Ultrasound-Assisted Extraction of Polyphenols from Olive Pomace: Scale Up from Laboratory to Pilot Scenario
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Ultrasound-Assisted Extraction
2.2.1. Laboratory-Scale: Batch
2.2.2. Medium-Scale: Continuous
2.2.3. Pilot-Scale: Continuous
2.2.4. Total Polyphenol Content (TPC) Determination
3. Results and Discussion
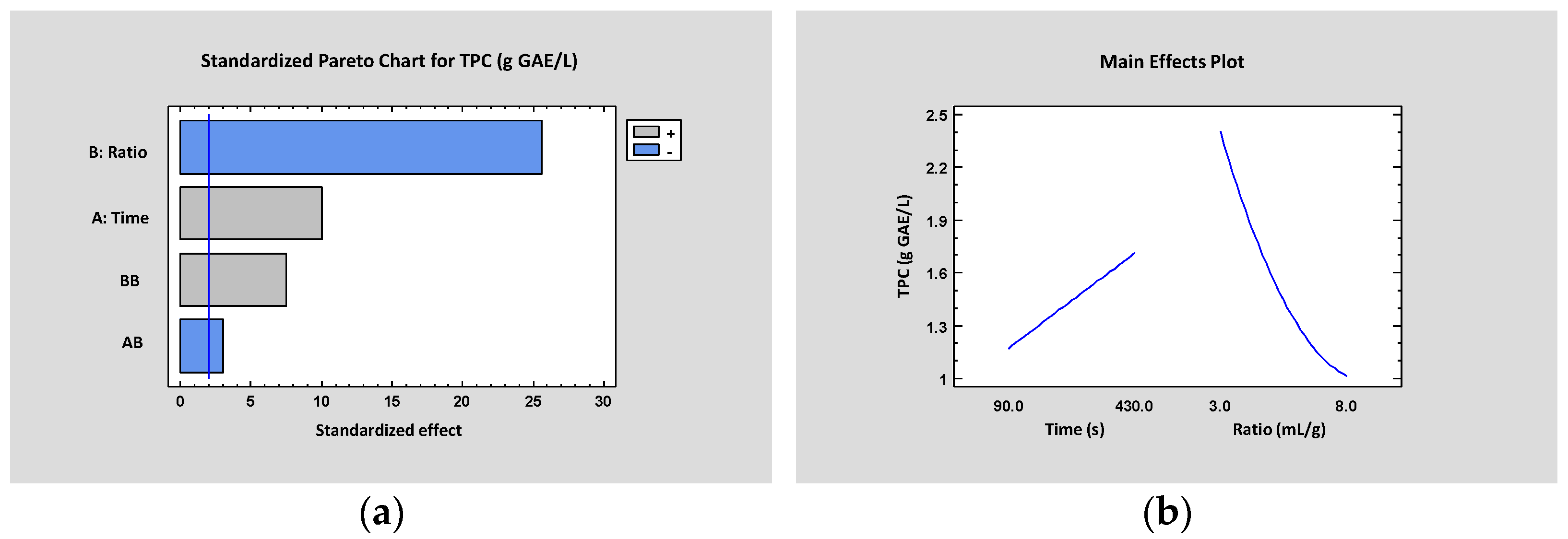
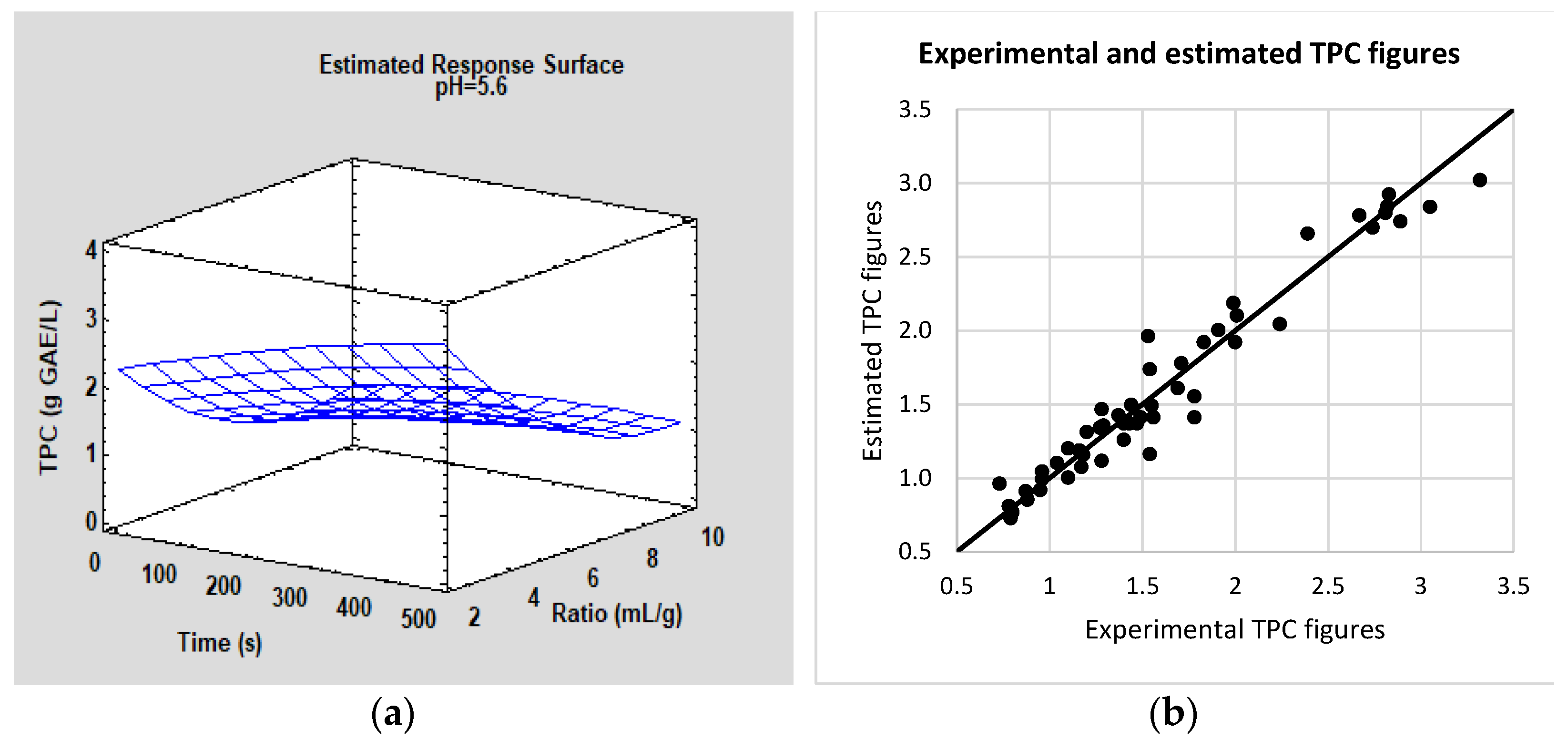
3.1. Laboratory-Scale: Batch
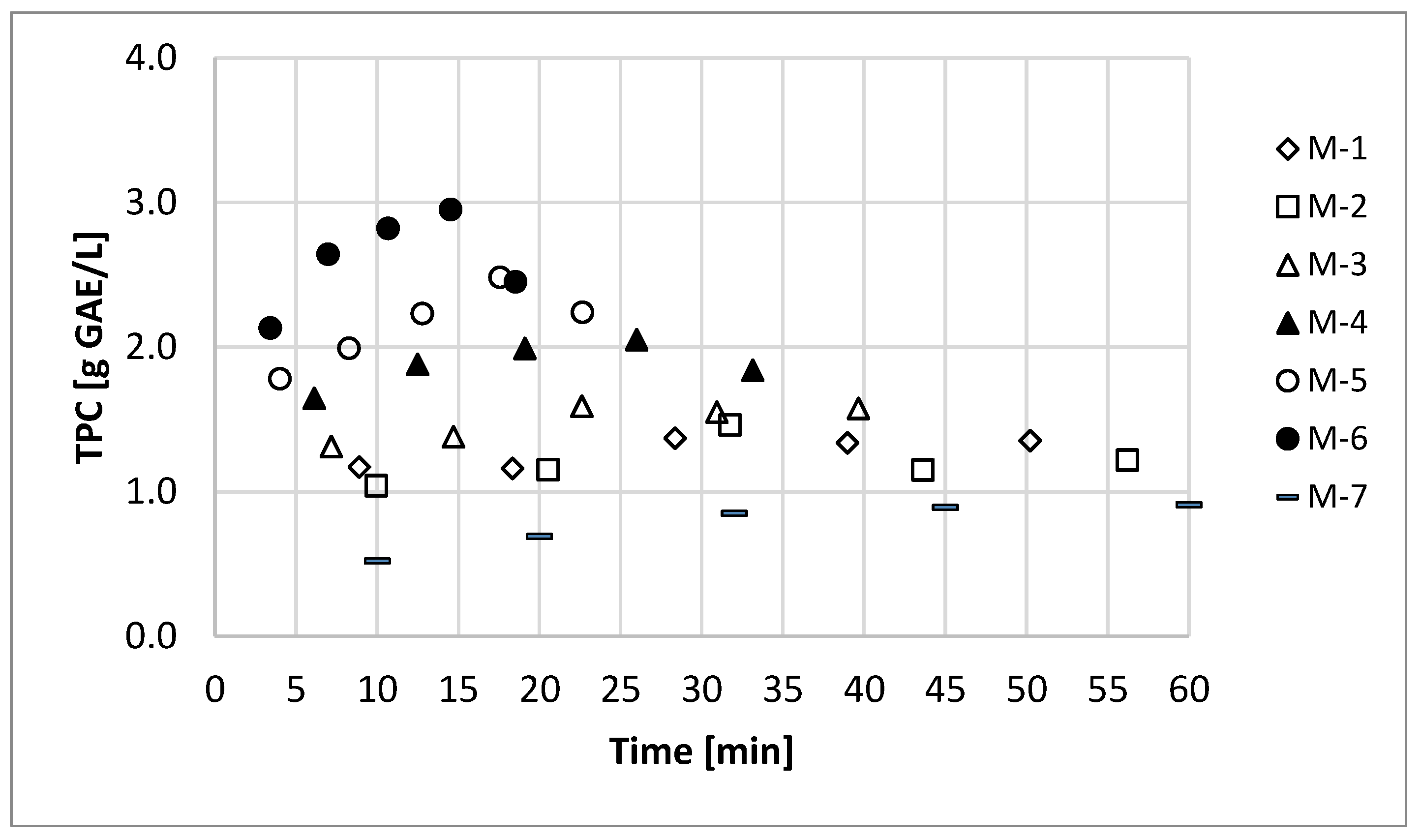
3.2. Medium-Scale: Continuous
3.3. Pilot-Scale: Continuous
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Rossi, R. The EU olive and olive oil sector. Eur. Parliam. Res. Serv. 2017, 608.690, 1–12. [Google Scholar]
- Şahin, S.; Şamlı, R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochem. 2013, 20, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Skaltsounis, A.-L.; Argyropoulou, A.; Aligiannis, N.; Xynos, N. Recovery of High Added Value Compounds from Olive Tree Products and Olive Processing Byproducts. Olive Olive Oil Bioact. Const. 2015, 333–356. [Google Scholar] [CrossRef]
- Albahari, P.; Jug, M.; Radić, K.; Jurmanović, S.; Brnčić, M.; Brnčić, S.R.; Čepo, D.V. Characterization of olive pomace extract obtained by cyclodextrin-enhanced pulsed ultrasound assisted extraction. LWT 2018, 92, 22–31. [Google Scholar] [CrossRef]
- Čepo, D.V.; Radić, K.; Jurmanović, S.; Jug, M.; Rajković, M.G.; Pedisić, S.; Moslavac, T.; Albahari, P. Valorization of Olive Pomace-Based Nutraceuticals as Antioxidants in Chemical, Food, and Biological Models. Molecules 2018, 23, 2070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batuecas, E.; Tommasi, T.; Battista, F.; Negro, V.; Sonetti, G.; Viotti, P.; Fino, D.; Mancini, G. Life Cycle Assessment of waste disposal from olive oil production: Anaerobic digestion and conventional disposal on soil. J. Environ. Manag. 2019, 237, 94–102. [Google Scholar] [CrossRef] [PubMed]
- European Commission. DIRECTIVE 2008/98/EC OF The European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives (Text with EEA Relevance). 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32008L0098 (accessed on 29 August 2022).
- Ahmad-Qasem, M.H.; Cánovas, J.; Barrajón-Catalán, E.; Micol, V.; Cárcel, J.A.; García-Pérez, J.V. Kinetic and compositional study of phenolic extraction from olive leaves (var. Serrana) by using power ultrasound. Innov. Food Sci. Emerg. Technol. 2013, 17, 120–129. [Google Scholar] [CrossRef]
- Pradal, D.; Vauchel, P.; Decossin, S.; Dhulster, P.; Dimitrov, K. Kinetics of ultrasound-assisted extraction of antioxidant polyphenols from food by-products: Extraction and energy consumption optimization. Ultrason. Sonochem. 2016, 32, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound Assisted Extraction for the Recovery of Phenolic Compounds from Vegetable Sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Patist, A.; Bates, D. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 2008, 9, 147–154. [Google Scholar] [CrossRef]
- Paniwnyk, L.; Alarcon-Rojo, A.; Rodriguez-Figueroa, J.C.; Toma, M. The Use of Ultrasound as an Enhancement Aid to Food Extraction; Elsevier Inc.: London, UK, 2017; pp. 399–440. [Google Scholar] [CrossRef]
- Kentish, S.E. Engineering Principles of Ultrasound Technology. In Ultrasound: Advances in Food Processing and Preservation; Elsevier Inc.: London, UK, 2017; pp. 1–13. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- De Bruno, A.; Romeo, R.; Fedele, F.L.; Sicari, A.; Piscopo, A.; Poiana, M. Antioxidant activity shown by olive pomace extracts. J. Environ. Sci. Health Part B 2018, 53, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Lafka, T.-I.; Lazou, A.E.; Sinanoglou, V.J.; Lazos, E.S. Phenolic and antioxidant potential of olive oil mill wastes. Food Chem. 2011, 125, 92–98. [Google Scholar] [CrossRef]
- Suárez, M.; Romero, M.-P.; Ramo, T.; Macià, A.; Motilva, M.-J. Methods for Preparing Phenolic Extracts from Olive Cake for Potential Application as Food Antioxidants. J. Agric. Food Chem. 2009, 57, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, M.; Namieśnik, J.; Konieczka, P. Ultrasound-Assisted Extraction. Appl. Green Solvents Sep. Process 2017, 301–324. [Google Scholar] [CrossRef]


| Test | Factors | Response | ||||
|---|---|---|---|---|---|---|
| Time [s] | Ratio [mL/g] | pH | TPC [g GAE/L] | TPC [g GAE/kg OP] | ΔT [°C] | |
| L-1 | 490 | 5.5 | 5.0 | 1.69 ± 0.15 | 33.1 ± 3.0 | 56 ± 3 |
| L-2 | 30 | 5.5 | 5.0 | 1.07 ± 0.10 | 21.1 ± 2.0 | 3 ± 1 |
| L-3 | 260 | 5.5 | 6.9 | 1.45 ± 0.21 | 28.6 ± 4.1 | 41 ± 2 |
| L-4 | 260 | 5.5 | 5.0 | 1.52 ± 0.05 | 30.0 ± 1.0 | 44 ± 3 |
| L-5 | 90 | 3.0 | 3.5 | 1.85 ± 0.27 | 19.9 ± 2.9 | 11 ± 1 |
| L-6 | 430 | 8.0 | 6.4 | 1.18 ± 0.09 | 33.8 ± 2.6 | 61 ± 1 |
| L-7 | 90 | 8.0 | 6.4 | 0.87 ± 0.09 | 24.9 ± 2.6 | 8 ± 1 |
| L-8 | 260 | 5.5 | 3.0 | 1.31 ± 0.12 | 25.8 ± 2.4 | 42 ± 4 |
| L-9 | 430 | 3.0 | 3.5 | 2.65 ± 0.23 | 28.5 ± 2.5 | 52 ± 2 |
| L-10 | 430 | 3.0 | 6.4 | 2.80 ± 0.11 | 30.1 ± 1.2 | 66 ± 3 |
| L-11 | 260 | 5.5 | 5.0 | 1.66 ± 0.20 | 32.7 ± 3.9 | 41 ± 2 |
| L-12 | 430 | 8.0 | 3.5 | 1.28 ± 0.12 | 36.7 ± 3.4 | 62 ± 3 |
| L-13 | 260 | 8.9 | 5.0 | 0.91 ± 0.16 | 29.0 ± 5.1 | 50 ± 4 |
| L-14 | 260 | 2.1 | 5.0 | 3.06 ± 0.26 | 23.0 ± 2.0 | 23 ± 2 |
| L-15 | 90 | 3.0 | 6.9 | 2.05 ± 0.17 | 22.0 ± 1.8 | 12 ± 1 |
| L-16 | 260 | 5.5 | 5.0 | 1.48 ± 0.08 | 29.2 ± 1.6 | 42 ± 1 |
| L-17 | 90 | 8.0 | 3.5 | 0.82 ± 0.04 | 23.5 ± 1.1 | 14 ± 3 |
| Source | Sum of Squares | Df | Mean Square | p-Value |
|---|---|---|---|---|
| A:Time | 2.58 | 1 | 2.58 | <0.0001 |
| B:Ratio | 16.85 | 1 | 16.85 | <0.0001 |
| C:pH | 0.06 | 1 | 0.06 | 0.1016 |
| AA | 0.07 | 1 | 0.07 | 0.0712 |
| AB | 0.23 | 1 | 0.23 | 0.0023 |
| AC | 0.02 | 1 | 0.02 | 0.3953 |
| BB | 1.43 | 1 | 1.43 | <0.0001 |
| BC | 0.06 | 1 | 0.06 | 0.1086 |
| CC | 0.08 | 1 | 0.08 | 0.0599 |
| Test | Variables | Responses | |||||
|---|---|---|---|---|---|---|---|
| Amplitude [µm] | Pressure [barg] | Power [W] | Intensity [W/cm2] | ΔT [°C] | TPC [g GAE/L] | TPC [g GAE/kg OP] | |
| M-1 | 41 | 0.0 | 217 ± 14 | 57.1 | 16 | 1.5 ± 0.2 | 10.8 ± 1.4 |
| M-2 | 46 | 0.0 | 235 ± 15 | 61.8 | 14 | 1.4 ± 0.2 | 10.0 ± 1.5 |
| M-3 | 57 | 0.0 | 288 ± 28 | 75.7 | 25 | 1.6 ± 0.1 | 11.5 ± 0.7 |
| M-4 | 57 | 1.0 | 343 ± 29 | 90.3 | 31 | 2.1 ± 0.2 | 15.1 ± 1.4 |
| M-5 | 43 | 0.0 | 528 ± 59 | 58.7 | 33 | 2.5 ± 0.1 | 17.9 ± 0.7 |
| M-6 | 43 | 1.0 | 632 ± 46 | 70.2 | 37 | 2.9 ± 0.1 | 20.8 ± 0.8 |
| M-7 | Conventional | 0 | 0 | 42 | 1.1 ± 0.1 | 7.9 ± 0.6 | |
| Test | Variables | Responses | |||||
|---|---|---|---|---|---|---|---|
| Amplitude [µm] | Pressure [barg] | Power [W] | Intensity [W/cm2] | ΔT [°C] | TPC [g GAE/L] | TPC [g GAE/kg OP] | |
| P-1 | 42 | 0.0 | 685 ± 197 | 30 ± 2 | 33 | 2.2 ± 0.3 | 11.0 ± 1.5 |
| P-2 | 42 | 1.0 | 1448 ± 140 | 58 ± 6 | 28 | 2.7 ± 0.2 | 13.5 ± 1.0 |
| P-3 | 30 | 0.0 | 755 ± 120 | 23 ± 4 | 30 | 2.4 ± 0.1 | 12.0 ± 0.5 |
| P-4 | 30 | 1.0 | 1464 ± 151 | 44 ± 5 | 31 | 3.1 ± 0.1 | 15.6 ± 0.4 |
| P-5 | 30 | 0.0 | 872 ± 170 | 26 ± 5 | 5 | 2.0 ± 0.2 | 10.0 ± 1.0 |
| P-6 | Conventional | 0 | 0 | 31 | 1.3 ± 0.3 | 6.5 ± 1.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, Ó.; Bona, S.; Stäbler, A.; Rodríguez-Turienzo, L. Ultrasound-Assisted Extraction of Polyphenols from Olive Pomace: Scale Up from Laboratory to Pilot Scenario. Processes 2022, 10, 2481. https://doi.org/10.3390/pr10122481
Rodríguez Ó, Bona S, Stäbler A, Rodríguez-Turienzo L. Ultrasound-Assisted Extraction of Polyphenols from Olive Pomace: Scale Up from Laboratory to Pilot Scenario. Processes. 2022; 10(12):2481. https://doi.org/10.3390/pr10122481
Chicago/Turabian StyleRodríguez, Óscar, Sergio Bona, Andreas Stäbler, and Laura Rodríguez-Turienzo. 2022. "Ultrasound-Assisted Extraction of Polyphenols from Olive Pomace: Scale Up from Laboratory to Pilot Scenario" Processes 10, no. 12: 2481. https://doi.org/10.3390/pr10122481







