The Cell Wall Regeneration of Tobacco Protoplasts Based on Microfluidic System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
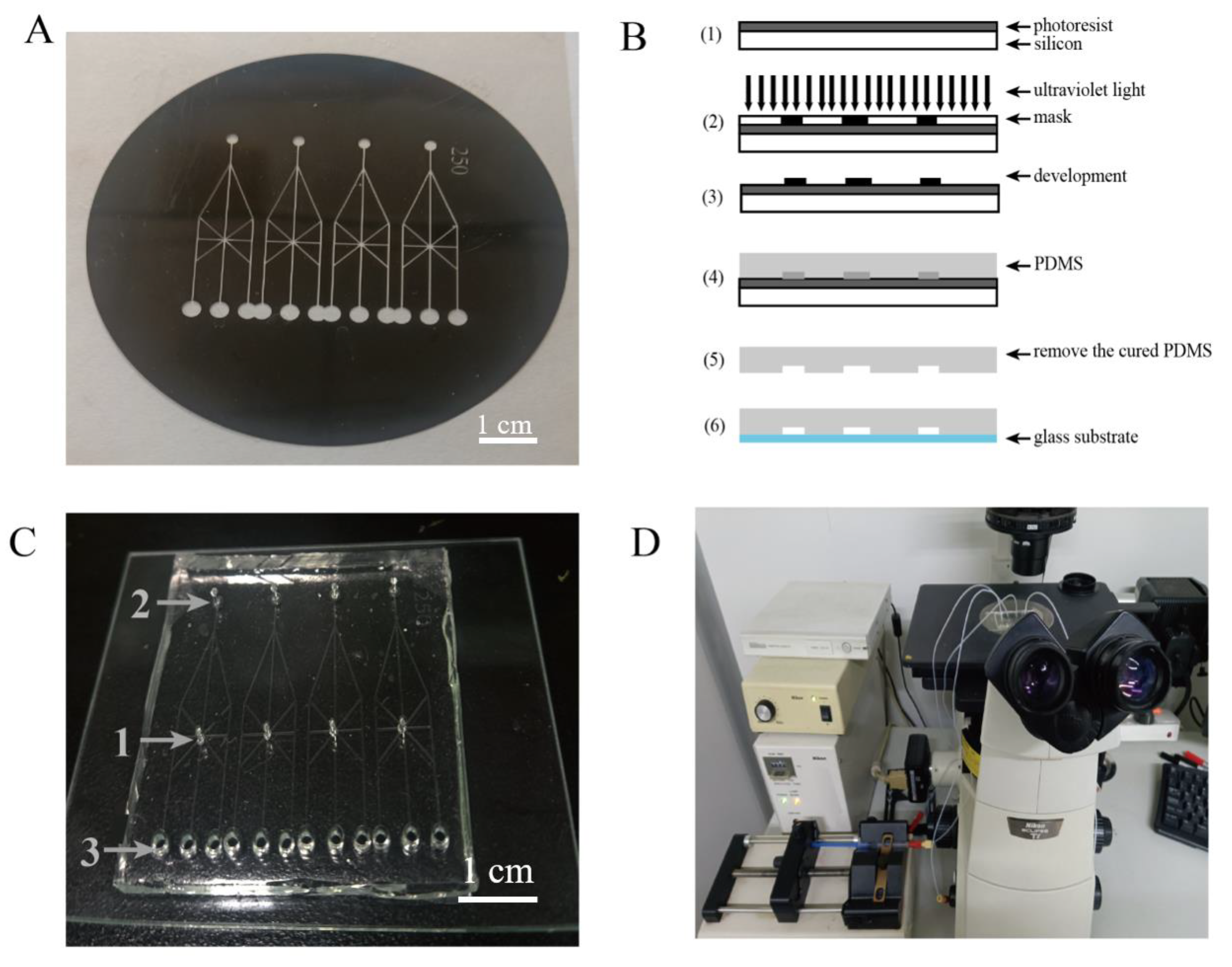
2.2. Microchip Fabrication
2.3. Extraction Process of Tobacco Protoplasts
2.4. The Separation and Cultivation of the Protoplasts in the Crossed Microfluidic Channels
2.5. The Observation of the Cell Wall Regeneration
2.6. Analysis of the Regeneration Ratio of the Plant Cell Wall
2.7. Statistical Analysis
3. Results and Discussion
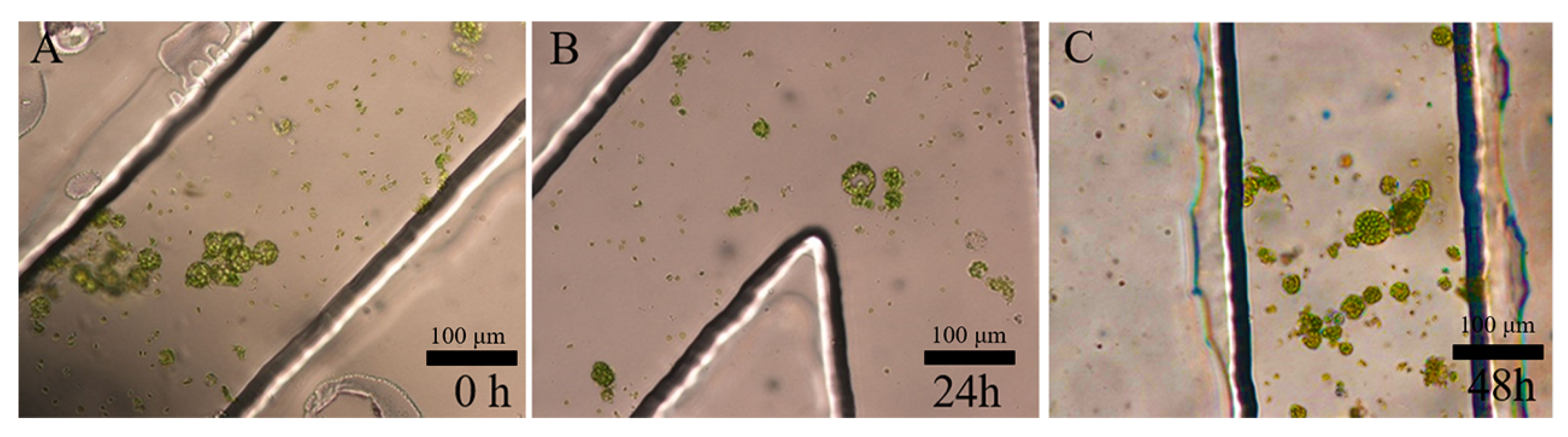
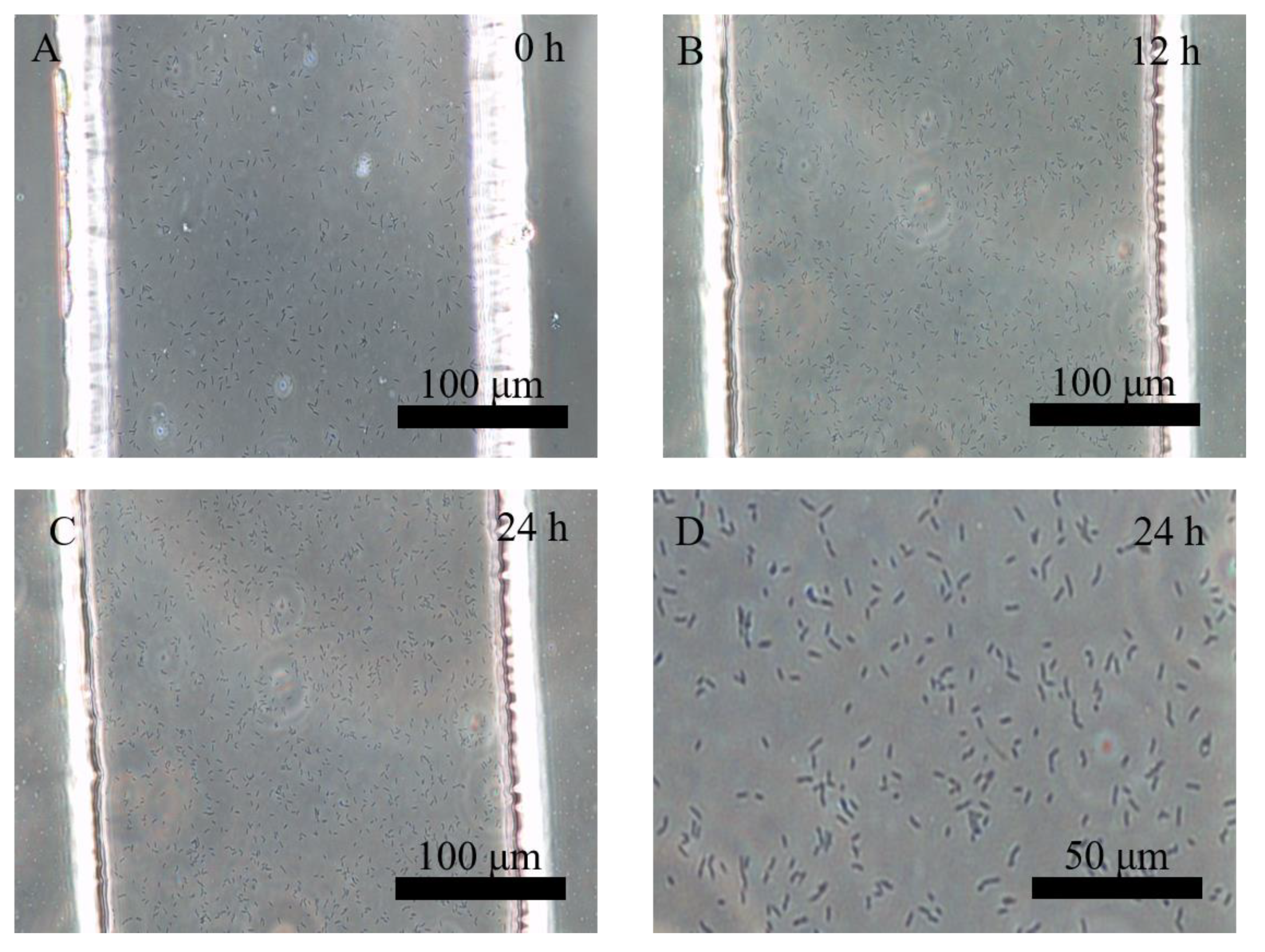
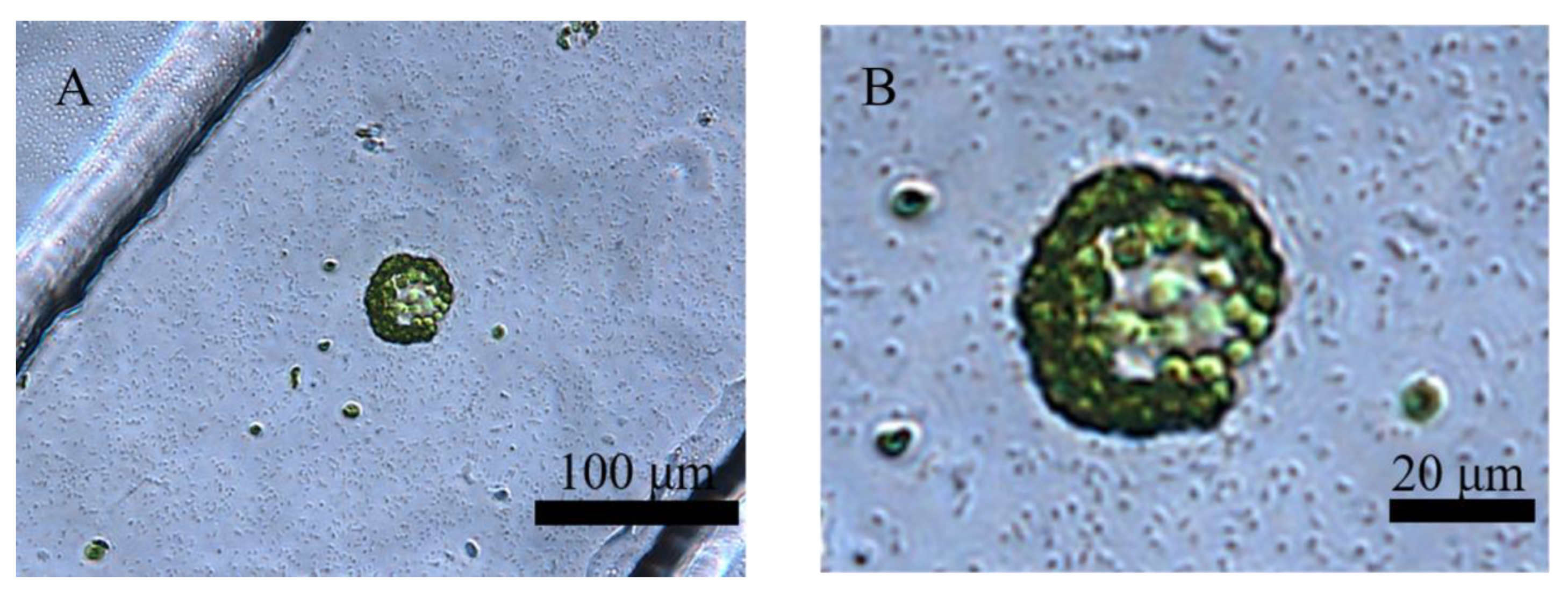
3.1. Separation and Cultivation of Protoplasts in Crossed Microfluidic Channels
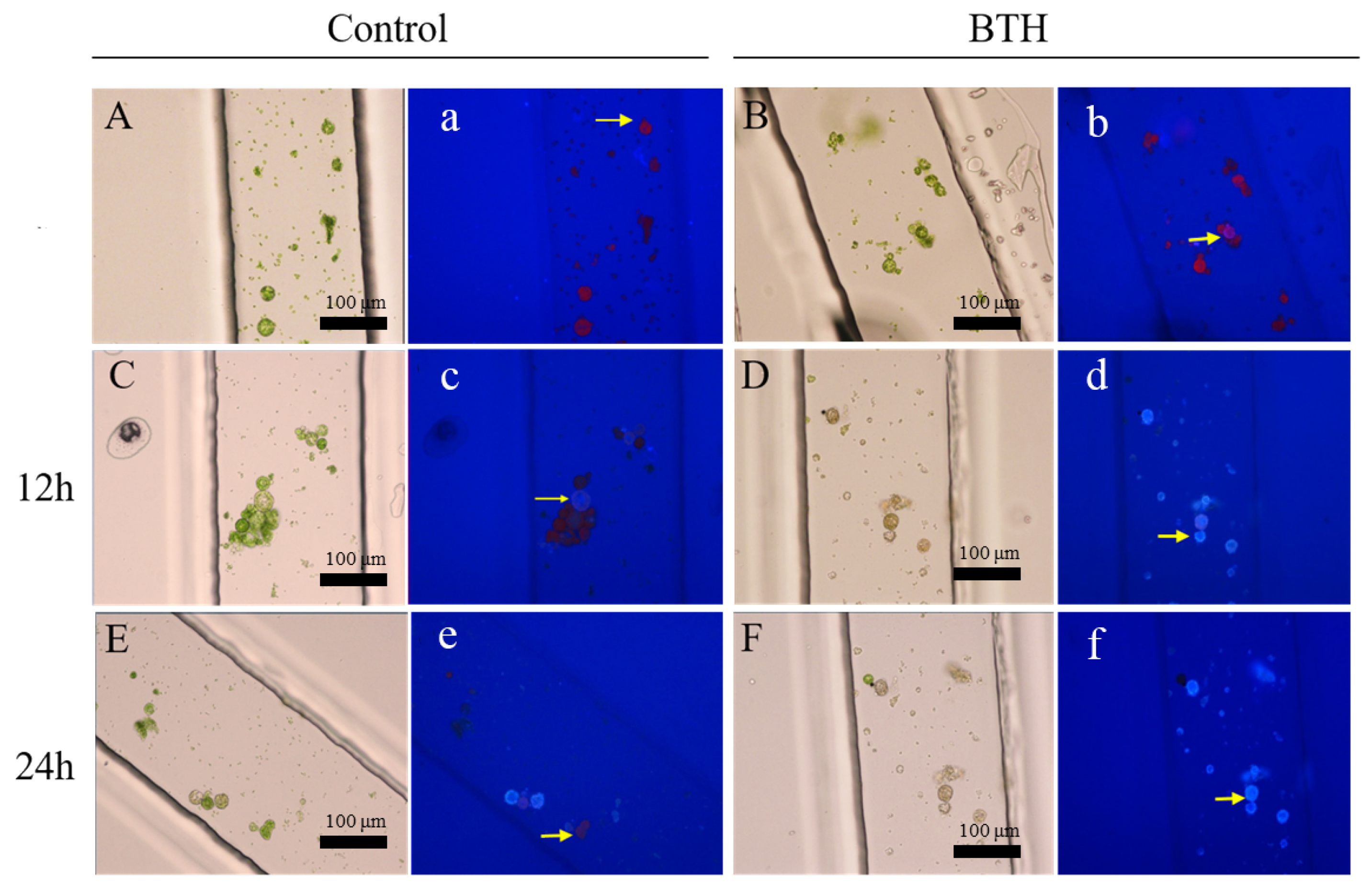
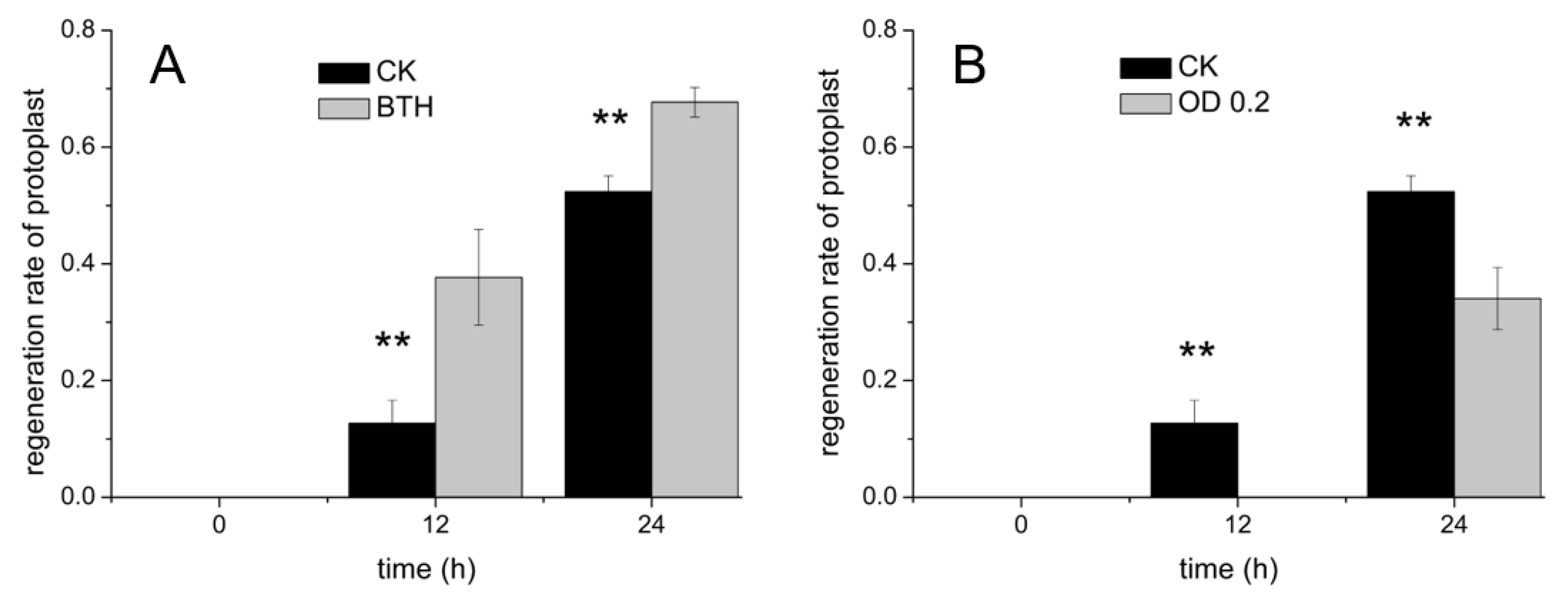
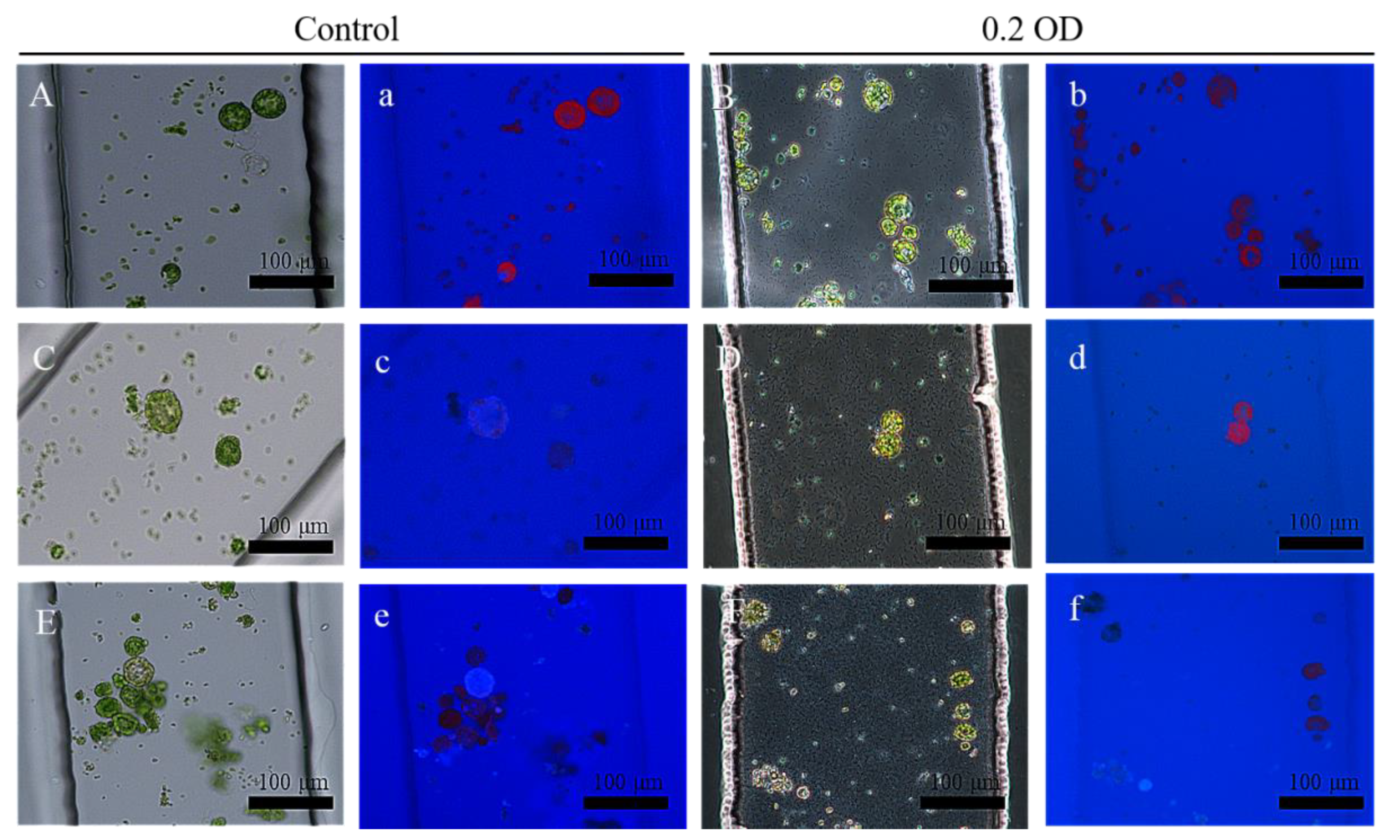
3.2. Regeneration of Cell Wall under BTH Treatment
3.3. Regeneration of Cell Wall under the PstDC3000 Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The plant cell wall: Biosynthesis, construction, and functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Vaahtera, L.; Schulz, J.; Hamann, T. Cell wall integrity maintenance during plant development and interaction with the environment. Nat. Plants 2019, 5, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Lin, W.; Zhou, X.; Guo, J.; Dang, X.; Li, B.; Lin, D.; Yang, Z. Mechano-transduction via the pectin-FERONIA complex activates ROP6 GTPase signaling in Arabidopsis pavement cell morphogenesis. Curr. Biol. 2022, 32, 508–517. [Google Scholar] [CrossRef]
- Bacete, L.; Mélida, H.; Miedes, E.; Molina, A. Plant cell wall-mediated immunity: Cell wall changes trigger disease resistance responses. Plant J. 2018, 93, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Z.; Wang, Y.; Wang, J.; Xiao, M.; Liu, H.; Quan, R.; Zhang, H.; Huang, R.; Zhu, L.; et al. Cellulose synthase-like protein OsCSLD4 plays an important role in the response of rice to salt stress by mediating abscisic acid biosynthesis to regulate osmotic stress tolerance. Plant Biotechnol. J. 2022, 20, 468–484. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, W.; Zhang, H.; Gu, L. Research progress on regeneration of cell wall from forest tree protoplasts. Mol. Plant Breed. 2021. Available online: http://kns.cnki.net/kcms/detail/46.1068.S.20210430.1320.006.html (accessed on 1 November 2022).
- Sahab, S.; Hayden, M.J.; Mason, J.; Spangenberg, G. Mesophyll Protoplasts and PEG-Mediated Transfections: Transient Assays and Generation of Stable Transgenic Canola Plants. In Transgenic Plants; Kumar, S., Barone, P., Smith, M., Eds.; Humana Press: New York, NY, USA, 2019; Volume 1864, pp. 131–132. [Google Scholar]
- Borgato, L.; Pisani, F.; Furini, A. Plant regeneration from leaf protoplasts of Solanum virginianum L. (Solanaceae). Plant Cell Tissue Organ Cult. 2007, 88, 247–252. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Piwowarczyk, B. Studies on cell wall regeneration in protoplast culture of legumes–the effect of organic medium additives on cell wall components. Czech. J. Genet. Plant Breed. 2014, 50, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Lucchetta, E.M.; Lee, J.H.; Fu, L.A.; Patel, N.H.; Ismagilov, R.F. Dynamics of Drosophila embryonic patterning network perturbed in space and time using microfluidics. Nature 2005, 434, 1134–1138. [Google Scholar] [CrossRef] [Green Version]
- Badri, A.M.; Coenen, K.; Vaillancourt, L.P.; Goulet, C.; Michaud, D. On-chip detection of low-molecular-weight recombinant proteins in plant crude extracts by SELDI-TOF MS. Methods Mol. Biol. 2009, 483, 313–324. [Google Scholar]
- Grossmann, G.; Guo, W.J.; Ehrhardt, D.W.; Frommer, W.B.; Sit, R.V.; Quake, S.R.; Meier, M. The RootChip: An integrated microfluidic chip for plant science. Plant Cell 2011, 23, 4234–4240. [Google Scholar] [CrossRef] [Green Version]
- Agudelo, C.G.; Sanati, N.A.; Ghanbari, M.; Naghavi, M.; Packirisamy, M.; Geitmann, A. TipChip: A modular, MEMS-based platform for experimentation and phenotyping of tip-growing cells. Plant J. 2013, 73, 1057–1068. [Google Scholar] [CrossRef]
- Sanati, N.A. Microfluidic platforms for plant cells studies. Lab Chip 2014, 14, 3262–3274. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, N.; Kozgunova, E.; Grossmann, G.; Geitmann, A.; Higashiyama, T. Microfluidics-Based Bioassays and Imaging of Plant Cells. Plant Cell Physiol. 2021, 62, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, W.; Tu, Q.; Song, N.; Li, L.; Wang, J.; Wang, J. Culture and chemical-induced fusion of tabacco mesophy11 protoplasts in a microfluidic device. Microfluid. Nanofluid. 2011, 10, 867–876. [Google Scholar] [CrossRef]
- Yu, Z.; Boehm, C.R.; Hibberd, J.M.; Abell, C.; Haseloff, J.; Burgess, S.J.; Reyna-Llorens, I. Droplet-based microfluidic analysis and screening of single plant cells. PLoS ONE 2018, 13, e0196810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, K.H.; Huang, L.; Kang, H.M.; Schiefelbein, J. Single-Cell RNA Sequencing Resolves Molecular Relationships Among Individual Plant Cells. Plant Physiol. 2019, 179, 1444–1456. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lee, A.P. Chapter 2-high-throughput microfluidic single-cell trapping arrays for biomolecular and imaging analysis. Method Cell Biol. 2018, 148, 35–50. [Google Scholar]
- Xu, X.; Wang, J.X.; Wu, L.L.; Guo, J.J.; Song, Y.L.; Tian, T.; Wang, W.; Zhu, Z.; Yang, C.Y. Microfluidic Single-Cell Omics Analysis. Small 2020, 16, 1903905. [Google Scholar] [CrossRef]
- Sun, L.; Liu, L.; Lin, X.; Xia, Z.; Cao, J.; Xu, S.; Gu, H.; Yang, H.; Bao, N. Microfluidic Devices for Monitoring the Root Morphology of Arabidopsis Thaliana in situ. Anal. Sci. 2021, 37, 605–611. [Google Scholar] [CrossRef]
- Sun, Y.; Xue, Q.; Ding, C.; Zhang, X.; Zhang, L.; Wang, W.; Ali, S. Somatic cybridization between Nicotiana tabacum and N. repanda based on a single inactivation procedure of nuclear donor parental protoplasts. Plant Sci. 2005, 168, 303–308. [Google Scholar] [CrossRef]
- Gao, H.; Guo, M.; Song, J.; Ma, Y.; Xu, Z. Signals in systemic acquired resistance of plants against microbial pathogens. Mol. Biol. Rep. 2021, 48, 3747–3759. [Google Scholar] [CrossRef] [PubMed]
- Lawton, K.A.; Friedrich, L.; Hunt, M.; Weymann, K.; Delaney, T.; Kessmann, H.; Staub, T.; Ryals, J. Benzothiadiazole induces disease resistance in Arabidopsis by activation of the systemic acquired resistance signal transduction pathway. Plant J. 1996, 10, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Shine, M.B.; Xiao, X.; Kachroo, P.; Kachroo, A. Signaling mechanisms underlying systemic acquired resistance to microbial pathogens. Plant Sci. 2019, 279, 81–86. [Google Scholar] [CrossRef]
- Cooper, B.; Beard, H.S.; Garrett, W.M.; Campbell, K.B. Benzothiadiazole Conditions the Bean Proteome for Immunity to Bean Rust. Mol. Plant-Microbe Interact. 2020, 33, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Burlini, N.; Iriti, M.; Daghetti, A.; Faoro, F.; Ruggiero, A.; Bernasconi, S. Benzothiadiazole (BTH) activates sterol pathway and affects vitamin D3 metabolism in Solanum malacoxylon cell cultures. Plant Cell Rep. 2011, 30, 2131–2141. [Google Scholar] [CrossRef]
- Barilli, E.; Rubiales, D.; Amalfitano, C.; Evidente, A.; Prats, E. BTH and BABA induce resistance in pea against rust (Uromyces pisi) involving differential phytoalexin accumulation. Planta 2015, 242, 1095–1106. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Ruiz-García, Y.; Williams, P.; Gil-Muñoz, R.; Gómez-Plaza, E.; Doco, T. Preharvest Application of Elicitors to Monastrell Grapes: Impact on Wine Polysaccharide and Oligosaccharide Composition. J. Agric. Food Chem. 2018, 66, 11151–11157. [Google Scholar] [CrossRef]
- Li, X.; Bi, Y.; Wang, J.; Dong, B.; Li, H.; Gong, D.; Zhao, Y.; Tang, Y.; Yu, X.; Shang, Q. BTH treatment caused physiological, biochemical and proteomic changes of muskmelon (Cucumis melo L.) fruit during ripening. J. Proteom. 2015, 120, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.F.; He, S.Y. Pseudomonas syringae pv. tomato DC3000: A model pathogen for probing disease susceptibility and hormone signaling in plants. Annu. Rev. Phytopathol. 2013, 51, 473–498. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Cai, G.; Jiang, S.; Sun, L.; Li, D. Response of tobacco to the Pseudomonas syringae pv. tomato DC3000 is mainly dependent on salicylic acid signaling pathway. FEMS Microbiol. Lett. 2013, 344, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Hou, S.; Wu, Q.; Lin, M.; Acharya, B.R.; Wu, D.; Zhang, W. IDL6-HAE/HSL2 impacts pectin degradation and resistance to Pseudomonas syringae pv. tomato DC3000 in Arabidopsis leaves. Plant J. 2017, 89, 250–263. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Sun, Z.; Liu, L.; Yang, Y.; Zhang, S.; Li, Y.; Bao, N.; Zhang, Y.; Sun, L. The Cell Wall Regeneration of Tobacco Protoplasts Based on Microfluidic System. Processes 2022, 10, 2507. https://doi.org/10.3390/pr10122507
Xu S, Sun Z, Liu L, Yang Y, Zhang S, Li Y, Bao N, Zhang Y, Sun L. The Cell Wall Regeneration of Tobacco Protoplasts Based on Microfluidic System. Processes. 2022; 10(12):2507. https://doi.org/10.3390/pr10122507
Chicago/Turabian StyleXu, Songzhi, Zhanghua Sun, Lili Liu, Ying Yang, Shuangyu Zhang, Ying Li, Ning Bao, Yali Zhang, and Lijun Sun. 2022. "The Cell Wall Regeneration of Tobacco Protoplasts Based on Microfluidic System" Processes 10, no. 12: 2507. https://doi.org/10.3390/pr10122507
APA StyleXu, S., Sun, Z., Liu, L., Yang, Y., Zhang, S., Li, Y., Bao, N., Zhang, Y., & Sun, L. (2022). The Cell Wall Regeneration of Tobacco Protoplasts Based on Microfluidic System. Processes, 10(12), 2507. https://doi.org/10.3390/pr10122507





