1. Introduction
Water vapor in the atmosphere is important to human life. The essential reason is that the heat and mass transfer processes of water vapor are crucial to many engineering practices—for example, cooling [
1], dehumidification [
2], thermal power plant [
3], water harvesting [
4], cryogenic wind tunnels [
5], etc. For the safety monitoring of cryogenic fuels, such as LH2 (liquid hydrogen), LOX (liquid oxygen), LN2 (liquid nitrogen), and LNG (liquid natural gas), water vapor in the air also plays an important role.
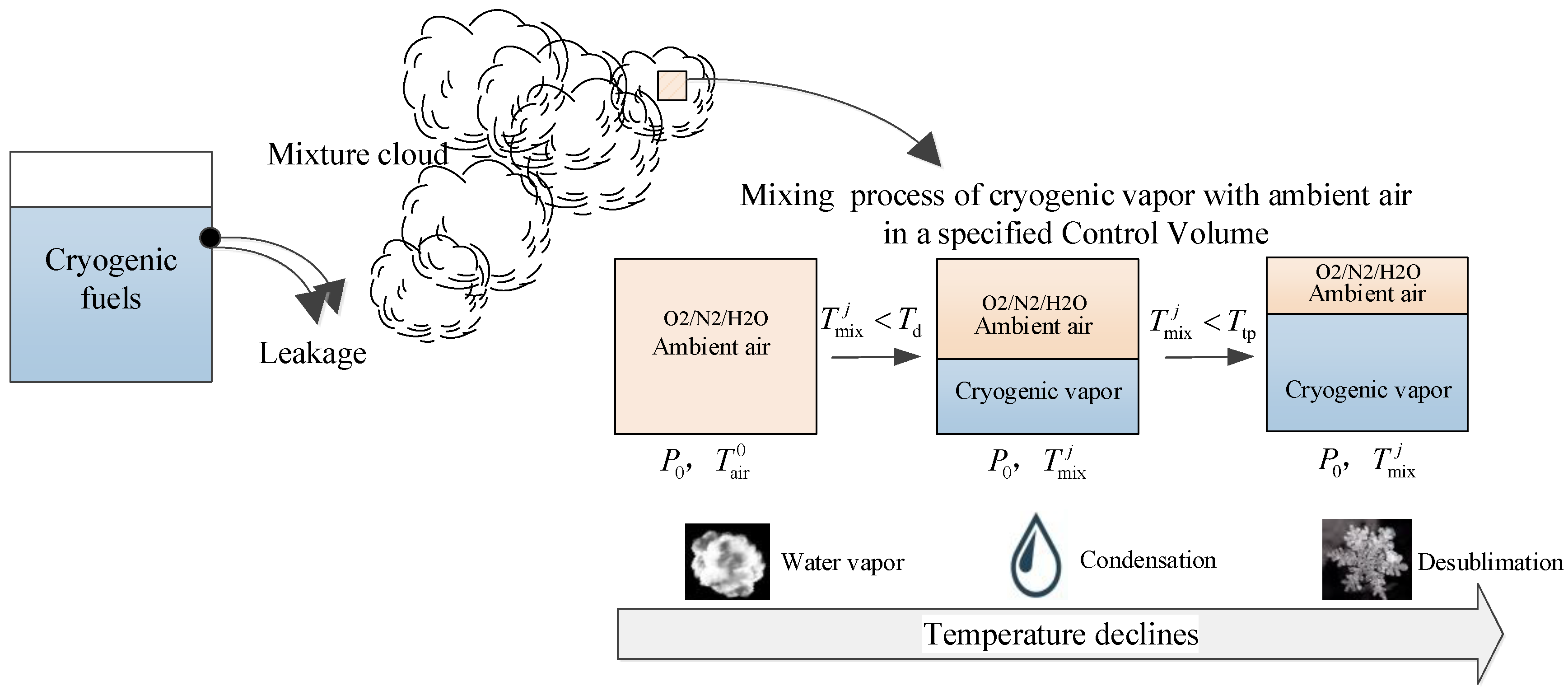
The cooling load induced by the cryogenic vapor will cause a temperature drop in the air, and a phase change in the water vapor (as shown in
Figure 1). Condensation and desublimation will occur when the air temperature is lower than the dew point
Td and triple point
Ttp, respectively. Because the low local temperature and high local humidity exceed the work range of commercial sensors, cryogenic gas detection has been a challenge for a long time [
6,
7,
8,
9]. For example, the gas sensors were severely affected by the condensed water vapor and the low temperature in both NASA [
6] and HSL [
7] liquid hydrogen release experiments. In these two experiments, hydrogen concentration data were obtained by adiabatic mixing calculations using the off-line temperature values. Unfortunately, a detailed model cannot be found in publications. Moreover, in the latest experimental program of HSL [
8], cryogenic hydrogen gas was extracted remotely from the sampling point through a pneumatic tube for analysis by the sensor. Besides this, two adiabatic mixing models have been respectively developed by Venetsanos et al. [
9] and Shao et al. [
10] in recent years, giving us a temporary solution to cryogenic hydrogen detection based on experimental temperature data.
In our previous study [
10], a calculation model based on adiabatic mixing theory for deriving cryogenic gas concentration was presented. To use the model in an engineering application, the authors conducted a real-time monitoring platform R&D program of cryogenic hydrogen detection. In Ref. [
10], the density of the water vapor is a key parameter for cryogenic gas concentration determination, the value of which was obtained using the software of REFPORP V9.1, which is a thermophysical properties database developed by NIST (National Institute of Standards and Technology) [
11]. However, for real-time monitoring systems, the expected fast response time will not realizable when the NIST database is continuously called. At the same time, the NIST database may be inapplicable to many engineering applications. Although IAPWS (International Association for the Properties of Water and Steam) formulation 95 has been proposed for general and scientific use [
12], there is no doubt that its high complexity (56 coefficients, and the parameters of the residual part of the dimensionless Helmholtz free energy) limits its industrial utilization.
As discussed above, an accurate and simple approach to the estimation of the density of water vapor is required for industrial utilization, especially for cryogenic fuel leakage monitoring, and this is our motivation in conducting this study. In this paper, the authors present an approach to water vapor density estimation, and satisfactory results have been obtained.
2. Methods
2.1. Dew Point Calculation
Under the adiabatic mixing assumption, a control volume inside a mixture cloud is selected as the investigation object. The control volume is thought to be in a quasi-steady state, the control volume is treated as an open adiabatic system, and the temperature drop is caused by the intake of some saturated cryogenic vapor (H2) into the control volume under the leakage condition.
As shown in
Figure 1, once the cryogenic fuel leaks into the environment, the affected ambient air will be cooled by the cryogenic vapor (
), and condensation/desublimation will occur with continuous temperature reduction. In
Figure 1,
is the temperature of the mixture cloud at time
j,
is the initial ambient air temperature,
is the ambient pressure, and
and
respectively denote the dew point and triple point of water vapor in the air. This process is shown in the P-T phase diagram of water in
Figure 2.
Assume that the initial state of the ambient air is located at point P1, and the temperature and relative humidity are and , respectively. If the ambient air is continuously cooled by cryogenic vapor (), the location of the water vapor moves from P1 to the vapor–liquid saturation line horizontally. The cross point of the temperature drop line and the saturation line is the so-called dew point, . Here, we define this cross point as , which is the dew point of the ambient air in the initial state. With further continuous cooling, the temperature drops along the saturation line, and condensation and desublimation will respectively occur in zone (1) and zone (2). That is to say, when the temperature drops along the saturation line, every temperature point is equal to the corresponding dew point at time level j ().
For the initial dew point
calculation, the correlation developed by Qian [
13] was adopted, which combines temperature
T with relative humidity
φ.
2.2. Partial Pressure Calculation
As we know, the gas state is simultaneously determined by temperature and pressure. However, the partial pressure of water vapor in air cannot be directly obtained by sensors. Thus, the partial pressure of water vapor is crucial for its density estimation. Here, an indirect method to obtain the water vapor’s partial pressure is proposed. The steps are as follows:
During the temperature reduction process shown in
Figure 1 and
Figure 2, the saturation pressure of water vapor
Ps,H2O at temperature
T can be calculated by the Goff–Gratch formulation [
14]. This formulation has been proposed by the WMO (World Meteorological Organization) and adopted by the China Meteorological Administration. For temperature
T within the range 273–350 K, the formulation of water saturation pressure on the liquid surface is adopted,
and for temperature
T within the range 200–273 K, the formulation of water saturation pressure on an ice surface is adopted,
where
T1 = 273.16 K. Note that the unit of
Ps,H2O is hPa here, and is Pa elsewhere in this paper. Compared with the NIST values, this formulation could obtain highly accurate predictions (
Figure 3). The mean deviation is −0.10%, and the maximum deviation is −0.146%.
As shown in the P-T phase diagram of water (
Figure 2), if the temperature exceeds the initial dew point
, the water vapor partial pressure
PH2O does not change with the decreased temperature, but the saturation water vapor pressure
Ps,H2O declines. When the temperature
drops to the saturation line, the initial dew point
is first reached, and the saturation water vapor pressure
Ps,H2O is equal to the water vapor partial pressure
PH2O. Hereafter, the water vapor partial pressure
PH2O begins to decline with continuous water vapor condensation during the temperature drop process along the saturation line. In addition, the dew point
continuously declines with water vapor condensation. Thus, the correlation between the water vapor partial pressure
PH2O(
T) and the saturation water vapor pressure on the dew point
Ps,H2O(
Td) is given as follows:
2.3. Density Calculation
The partial pressure of water vapor in air is very low, so the ideal gas law is applicable. Therefore, the equation of state (EOS) of ideal gas can be used to derive the water vapor density,
ρH2O:
Here, MH2O is the molecular weight of water, 18.015 kg/kmol, and R is the universal gas constant, 8314 J/(kmol∙K).
2.4. Procedure
In summary, the procedure of water vapor density estimation with the adiabatic mixing of cryogenic vapor and moist air can be seen in
Figure 4. The first step is deriving the initial conditions of the ambient air,
and
. Secondly, the initial dew point can be solved by Equation (1). The third step regards the water vapor partial pressure solution, which is based on temperature comparisons between the mixture cloud temperature, the dew point and the triple point. Then, Equations (2) and (3) are used for larger and lower than triple point cases, respectively. Fourthly, the water vapor density can be solved by EOS.
3. Results
3.1. Validation Dataset
To validate the estimation approach mentioned in
Section 2, it is necessary to construct a validation dataset. Because the temperature drop process is determined by the location of P1 in
Figure 2, it is ineffective to validate the approach using only a few locations for the real conditions. However, any location of P1 is determined by the water vapor state’s temperature
T and partial pressure
PH2O, and the partial pressure is correlated with the relative humidity
φ. Thus, a random temperature
T and relative humidity
φ can be defined, and we can derive the partial pressure via the definition of relative humidity
φ (Equation (6)).
Using the random generation function, a validation sample dataset including 10,000 state points was constructed. The samples are within the ranges of 273–320 K and 0–100% RH, respectively, and have an even distribution, which implies a fair validation basis. The water vapor saturation pressure Ps,H2O(T) was first obtained by NIST, while PH2O(T, φ) was solved by Equation (6), and ρH2O with 10,000 points can be obtained by NIST. These values were adopted as the validation dataset.
3.2. Validation Result
Table 1 shows a comparison of the present model with IAPWS 95. The present model has a mean relative error (
MRE) of 0.08%, which is very close to the level of IAPWS 95 of 0.03%.
Here, MRE is the mean relative error, i is the index of sample point, m is the number of sample points, is the value predicted by the model, and is the true value.
The
MRE distribution in various deviation regions is firstly shown in
Table 2. The maximum deviation of the model is 0.35%. At the same time, the most concentrated area is in the 0–0.1% region, which implies the most robust accuracy of the models.
Figure 5 displays the distribution density and deviation versus the temperature and relative humidity
φ. A further exploration of the validation results shows that the presented model has higher sensitivity to humidity
φ than temperature
T. It can be found that the model gives a high accuracy of
MRE < 0.03% in the low-humidity region (
φ < 10% RH), and gives relatively larger deviations of 0.002–0.35% in the medium and high humidity regions (
φ > 10% RH). However, temperature is not significantly sensitive to
MRE. The reason for this is that the calculated water vapor’s partial pressure value is strongly affected by the relative humidity, and the influence of temperature is only reflected in the saturated water vapor pressure, which does not vary dramatically in the region of 273–320 K. Thus, the yielded water vapor density is sensitive to relative humidity, and the sensitivity to temperature is very slight.
The computation time comparisons were conducted on the monitoring platform, which was developed using the matlab 2017a code and has nine temperature signal channels. In the base case (case A), all the density and enthalpy values of the hydrogen and moist air components were obtained by continually calling the NIST database. In case C, the water vapor density was determined by the model in this paper, and the other thermal properties were calculated by fitted correlations. The only difference between case B and case C is that the water vapor density was obtained by calling the NIST database. The results shown that the response times in case A, case B and case C are 35 ms, 5 ms, and 3 ms, respectively. This indicates that the model presented in this paper could bring a computational efficiency increase of +67% (case C vs. case B). Although the increase in the computation efficiency (+67%) is not dramatic, the model presented here facilitates the utilization of the adiabatic mixing model in engineering applications, because the NIST database may be inapplicable in industrial scenarios. Moreover, the advantages of this model will be enhanced if the scale of testing points is significantly increased.
4. Conclusions
In this paper, the estimation of ambient water vapor was the focus, and we sought to facilitate the engineering utilization of the adiabatic mixing model for cryogenic fuel leakage monitoring. A calculation model was presented based on the detection of temperature and the determination of water vapor partial pressure.
The validation ranges of temperature and relative humidity are 273.16–320 K and 0–100% RH, respectively. The results show that the present model has a high estimation accuracy. The mean relative error of the present model (0.08%) is close to the IAPWS 95 of 0.03%, and the maximum MRE is 0.35%. It is confirmed that the model could yield a computation efficiency increase of +67%.
However, the present model shows relatively larger deviations in the region of relative humidity exceeding 10% RH; this issue should be addressed in the future. Moreover, the accuracy in the temperature region lower than triple point should also be validated.