Chemical Analysis and Quality Assessment of Honey Obtained from Different Sources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Organoleptic Analysis
2.3. Determination of Water Content (wc)
2.4. pH and Free Acidity
2.5. Electrical Conductivity (EC)
2.6. Soluble Solids Content (SSC)
2.7. Protein Content Determination
2.8. Determination of Cu, Cr, Mn, Co, Cd, Ni, Pb, Fe by Graphite Atomic Absorption Spectrometry
2.9. Statistical Analysis
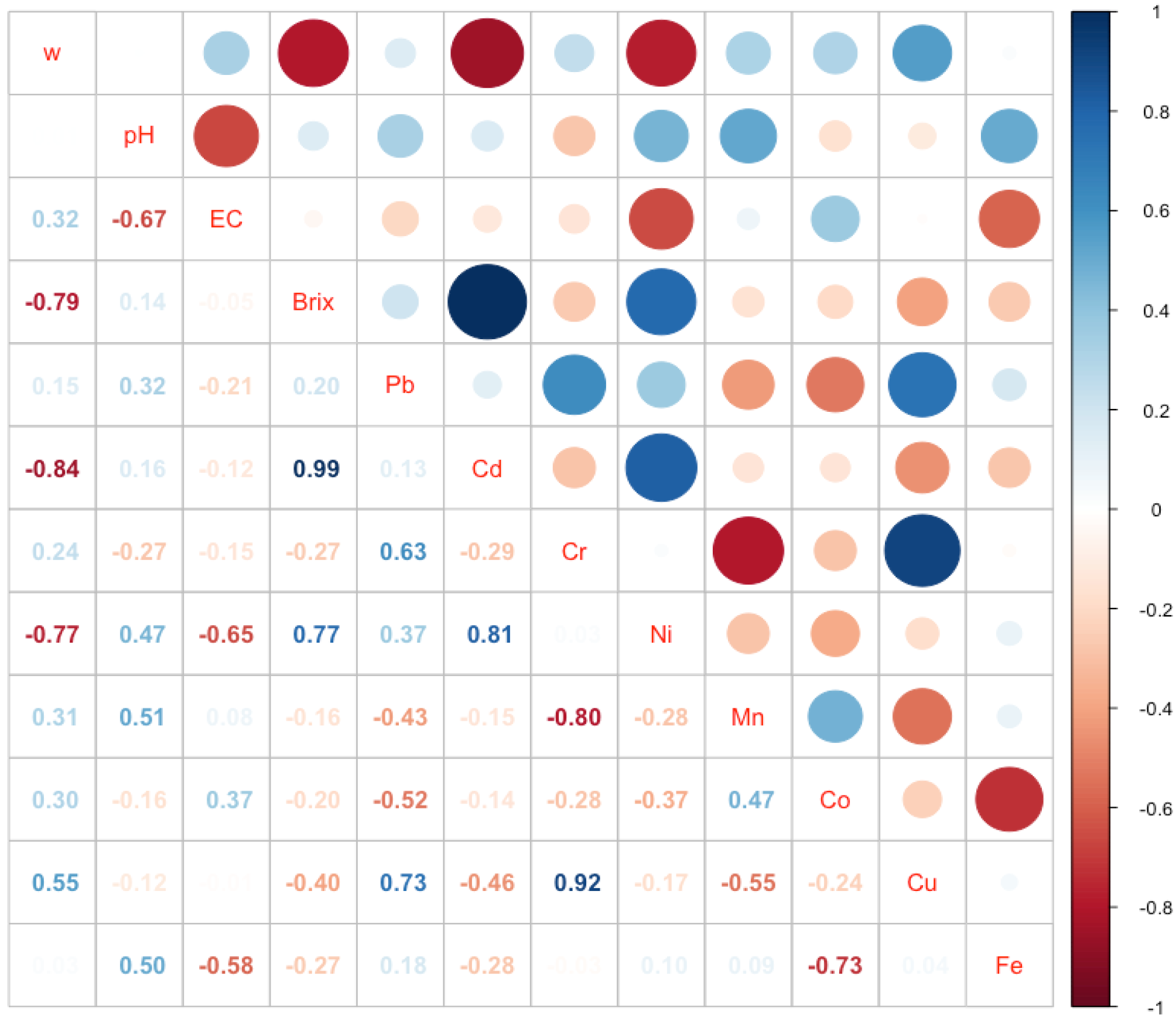
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- The Editors of Encyclopaedia Britannica. Honey. Available online: https://www.britannica.com/topic/honey (accessed on 22 May 2022).
- Codex Alimentations. Draft revised standard for standard for honey (at step 10 of the Codex procedure). Alinorm 2001, 1, 19–26. [Google Scholar]
- Tischer-Seraglio, S.K.; Silva, B.; Bergamo, G.; Brugnerotto, P.; Valdemiro-Gonzaga, L.; Fett, R.; Costa, A.C.O. An overview of physicochemical characteristics and health-promoting properties of honeydew honey. Food Res. Int. 2019, 119, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, T.; Stamatovska, V.; Kalevska, T.; Dimov, I.; Nakov, G. Quality characteristics of honey: A review. Proc. Univ. Ruse 2018, 57, 31–57. [Google Scholar]
- Conti, M.E.; Finoia, M.G.; Fontana, L.; Mele, G.; Botre, F.; Iavicoli, I. Characterization of Argentine honeys on the basis of their mineral content and some typical quality parameters. Chem. Cent. J. 2014, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Aljohar, H.I.; Maher, H.M.; Albaqami, J.; Al-Mehaizie, M.; Orfali, R.; Orfali, R.; Alrubia, S. Physical and chemical screening of honey samples available in the Saudi market: An important aspect in the authentication process and quality assessment. Saudi Pharm. J. 2018, 26, 932–942. [Google Scholar] [CrossRef]
- Alghamdi, B.A.; Alshumrani, E.S.; Saeed, M.S.B.; Rawas, G.M.; Alharthi, N.T.; Baeshen, M.N.; Helmi, N.M.; Alam, M.Z.; Suhail, M. Analysis of sugar composition and pesticides using HPLC and GC–MS techniques in honey samples collected from Saudi Arabian markets. Saudi J. Biol. Sci. 2020, 27, 3720–3726. [Google Scholar] [CrossRef]
- El Sohaimy, S.A.; Masry, S.H.D.; Shehata, M.G. Physicochemical characteristics of honey from different origins. Ann. Agric. Sci. 2015, 60, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Missio da Silva, P.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Ghramh, H.A.; Khan, K.A.; Ahmed, Z.; Ansari, M.J. Quality evaluation of Saudi honey harvested from the Asir province by using high-performance liquid chromatography (HPLC). Saudi J. Biol. Sci. 2020, 27, 2097–2105. [Google Scholar] [CrossRef]
- Bittar, D.B.; Catelani, T.A.; Pezza, L.; Pezza, H.R. A fast method for the determination of lead in honey samples using stabilizer-free silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 189, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Lazarus, M.; Lovaković, B.T.; Orct, T.; Sekovanić, A.; Bilandžić, N.; Ðokić, M.; Kolanović, B.S.; Varenina, I.; Jurič, A.; Lugomer, M.D.; et al. Difference in pesticides, trace metal(loid)s and drug residues between certified organic and conventional honeys from Croatia. Chemosphere 2021, 266, 128954. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Chowdhury, M.A.Z.; Rahman, M.A.; Sulaiman, S.A.; Gan, S.H. Determination of Mineral, Trace Element, and Pesticide Levels in Honey Samples Originating from Different Regions of Malaysia Compared to Manuka Honey. Biomed. Res. Int. 2014, 2014, 359890. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.N.; Soltan, M.E. Major and trace elements in different types of Egyptian mono-floral and non-floral bee honeys. J. Food Compos. Anal. 2004, 17, 725–735. [Google Scholar] [CrossRef]
- Fiorentini, E.F.; Oviedo, M.N.; Wuilloud, R.G. Ultra-trace Cr preconcentration in honey samples by magnetic ionic liquid dispersive liquid-liquid microextraction and electrothermal atomic absorption spectrometry. Spectrochim. Acta Part B 2020, 169, 105879. [Google Scholar] [CrossRef]
- Fakhria, Y.; Abtahib, M.; Atamalekia, A.; Raoofic, A.; Atabatid, H.; Asadie, A.; Mirif, A.; Shamloog, E.; Alinejadh, A.; Keramatii, H.; et al. The concentration of potentially toxic elements (PTEs) in honey: A global systematic review and meta-analysis and risk assessment. Trends Food Sci. Technol. 2019, 91, 498–506. [Google Scholar] [CrossRef]
- Altun, S.K.; Dinç, H.; Paksoy, N.; Temamoğullar, F.K.; Savrunlu, M. Analyses of Mineral Content and Heavy Metal of Honey Samples from South and East Region of Turkey by Using ICP-MS. Int. J. Anal. Chem. 2017, 2017, 6391454. [Google Scholar] [CrossRef]
- Taliob, M.C.; Muñozb, V.; Acostab, M.; Fernándeza, L.P. Determination of lead traces in honey using a fluorimetric method. Food Chem. 2019, 298, 125049. [Google Scholar]
- Morgano, M.A.; Milani, R.F.; Martins, M.C.T.; Rodriguez-Amaya, D.B. Determination of water content in Brazilian honeybee-collected pollen by Karl Fischer titration. Food Control 2011, 22, 1604–1608. [Google Scholar] [CrossRef] [Green Version]
- SR 784/2:2009 Honey. Part 1. Requirements for Producers; Part 2: Quality Requirements at Sale; Part 3: Analysis Methods. 2009. Available online: https://e-standard.eu/en/standard/174481 (accessed on 8 January 2009).
- Association of Official Analytical Chemists (AOAC). Official Method of Analysis, 19th ed.; AOAC: Washington, DC, USA, 2012. [Google Scholar]
- Bogdanov, S.; Martin, P.; Luelmann, C. Harmonised methods of the European Honey Commission. Apidologie 1998, 28, 1–59. [Google Scholar]
- Lazarevic, K.B.; Andric, F.; Trifkovic, J.; Tesic, Z.; Milojkovic-Opsenica, D. Characterisation of Serbian unifloral honeys according to their physicochemical parameters. Food Chem. 2012, 132, 2060–2064. [Google Scholar] [CrossRef]
- Hu, B.; Jia, X.; Hu, J.; Xu, D.; Xia, F.; Li, Y. Assessment of heavy metal pollution and health risks in the soil-plant-human system in the Yangtze river delta, China. Int. J. Environ. Res. Public Health 2017, 14, 1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Version 4.2.1 (2022-06-23), Copyright (C) 2022 The R Foundation for Statistical Computing Platform: aarch64-apple-darwin21.6.0 (64-bit). Available online: https://www.r-project.org/ (accessed on 23 June 2022).
- Šereviciene, V.; Zigmontiene, A.; Paliulis, D. Heavy Metals in Honey Collected from Contaminated Locations: A Case of Lithuania. Sustainability 2022, 14, 9196. [Google Scholar] [CrossRef]
- Laaroussi, H.; Bouddine, T.; Bakour, M.; Ousaaid, D.; Lyoussi, B. Physicochemical Properties, Mineral Content, Antioxidant Activities, and Microbiological Quality of Bupleurum spinosum Gouan Honey from the Middle Atlas in Morocco. J. Food Qual. 2020, 2020, 7609454. [Google Scholar] [CrossRef] [Green Version]
- Colucci, G.; De Vito, V.; Varricchio, E.; De Cunzo, F.; Coccia, E.; Paolucci, M.; Di Stasio, M.; Boscaino, F.; Viola, C.; Volpe, M.G. Identification of Traceability Markers in Italian Unifloral Honeys of different Botanical Origin. J. Nutr. Food Sci. 2016, 6, 1000462. [Google Scholar]
- Bartha, S.; Taut, I.; Goji, G.; Andravlad, I.; Dinulică, F. Heavy Metal Content in Polyfloralhoney and Potential Health Risk. A Case Study of Copsa Mică, Romania. Int. J. Environ. Res. Public Health 2020, 17, 1507. [Google Scholar] [CrossRef]
- EU Council. Council directive 2001/11 O/EC of 20 December 2001 relating to honey. Off. J. Eur. Communities 2002, L10, 47–52. [Google Scholar]
- Nanda, V.; Sarkar, B.C.; Sharma, H.K.; Bawa, A.S. Physico-chemical properties and estimation of mineral content in honey produced from different plants in Northern India. J. Food Compos. Anal. 2003, 16, 613–619. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Revised Codex Standard for Honey, Codex STAN 12-1981, Rev.1 (1987), Rev.2. 2001. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/fr/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B12-1981%252FCXS_012e.pdf (accessed on 23 June 2022).
- Habib, H.M.; Al Meqbali, F.T.; Kamal, H.; Souka, U.D.; Ibrahim, W.H. Physicochemical and biochemical properties of honeys from arid regions. Food Chem. 2014, 153, 35–43. [Google Scholar] [CrossRef]
- Mato, I.; Huidobro, J.F.; Simal-Lozano, J.; Sancho, M.T. Significance of nonaromatic organic acids in honey. J. Food Prot. 2003, 66, 2371–2376. [Google Scholar] [CrossRef]
- Malika, N.; Mohamed, F.; Chakib, E.A. Microbiological and physicochemical properties of Moroccan honey. Int. J. Agric. Biol. 2005, 7, 773–776. [Google Scholar]
- Gebru, E.; Berhanu, A.; Hayal, A.; Solomon, A. Physicochemical characterization of honey from Debre-Nazret Kebelle of Tigray Region. World Appl. Sci. J. 2015, 33, 1806–1814. [Google Scholar]
- Adisu, G.; Malede, B. Chemical analysis of honey and major honey production challenges in and around Gondar, Ethiopia. Acad. J. Nutr. 2014, 3, 6–14. [Google Scholar]
- Bogdanov, S. Harmonised methods of the honey commission. Int. Honey Comm. 2002, 5, 1–62. [Google Scholar]
- Boussaid, A.; Chouaibi, M.; Rezig, L.; Hellal, R.; Donsi, F.; Ferrari, G.; Hamdi, S. Physicochemical and bioactive properties of six honey samples from various floral origins from Tunisia. Arab. J. Chem. 2018, 11, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Yadata, D. Detection of the electrical conductivity and acidity of honey from different areas of Tepi. Food Sci. Technol. 2014, 2, 59–63. [Google Scholar] [CrossRef]
- Albu, A.; Radu-Rusu, C.G.; Mircea Pop, I.; Frunza, G.; Nacu, G. Quality Assessment of Raw Honey Issued from Eastern Romania. Agriculture 2021, 11, 247. [Google Scholar] [CrossRef]
- Scripca, L.A.; Norocel, L.; Amariei, S. Comparison of physicochemical, microbiological properties and bioactive compounds content of grassland honey and other floral origin honeys. Molecules 2019, 24, 2932. [Google Scholar] [CrossRef] [Green Version]
- USDA. Extracted Honey Grading Manual. In Standards for Honey Grading; United States Department of Agriculture (USDA): Washington, DC, USA, 1985. [Google Scholar]
- Adebiyi, F.M.; Akpam, T.; Obiajunwa, E.T.; Olaniy, H.B. Chemical physical characterization of Nigeria Honey. Pak. J. Nutr. 2004, 3, 278–281. [Google Scholar]
- Lawal, R.A.; Lawal, A.K.; Adekalu, I.B. Physico-chemical studies on Adulteration of Honey in Nigeria. Pak. J. Biol. Sci. 2009, 12, 1080–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahnawaz, S.; Sheikh, A.; Hussain, M.; Razaq, A.; Khan, S.S. A study on the determination of physicochemical properties of honey from different valleys of Gilgit-Baltistan. Int. J. Agric. Sci. Res. 2013, 2, 49–53. [Google Scholar]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02006R1881-20160401&from=LV (accessed on 23 June 2022).
- Fredes, C.; Montenegro, G. Heavy metal and other trace elements contents in honey bee in Chile. Cienc. E Investig. Agrar. 2006, 33, 50–58. [Google Scholar] [CrossRef]
- Tuzen, M.; Silici, S.; Mendil, D.; Soylak, M. Trace element levels in honeys from different regions of Turkey. Food Chem. 2007, 103, 325–330. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Olowe, M.; Asemoloye, M.D. Phytoremediation Technology and Food Security Impacts of Heavy Metal Contaminated Soils: A Review of Literature. Chemosphere 2022, 288, 132555. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.K.; Louppis, A.P.; Kontakos, S.; Papastephanou, C.; Kontominas, M.G. Characterization and Geographical Discrimination of Greek Pine and Thyme Honeys Based on Their Mineral Content, Using Chemometrics. Eur. Food Res. Technol. 2017, 243, 101–113. [Google Scholar] [CrossRef]
| GTAAS | ||||||||
|---|---|---|---|---|---|---|---|---|
| Analytical Characteristic | Pb | Cd | Cr | Ni | Mn | Co | Cu | Fe |
| Λ (nm) | 217 | 228 | 357 | 232 | 279 | 240 | 324 | 248 |
| Linearity of calibration curves (μg/L) | 1–20 | 1–20 | 1–20 | 1–20 | 2–20 | 1–20 | 1–20 | 1–40 |
| Regression equation | y = 0.000980 + 0.0103x | y = 0.01705 + 0.0633x | y = 0.00126 + 0.02088x | y = 0.00150 + 0.00483x | y = 0.04583 + 0.055529x | y = 0.00053 + 0.0102x | y= 0.02866 + 0.05722x | y = 0.0714 + 0.028x |
| Coefficient of correlation | 0.9984 | 0.9963 | 0.9914 | 0.9980 | 0.9760 | 0.9949 | 0.9979 | 0.9831 |
| LOD (μg/L) | 0.650 | 0.9938 | 1.538 | 0.731 | 2.584 | 1.183 | 0.756 | 4.314 |
| LOQ (μg/L) | 2.481 | 3.698 | 5.538 | 2.776 | 9.001 | 4.346 | 2.664 | 14.37 |
| Sample | Color | Smell and Taste | Consistency |
|---|---|---|---|
| Acacia honey (beekeeper) | Golden yellow | Pleasant, sweet, characteristic of acacia honey | Fluid |
| Polyfloral honey (beekeeper) | Light yellow | Pleasant, sweet, characteristic of polyfloral honey | Finely crystallized |
| Acacia honey (market) | Light yellow | Pleasant, sweet, characteristic of acacia honey | Fluid |
| Polyfloral honey (market) | Golden yellow | Pleasant, sweet, characteristic of polyfloral honey | Fluid |
| Acacia (organic honey) | Light yellow | Pleasant, sweet, characteristic of acacia honey | Fluid |
| Polyfloral (organic honey) | Light yellow | Pleasant, sweet, characteristic of polyfloral honey | Finely crystallized |
| Sample | Water Content wc (%) | pH | Free Acidity (meq/kg) | EC (μS/cm) | SSC (%) |
|---|---|---|---|---|---|
| Acacia honey (beekeeper) | 6.43 | 4.5 | 40.2 | 296 | 77.5 |
| Polyfloral honey (beekeeper) | 9.31 | 4.1 | 35.5 | 655 | 75.1 |
| Acacia honey (market) | 9.68 | 4.0 | 18.9 | 370 | 69.7 |
| Polyfloral honey (market) | 10.36 | 5.0 | 25.6 | 278 | 68.0 |
| Acacia (organic honey) | 8.23 | 4.1 | 20.4 | 387 | 70.5 |
| Polyfloral (organic honey) | 10.18 | 4.0 | 26.7 | 569 | 67.2 |
| Sample | The Organic Nitrogen Content (%) | The Protein Content (%) |
|---|---|---|
| Acacia honey (beekeeper) | 0.0210 | 0.1313 |
| Polyfloral honey (beekeeper) | 0.0350 | 0.2171 |
| Acacia honey (market) | 0.0420 | 0.2627 |
| Polyfloral honey (market) | 0.0070 | 0.0437 |
| Acacia (organic honey) | 0.0140 | 0.0875 |
| Polyfloral (organic honey) | 0.0210 | 0.1313 |
| Sample | Concentration (µg/kg) ± sd | |||||||
|---|---|---|---|---|---|---|---|---|
| Pb | Cd | Cr | Ni | Mn | Co | Cu | Fe | |
| Acacia honey (beekeeper) | 3.85 ± 0.31 | 0.52 ± 0.09 | 3.45 ± 0.09 | 3.66 ± 0.27 | 0.87 ± 0.10 | 0.168 ± 0.01 | <LOD | 1.71 ± 0.14 |
| Polyfloral honey (beekeeper) | 4.36 ± 0.20 | 0.21 ± 0.01 | 3.93 ± 0.11 | 1.42 ± 0.09 | 1.04 ± 0.07 | 0.050 ± 0.01 | 8.76 ± 0.26 | 1.95 ± 0.09 |
| Acacia honey (market) | 5.28 ± 0.51 | 0.019 ± 0.00 | 12.75 ± 0.08 | 1.95 ± 0.11 | <LOD | 0.035 ± 0.02 | 24.67 ± 2.79 | 2.35 ± 0.12 |
| Polyfloral honey (market) | 4.46 ± 0.25 | 0.081 ± 0.04 | 3.66 ± 0.01 | 1.94 ± 0.08 | 1.86 ± 0.09 | 0.131 ± 0.03 | 9.84 ± 0.65 | 4.38 ± 0.34 |
| Acacia (organic honey) | 1.7 ± 0.22 | 0.042 ± 0.01 | 2.94 ± 0.04 | 1.48 ± 0.17 | 1.05 ± 0.28 | 0.052 ± 0.01 | <LOD | 3.88 ± 0.41 |
| Polyfloral (organic honey) | 2.06 ± 0.17 | 0.041 ± 0.01 | 3.43 ± 0.10 | 0.95 ± 0.07 | 1.60 ± 0.12 | 0.854 ± 0.04 | 5.124 ± 0.42 | <LOD |
| Pb | Cd | Cr | Ni | Cu | |
|---|---|---|---|---|---|
| Present study, mg/kg | 0.0017–0.0052 | 0.00001–0.0005 | 0.0029–0.012 | 0.00095–0.0036 | <LD-0.024 |
| Lithuania, mg/kg [26] | 0.008–1.649 | 0.002–0.013 | 0.019–0.051 | 0.012–0.087 | 0.019–0.051 |
| Morocco, mg/kg [27] | - | - | -- | 0.03–0.13 | 0.14–1.53 |
| Italy, mg/kg [28] | 0.86-0.99 | - | 0.0-0.24 | 0.05-0.14 | - |
| Romania, mg/kg [29] | 0.76–3.41 | 0.05–3.81 | - | - | 2.00–33.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrinas, S.; Soceanu, A.; Birghila, S.; Birghila, C.; Matei, N.; Popescu, V.; Constanda, L.M. Chemical Analysis and Quality Assessment of Honey Obtained from Different Sources. Processes 2022, 10, 2554. https://doi.org/10.3390/pr10122554
Dobrinas S, Soceanu A, Birghila S, Birghila C, Matei N, Popescu V, Constanda LM. Chemical Analysis and Quality Assessment of Honey Obtained from Different Sources. Processes. 2022; 10(12):2554. https://doi.org/10.3390/pr10122554
Chicago/Turabian StyleDobrinas, Simona, Alina Soceanu, Semaghiul Birghila, Corina Birghila, Nicoleta Matei, Viorica Popescu, and Luminita Mihaela Constanda. 2022. "Chemical Analysis and Quality Assessment of Honey Obtained from Different Sources" Processes 10, no. 12: 2554. https://doi.org/10.3390/pr10122554
APA StyleDobrinas, S., Soceanu, A., Birghila, S., Birghila, C., Matei, N., Popescu, V., & Constanda, L. M. (2022). Chemical Analysis and Quality Assessment of Honey Obtained from Different Sources. Processes, 10(12), 2554. https://doi.org/10.3390/pr10122554







