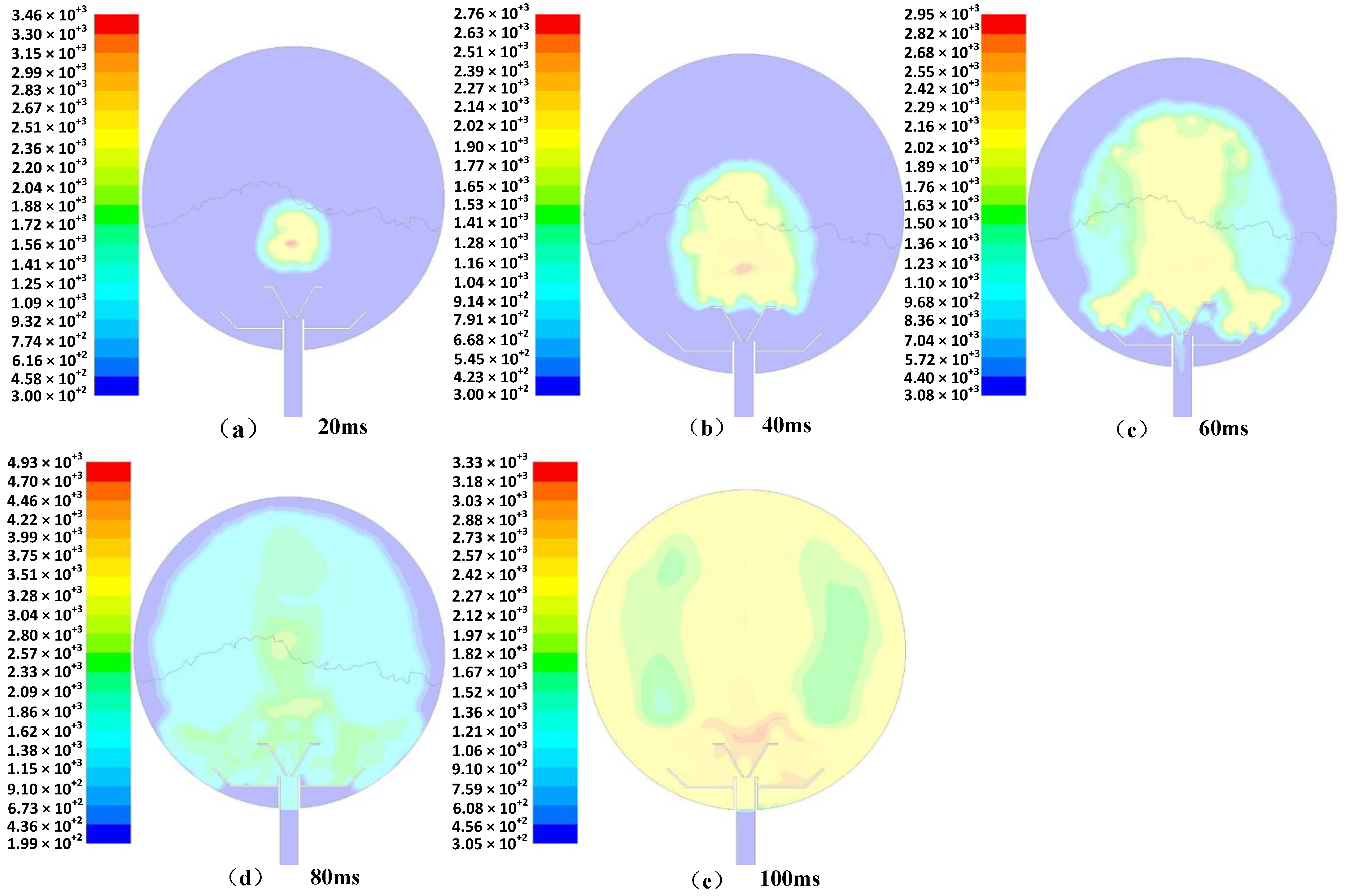
3.4. Ignition and Explosion Simulation
- (1)
- Basic governing equations
- (2)
- Simulation results and analysis of the ignition and deflagration process
4. Conclusions
- (1)
- With increasing ignition delay time, the explosion pressure gradually increased towards a peak, reaching a maximum value of 0.476 MPa at a corresponding ignition delay time of 120 Ms. At this time, the uniform dust in the ball was effectively ignited, and the cloud concentration reached its maximum value. The explosion reactions were also the most favorable, and the resulting pressure reached its maximum value. When the ignition delay time was greater than 120 Ms, the dust cloud that could effectively be burned in the ball gradually decreased, the heat and intensity of the combustion process decreased, and the explosion pressure also decreased. The relationship between time point of ignition and the increase in the maximum explosion pressure rate was random, and the maximum explosion index was 22.37 MPa∙M∙s−1.
- (2)
- With increasing dust concentration, the explosion pressure increased until reaching its maximum value, and then gradually decreased; within a certain range of dust quality, with appropriate injection pressure, the dust concentration was found to be proportional to the explosion pressure. The more unburned particles of dust there were in the combustion pellets, the less heat there was in the sphere, resulting in a lower explosion pressure due to the absorption of a lot of the heat by the unburned particles. The maximum rate of increase in explosion pressure was 13.84 MPa∙M∙s−1.
- (3)
- With increasing injection pressure, the initial acceleration in the dust became greater, and the diffusion dust became more uniform. When the pressure reached a certain value, the dust reached its maximally uniform value, the explosion was the most extensive, and the explosion pressure reached its highest value. However, when the pressure exceeded a certain value, acceleration increased, and the dust diffusion began to become uneven.
- (4)
- The numerical simulation was able to reflect the physical movement law of dust diffusion in a 20 L spherical tank, as well as the whole process of temperature, pressure and turbulence from ignition to explosion and extinction.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, B.; Shao, Z.; Xu, B.; Wei, H.; Wang, T. Analysis of explosion pressure and residual gas characteristics of micro-nano coal dust in confined space. J. Loss Prev. Process Ind. 2020, 64, 104056. [Google Scholar] [CrossRef]
- Saha, L.K.; Bala, S.K.; Roy, N.C.; Molla, M. Numerical simulation of natural convection of dusty nanofluids within a curve-shaped enclosure. AIP Adv. 2020, 10, 105304. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, X.; Xu, M.; An, S.; Li, C.; Huang, C.; Chai, K.; Wang, S.; Liu, Y. Dietary fibres from cassava residue: Physicochemical and enzymatic improvement, structure and physical properties. AIP Adv. 2018, 8, 105035. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Pan, Y. Preparation, Properties and Application of Gel Materials for Coal Gangue Control. Energies 2022, 15, 557. [Google Scholar] [CrossRef]
- Yang, L.G.; Yuan, H.X.; Eckhoff, R.K. A catastrophic aluminium-alloy dust explosion in China. J. Loss Prev. Process. Ind. 2016, 39, 121–130. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Z.; Peng, X.; Zhang, Y.; Cao, W.; Chen, W.; Jun, L. Effect of the ignition delay time on explosion severity parameters of coal dust/air mixtures. Powder Technol. 2018, 342, 509–516. [Google Scholar] [CrossRef]
- Han, F.; Zhang, J.; Zhao, Y.; Han, J. Experimental investigation on the interaction process between coal particles and foam. AIP Adv. 2019, 9, 015023. [Google Scholar] [CrossRef] [Green Version]
- Stahmer, K.-W.; Gerhold, M. Study of the explosion reactions of sucrose, activated charcoal, polyethylene and lignite part 2: Study of the gas phase following the explosion reaction. J. Loss Prev. Process Ind. 2017, 48, 216–222. [Google Scholar] [CrossRef]
- ASTM E1226-12a; Standard Test Method for Explosibility of Dust Clouds. ASTM International: West Conshohocken, PA, USA, 2020; pp. 1–15.
- Cao, W.; Gao, W.; Liang, J.; Xu, S.; Pan, F. Flame-propagation behavior and a dynamic model for the thermal-radiation effects in coal-dust explosions. J. Loss Prev. Process Ind. 2014, 29, 65–71. [Google Scholar] [CrossRef]
- Cao, W.; Cao, W.; Peng, Y.; Qiu, S.; Miao, N.; Pan, F. Experimental study on the combustion sensitivity parameters and pre-combusted changes in functional groups of lignite coal dust. J. Powder Technol. 2015, 283, 512–518. [Google Scholar] [CrossRef]
- Cao, W.; Qin, Q.; Cao, W.; Lan, Y.; Chen, T.; Xu, S.; Cao, X. Experimental and numerical studies on the explosion severities of coal dust/air mixtures in a 20-L spherical vessel. Powder Technol. 2017, 310, 17–23. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Wang, H.; Jiang, R.; Zhang, Y.; Zhao, M.; Li, Y.; Shi, T.; Cao, W. Hazard evaluation of explosion venting behaviors for aluminum powder/air fuels using experimental and numerical approach. J. Powder Technol. 2020, 364, 78–87. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.; Zhang, Q.; Wang, L.; Ji, K.; Peng, L.; Gao, W. Combustion behaviors and flame microstructures of micro- and nano-titanium dust explosions. Fuel 2016, 181, 785–792. [Google Scholar] [CrossRef]
- Zhong, S.; Deng, X. Modeling of maize starch explosions in a 12 m3 silo. J. Loss Prev. Process Ind. 2000, 13, 299–309. [Google Scholar] [CrossRef]
- Zhong, S.; Teodorczyk, A.; Deng, X.; Dang, J. Modeling and simulation of coal dust explosions. J. Phys. Colloq. 2002, 12, 141–147. [Google Scholar] [CrossRef]
- Ren, J.; Chang, C.; Rao, G.; Bai, C.; Jing, Q.; Peng, X.; Xiao, Q. Experimental and numerical simulation study on the effect of ignition delay time on dust explosion in the 1 m3 vessel. Journal of Loss Prevention in the Process Industries. 2022, 78, 104801. [Google Scholar] [CrossRef]
- Wang, D.; Qian, X.; Wu, D.; Ji, T.; Zhang, Q.; Huang, P. Numerical study on hydrodynamics and explosion hazards of corn starch at high-temperature environments. Powder Technol. 2019, 360, 1067–1078. [Google Scholar] [CrossRef]
- Jing, Q.; Wang, D.; Liu, Q.; Shen, Y.; Wang, Z.; Chen, X.; Zhong, Y. Transient reaction process and mechanism of cornstarch/air and CH4/cornstarch/air in a closed container: Quantitative research based on experiments and simulations. J. Hazard. Mater. 2020, 409, 124475. [Google Scholar] [CrossRef]
- Jing, Q.; Wang, D.; Liu, Q.; Chen, X.; Shen, Y.; Wang, Z.; Zhong, Y. Inhibition effect and mechanism of ultra-fne water mist on CH4/air detonation: Quantitative research based on CFD technology. J. Process Saf. Environ. Protect. 2021, 148, 75–92. [Google Scholar] [CrossRef]
- Jing, Q.; Huang, J.; Liu, Q.; Wang, D.; Chen, X.; Wang, Z.; Liu, C. The flame propagation characteristics and detonation parameters of ammonia/oxygen in a large-scale horizontal tube: As a carbon-free fuel and hydrogen-energy carrier. Int. J. Hydrogen Energy 2021, 46, 19158–19170. [Google Scholar] [CrossRef]
- Jing, Q.; Wang, D.; Liu, Q.; Ren, L.; Wang, Y.; Liu, C.; Shen, Y.; Wang, Z. Study on transient reaction mechanism and explosion intensity parameters of micron-sized flake aluminum dust in air. Chem. Eng. Sci. 2021, 246, 116884. [Google Scholar] [CrossRef]
- Jing, Q.; Wang, D.; Liu, Q.; Liu, C.; Wang, Z.; He, Z.; Yang, Z. Deflagration evolution characteristic and chemical reaction kinetic mechanism of JP-10/DEE mixed fuel in a large-scale tube. Fuel 2022, 322, 124238. [Google Scholar] [CrossRef]
- Huang, L. Study on Dust Explosion Characteristics of Lycopodium Powder. Doctoral Dissertation, Nanjing University of Science and Technology, Nanjing, China, 2014. [Google Scholar]
- Xie, C.; Xiong, G.; Chen, Z. Using CFD to Simulate the Concentration of Polluting and Harmful Gases in the Roadway of Non-Metallic Mines Reveals Its Migration Law. Sustainability 2022, 14, 13349. [Google Scholar] [CrossRef]
- Cao, J.; Xie, C.; Hou, Z. Ecological evaluation of heavy metal pollution in the soil of Pb-Zn mines. Ecotoxicology 2022, 31, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, X.; Deng, J.; Shu, C.-M.; Kuo, C.-H.; Yu, Y.; Hu, X. CFD analysis and experimental study on the effect of oxygen level, particle size, and dust concentration on the flame evolution characteristics and explosion severity of cornstarch dust cloud deflagration in a spherical chamber. Powder Technol. 2020, 372, 585–599. [Google Scholar] [CrossRef]
- Di Sarli, V.; Russo, P.; Sanchirico, R.; Di Benedetto, A. CFD simulations of dust dispersion in the 20 L vessel: Effect of nominal dust concentration. J. Loss Prev. Process Ind. 2013, 27, 8–12. [Google Scholar] [CrossRef]
- Xie, C.; Nguyen, H.; Choi, Y.; Armaghani, D.J. Optimized functional linked neural network for predicting diaphragm wall deflection induced by braced excavations in clays. Geosci. Front. 2022, 13, 101313. [Google Scholar] [CrossRef]


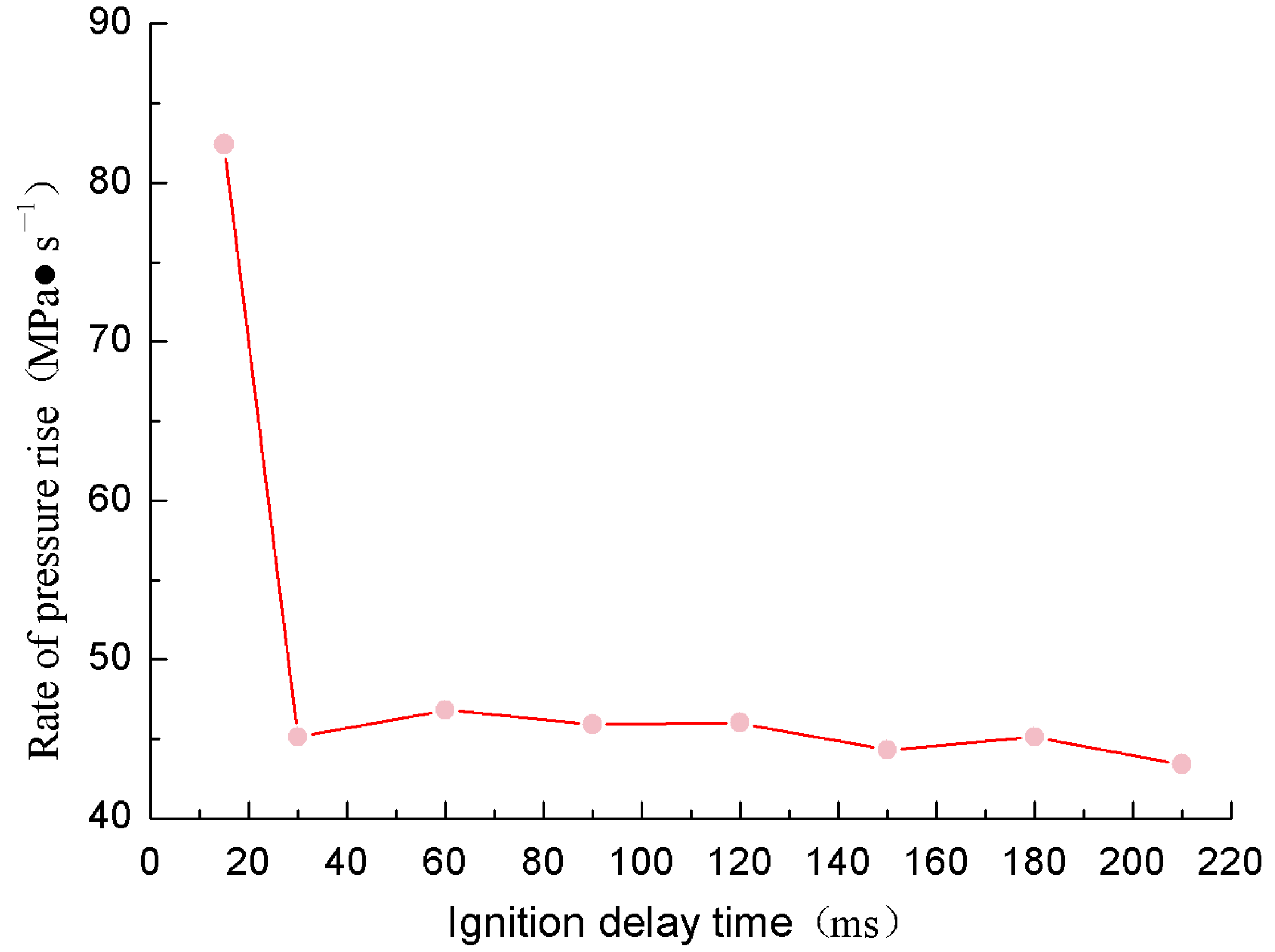


| Ignition Delay Time (ms) | Explosion Pressure (MPa) | |||
|---|---|---|---|---|
| 1st | 2nd | 3rd | Average Value | |
| 15 | 0.381 | 0.345 | 0.429 | 0.385 |
| 30 | 0.365 | 0.495 | 0.334 | 0.398 |
| 60 | 0.345 | 0.396 | 0.329 | 0.428 |
| 90 | 0.457 | 0.478 | 0.451 | 0.462 |
| 120 | 0.412 | 0.560 | 0.458 | 0.476 |
| 150 | 0.459 | 0.398 | 0.406 | 0.421 |
| 180 | 0.431 | 0.361 | 0.435 | 0.409 |
| 210 | 0.331 | 0.334 | 0.192 | 0.285 |
| Dust Concentration (g∙m−3) | Explosion Pressure (MPa) | |||
|---|---|---|---|---|
| 1st | 2nd | 3rd | Average Value | |
| 200 | 0.265 | 0.261 | 0.193 | 0.380 |
| 250 | 0.412 | 0.560 | 0.458 | 0.476 |
| 400 | 0.585 | 0.607 | 0.643 | 0.611 |
| 500 | 0.634 | 0.685 | 0.627 | 0.649 |
| 750 | 0.696 | 0.689 | 0.699 | 0.695 |
| 1000 | 0.666 | 0.668 | 0.648 | 0.661 |
| 1250 | 0.630 | 0.680 | 0.672 | 0.660 |
| Dust Pressure (MPa) | Explosion Pressure (MPa) | |||
|---|---|---|---|---|
| 1st | 2nd | 3rd | Average Value | |
| 0.9 | 0.442 | 0.390 | 0.378 | 0.403 |
| 1.1 | 0.387 | 0.498 | 0.412 | 0.432 |
| 1.3 | 0.412 | 0.56 | 0.458 | 0.476 |
| 1.5 | 0.396 | 0.520 | 0.488 | 0.467 |
| 1.7 | 0.504 | 0.439 | 0.341 | 0.428 |
| 1.9 | 0.369 | 0.289 | 0.364 | 0.340 |
| Explosive Index Range | Explosion Hazard Class |
|---|---|
| St1 | |
| St2 | |
| St3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Xie, C. Revealing Impact Characteristics of the Cassava Dust Explosion Process: Experimental and Numerical Research. Processes 2022, 10, 2419. https://doi.org/10.3390/pr10112419
Zhang R, Xie C. Revealing Impact Characteristics of the Cassava Dust Explosion Process: Experimental and Numerical Research. Processes. 2022; 10(11):2419. https://doi.org/10.3390/pr10112419
Chicago/Turabian StyleZhang, Ruichong, and Chengyu Xie. 2022. "Revealing Impact Characteristics of the Cassava Dust Explosion Process: Experimental and Numerical Research" Processes 10, no. 11: 2419. https://doi.org/10.3390/pr10112419
APA StyleZhang, R., & Xie, C. (2022). Revealing Impact Characteristics of the Cassava Dust Explosion Process: Experimental and Numerical Research. Processes, 10(11), 2419. https://doi.org/10.3390/pr10112419






