Characterization of Malaysian Jatropha Seed Oil and Discovering the Process of Powdered Jatropha Leaves
Abstract
:1. Introduction
2. Materials and Methods
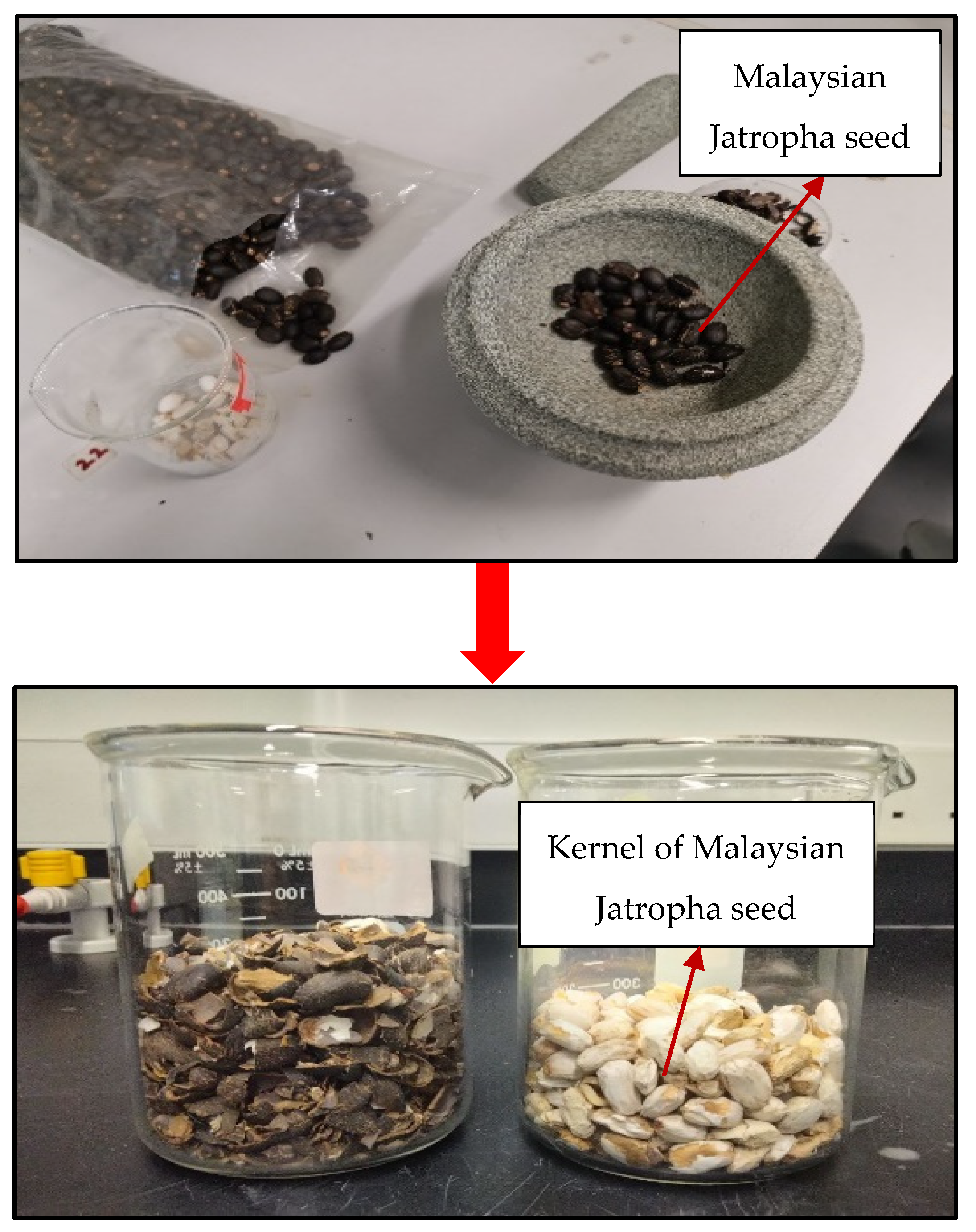
2.1. Extraction of Malaysian Jatropha Seed Oil
2.2. Characterization of Jatropha Seed Oil
2.3. Powdered Jatropha Leaves
3. Results and Discussion
3.1. Malaysian Jatropha Seed Oil
3.2. Gas Chromatography–Mass Spectrometry
3.3. Fourier Transformed Infrared Spectroscopy
3.4. Effectiveness of Malaysian Jatropha Seed Oil and Powdered Jatropha Leaves
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, E.; Lu, Y.; Daraboina, N.; Sarica, C. Thermal methods in flow assurance: A review. J. Nat. Gas Sci. Eng. 2021, 88, 103798. [Google Scholar] [CrossRef]
- Alnaimat, F.; Ziauddin, M. Wax deposition and prediction in petroleum pipelines. J. Pet. Sci. Eng. 2020, 184, 106385. [Google Scholar] [CrossRef]
- El-Dalatony, M.M.; Jeon, B.H.; Salama, E.S.; Eraky, M.; Kim, W.B.; Wang, J.; Ahn, T. Occurrence and characterization of paraffin wax formed in developing wells and pipelines. Energies 2019, 12, 967. [Google Scholar] [CrossRef] [Green Version]
- Chala, G.T.; Sulaiman, S.A.; Japper-Jaafar, A. Flow start-up and transportation of waxy crude oil in pipelines—A review. J. Nonnewton. Fluid Mech. 2018, 251, 69–87. [Google Scholar] [CrossRef]
- Akinyemi, O.P.; Udonne, J.D.; Oyedeko, K.F. Study of effects of blend of plant seed oils on wax deposition tendencies of Nigerian waxy crude oil. J. Pet. Sci. Eng. 2018, 161, 551–558. [Google Scholar] [CrossRef]
- Sivakumar, P.; Sircar, A.; Deka, B.; Silviya Anumegalai, A.; Suresh Moorthi, P.; Yasvanthrajan, N. Flow improvers for assured flow of crude oil in midstream pipeline—A review. J. Pet. Sci. Eng. 2018, 164, 24–30. [Google Scholar] [CrossRef]
- Subramanie, P.A.P.; Padhi, A.; Ridzuan, N.; Adam, F. Experimental study on the effect of wax inhibitor and nanoparticles on rheology of Malaysian crude oil. J. King Saud. Univ. Eng. Sci. 2020, 32, 479–483. [Google Scholar] [CrossRef]
- Alpandi, A.H.; Husin, H.; Sidek, A. A critical review on the development of wax inhibiting agent in facilitating remediation process of contaminated groundwater. Environ. Sci. Pollut. Res. 2021, 29, 51030–51040. [Google Scholar] [CrossRef]
- Ragunathan, T.; Husin, H.; Wood, C.D. Effects of crude palm oil and crude palm kernel oil upon wax inhibition. ACS Omega 2020, 5, 19342–19349. [Google Scholar] [CrossRef]
- Peng, S.; Tang, X.B.; Wang, H.; Wei, Y.; Chen, G. Preparation of poly-Hydrazide and use as pour point depressor for heavy oil. Mater. Sci. Forum 2018, 943, 135–140. [Google Scholar] [CrossRef]
- Alpandi, A.H.; Husin, H.; Jeffri, S.I.; Sidek, A.; Mingyuan, L. Investigation on wax deposition reduction using natural plant-based additives for sustainable energy production from Penara oilfield Malaysia basin. ACS Omega 2022, 7, 30730–30745. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, O.P.; Udonne, J.D.; Efeovbokhan, V.E.; Ayoola, A.A. A study on the use of plant seed oils, triethanolamine and xylene as flow improvers of Nigerian waxy crude oil. J. Appl. Res. Technol. 2016, 14, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Yusuff, A.S. Parametric optimization of solvent extraction of Jatropha curcas seed oil using design of experiment and its quality characterization. South Afr. J. Chem. Eng. 2021, 35, 60–68. [Google Scholar] [CrossRef]
- Singh, R.K.; Padhi, S.K. Characterization of jatropha oil for the preparation of biodiesel. Indian J. Nat. Prod. Resour. 2009, 8, 127–132. [Google Scholar]
- García-Dávila, J.; Ocaranza-Sánchez, E.; Sánchez, C.; Ortega-Sánchez, E.; Tlecuitl-Beristaín, S.; Martínez-Ayala, A.L. Ftir analysis of hydrotreated Jatropha curcas L. seed oil over ni-mo catalyst for biofuel production. Rev. Mex. Ing. Quim. 2017, 16, 337–345. [Google Scholar]
- Soni, H.P.; Kiranbala; Agrawal, K.S.; Nagar, A.; Bharambe, D.P. Designing maleic anhydride-α-olifin copolymeric combs as wax crystal growth nucleators. Fuel Process. Technol. 2010, 91, 997–1004. [Google Scholar] [CrossRef]
- Nayak, B.S.; Patel, K.N. Physicochemical characterization of seed and seed oil of Jatropha curcas L. Collected from bardoli (South Gujarat). Sains Malays. 2010, 39, 951–955. [Google Scholar]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret ftir spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef] [Green Version]
- Tapanes, N.C.; Gomes Aranda, D.A.; de Mesquita Carneiro, J.W.; Ceva Antunes, O.A. Transesterification of Jatropha curcas oil glycerides: Theoretical and experimental studies of biodiesel reaction. Fuel 2008, 87, 2286–2295. [Google Scholar] [CrossRef]
- Abdulla, R.; Chan, E.S.; Ravindra, P. Biodiesel production from Jatropha curcas: A critical review. Crit. Rev. Biotechnol. 2011, 31, 53–64. [Google Scholar] [CrossRef]
- Emil, A.; Yaakob, Z.; Kumar, M.N.S.; Jahim, J.M.; Salimon, J. Comparative evaluation of physicochemical properties of jatropha seed oil from Malaysia, Indonesia and Thailand. JAOCS J. Am. Oil Chem. Soc. 2010, 87, 689–695. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. Encycl. Anal. Chem. 2004, 1–23. [Google Scholar]
- Kiefer, J. Recent advances in the characterization of gaseous and liquid fuels by vibrational spectroscopy. Energies 2015, 8, 3165–3197. [Google Scholar] [CrossRef]












| Reference | Seeds Origin | Oleic Acid Composition (%) | Factor Difference of Oleic Acid Content |
|---|---|---|---|
| This study | Malaysia | 44.91 |
|
| Akinyemi et al. [12] | Nigeria | 43.11 | |
| Emil et al. [21] | Indonesia | 42.40 |
| Wavenumber (cm−1) | Appearance/Intensity | Functional Group | Compound Class | Comments |
|---|---|---|---|---|
| 3007.84 | Weak, broad | O-H stretching | Alcohol | Intramolecular bonded |
| 2955.91 | Medium | C-H stretching | Alkane | - |
| 2923.36 | Medium | C-H stretching | Alkane | - |
| 2854.51 | Medium | C-H stretching | Alkane | - |
| 1746.48 | Strong | C=O stretching | Esters | 6-membered lactone |
| 1462.03 | Medium | C-H bending | Alkane | Methylene group |
| 1377.68 | Medium | O-H bending | Phenol | - |
| 1237.65 | Medium | C-N stretching | Amine | - |
| 1161.52 | Strong | C-O stretching | Tertiary alcohol | - |
| 1118.47 | Strong | C-O stretching | Secondary alcohol | - |
| 722 | Strong | C=C bending | Alkene | Disubstituted (cis) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alpandi, A.H.; Husin, H.; Sidek, A.; Abdurrahman, M. Characterization of Malaysian Jatropha Seed Oil and Discovering the Process of Powdered Jatropha Leaves. Processes 2022, 10, 2577. https://doi.org/10.3390/pr10122577
Alpandi AH, Husin H, Sidek A, Abdurrahman M. Characterization of Malaysian Jatropha Seed Oil and Discovering the Process of Powdered Jatropha Leaves. Processes. 2022; 10(12):2577. https://doi.org/10.3390/pr10122577
Chicago/Turabian StyleAlpandi, Amni Haslinda, Hazlina Husin, Akhmal Sidek, and Muslim Abdurrahman. 2022. "Characterization of Malaysian Jatropha Seed Oil and Discovering the Process of Powdered Jatropha Leaves" Processes 10, no. 12: 2577. https://doi.org/10.3390/pr10122577






