Supercritical CO2 Impregnation of Clove Extract in Polycarbonate: Effects of Operational Conditions on the Loading and Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
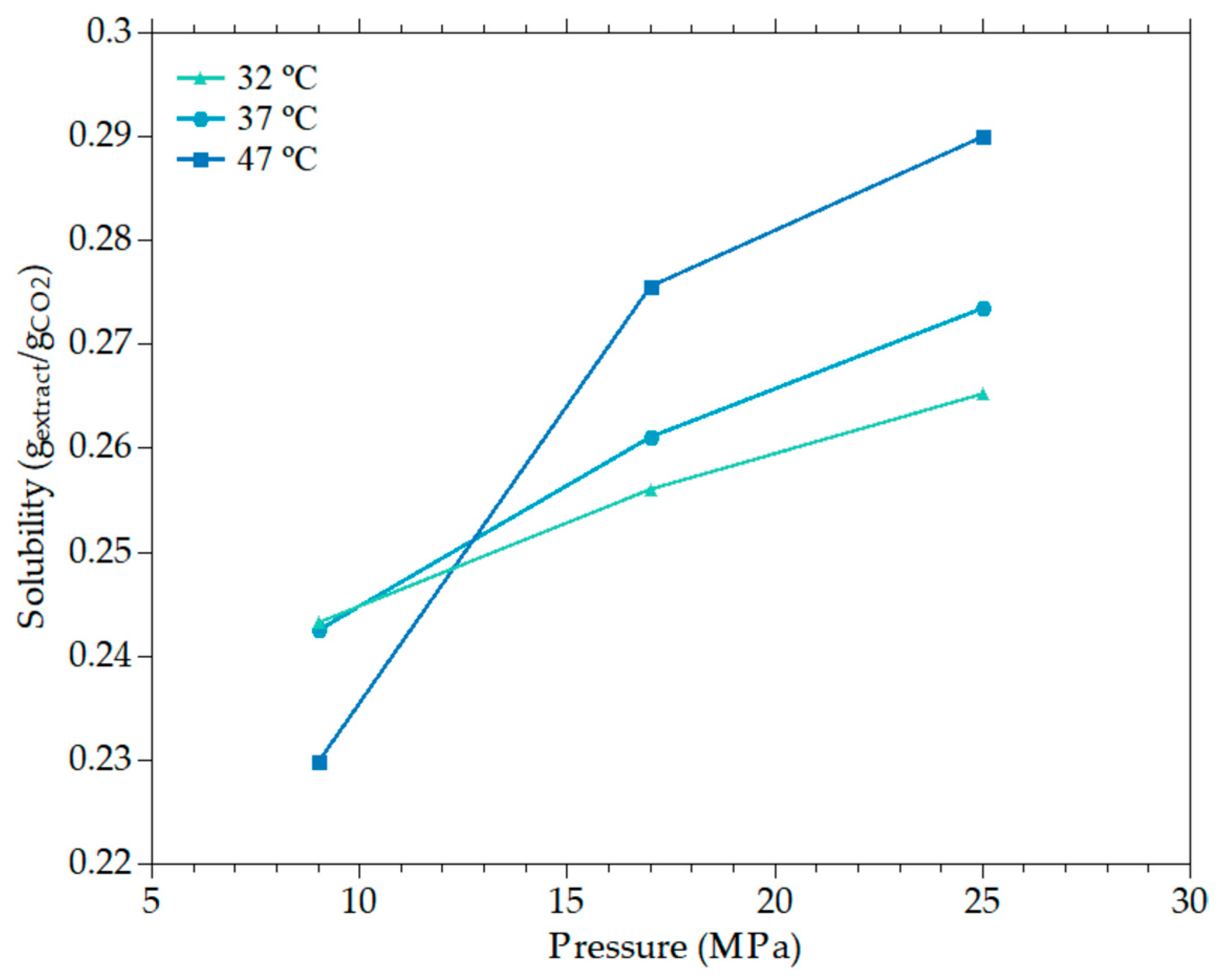
2.2. Supercritical CO2 Extraction
2.3. Characterization of PC Chemical Structure by 1H NMR
2.4. Preparation of PC Films
2.5. scCO2 Impregnation Method
2.6. Design of Experiment
2.7. Clove Extract Loading
2.8. Composition of the Impregnated Extract
2.9. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy
2.10. Thermal Analysis
2.11. Scanning Electron Microscopy
3. Results and Discussion
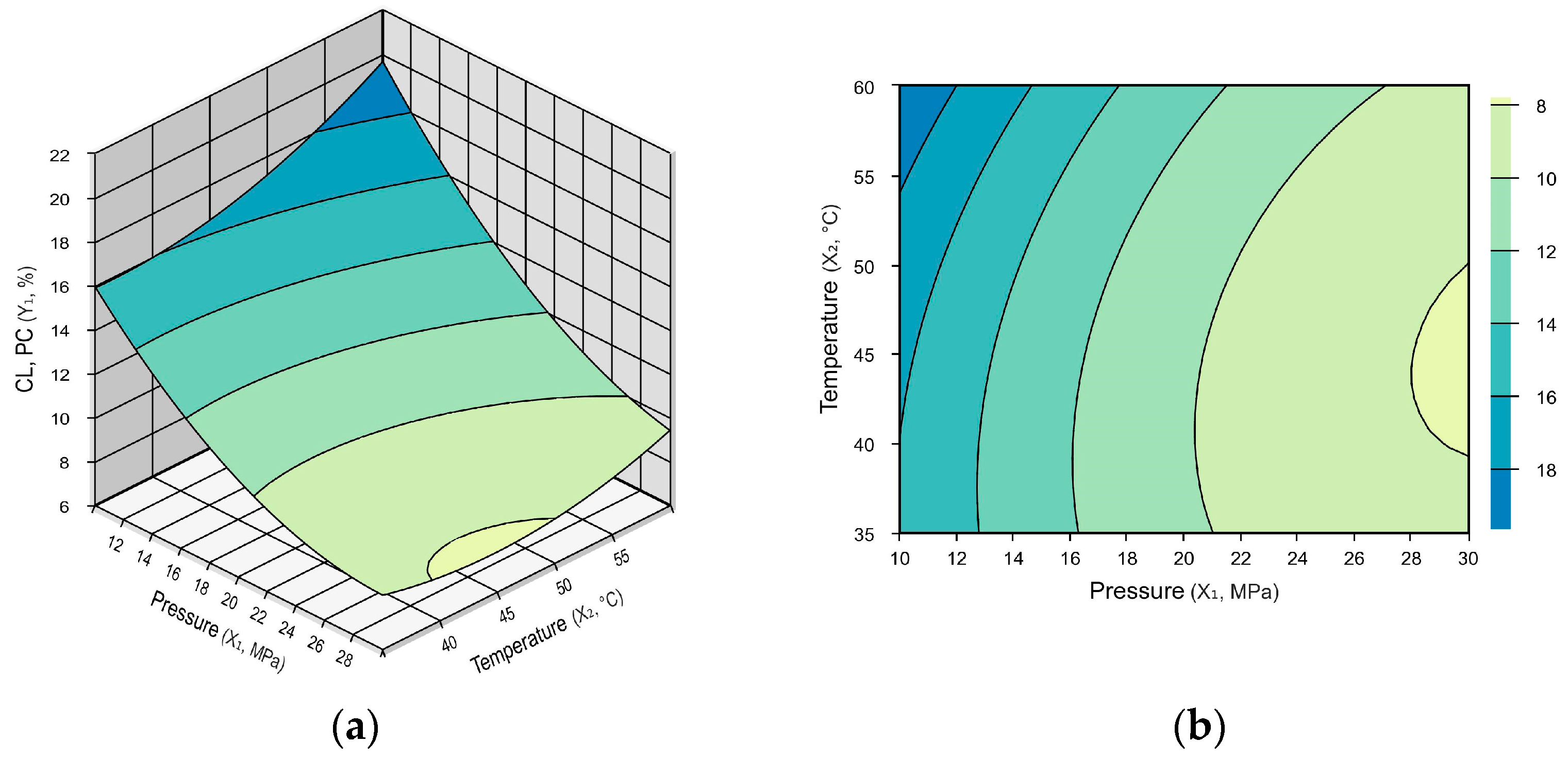
3.1. Influences of the Impregnation Conditions on the Clove Extract Loading
3.1.1. Effects of Pressure
3.1.2. Effects of Temperature
3.1.3. Optimum Conditions for the Clove Extract Loading
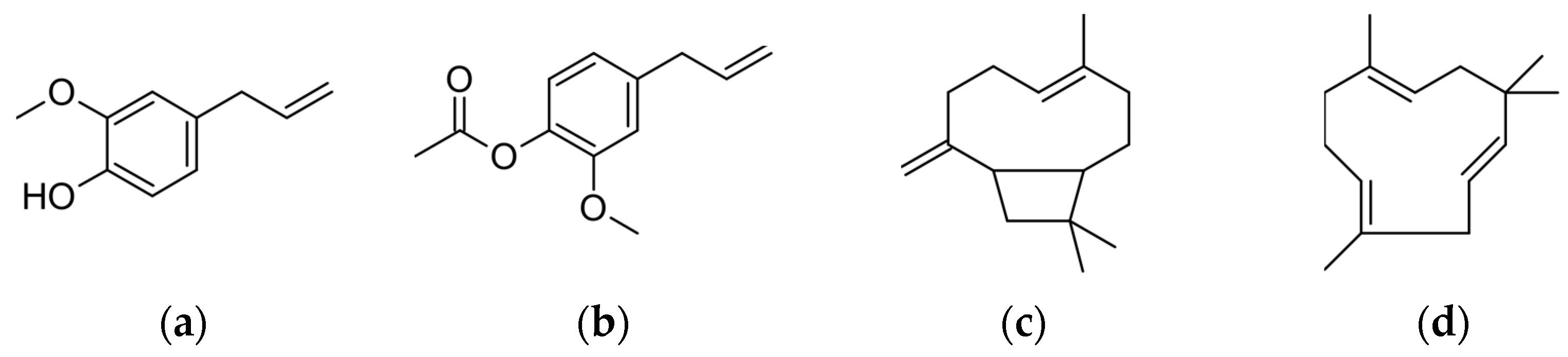
3.2. Influences of the Impregnation Conditions on the Composition of the Loaded Extract
| Polymer/Compound | δd (Mpa0.5) | δp (MPa0.5) | δh (MPa0.5) | Solubility Parameter (δt) (MPa0.5) | |δt PC − δt compound| (MPa0.5) |
|---|---|---|---|---|---|
| PC | 17.9 | 3.1 | 6.9 | 19.4 | - |
| Eugenol | 18.5 | 4.1 | 12.2 | 22.5 | 3.1 |
| Eugenyl Acetate | 17.5 | 3.3 | 7.2 | 19.3 | 0.1 |
| β-caryophyllene | 15.2 | 0.0 | 0.0 | 15.2 | 4.2 |
| α-humulene | 15.7 | 0.0 | 0.0 | 15.7 | 3.7 |
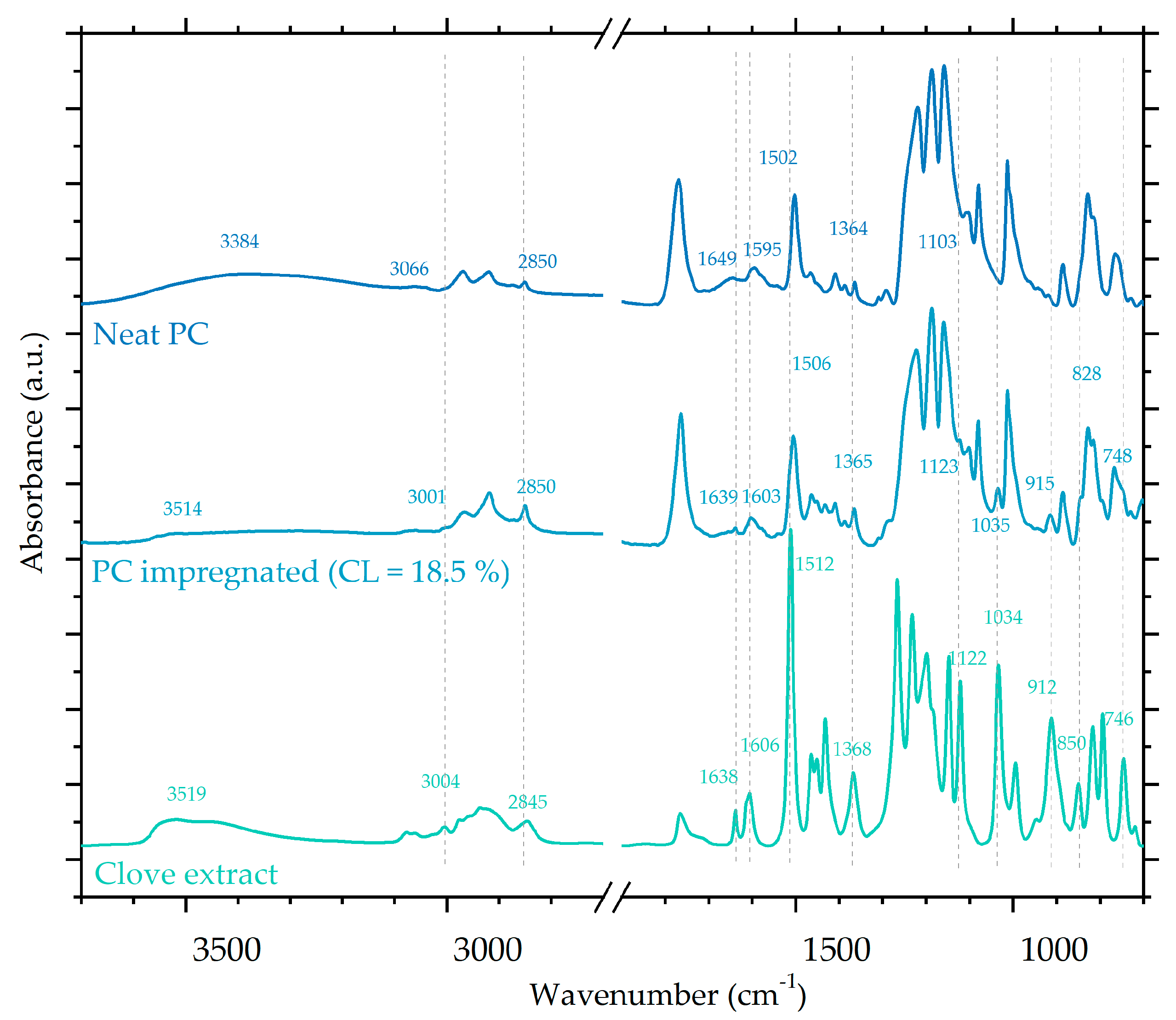
3.3. ATR-FTIR
3.4. Film Morphology
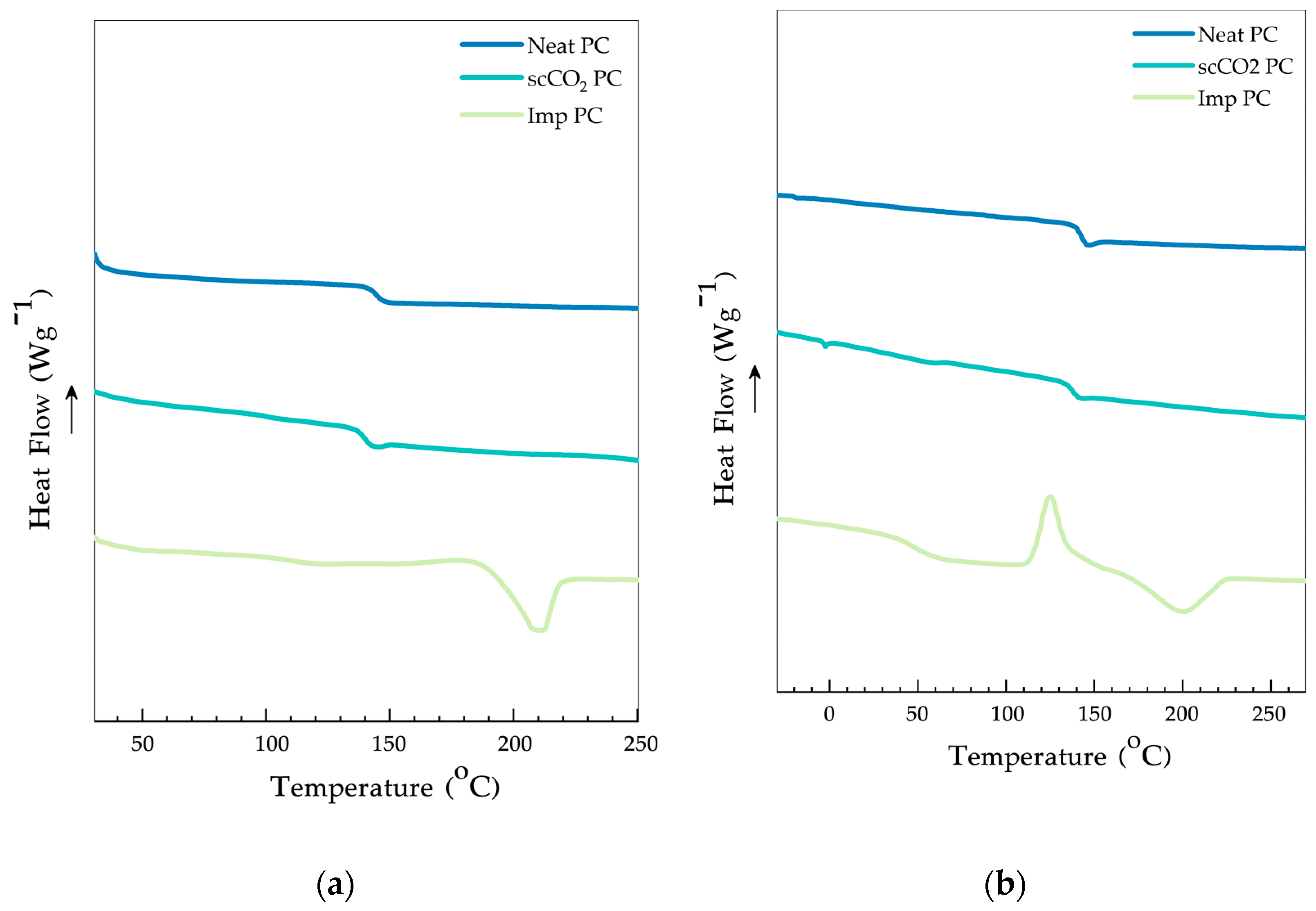
3.5. DSC Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukurumbira, A.R.; Shellie, R.A.; Keast, R.; Palombo, E.A.; Jadhav, S.R. Encapsulation of Essential Oils and Their Application in Antimicrobial Active Packaging. Food Control 2022, 136, 108883. [Google Scholar] [CrossRef]
- Maisanaba, S.; Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Pichardo, S.; Puerto, M.; Prieto, A.I.; Jos, A.; Cameán, A.M. New Advances in Active Packaging Incorporated with Essential Oils or Their Main Components for Food Preservation. Food Rev. Int. 2017, 33, 447–515. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential Oils as Additives in Active Food Packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Essential, D. Oils, Oleoresins (Solventfree), and Natural Extractives (Including Distillates), 2014. In Code of Federal Regulations, Title 21; 2014. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=182.20 (accessed on 7 December 2022).
- Cejudo Bastante, C.; Cran, M.J.; Casas Cardoso, L.; Mantell Serrano, C.; Martínez de la Ossa, E.J.; Bigger, S.W. Effect of Supercritical CO2 and Olive Leaf Extract on the Structural, Thermal and Mechanical Properties of an Impregnated Food Packaging Film. J. Supercrit. Fluids 2019, 145, 181–191. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Supercritical Impregnation of Food Packaging Films to Provide Antioxidant Properties. J. Supercrit. Fluids 2017, 128, 200–207. [Google Scholar] [CrossRef]
- Milovanovic, S.; Hollermann, G.; Errenst, C.; Pajnik, J.; Frerich, S.; Kroll, S.; Rezwan, K.; Ivanovic, J. Supercritical CO2 Impregnation of PLA/PCL Films with Natural Substances for Bacterial Growth Control in Food Packaging. Food Res. Int. 2018, 107, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.M.A.; Rey-Rico, A.; Oliveira, R.A.; Marceneiro, S.; Alvarez-Lorenzo, C.; Concheiro, A.; Júnior, R.N.C.; Braga, M.E.M.; de Sousa, H.C. Wound Dressings Loaded with an Anti-Inflammatory Jucá (Libidibia Ferrea) Extract Using Supercritical Carbon Dioxide Technology. J. Supercrit. Fluids 2013, 74, 34–45. [Google Scholar] [CrossRef] [Green Version]
- da Silva, C.V.; Pereira, V.J.; Rosa, P.T.V.; Cabral-Albuquerque, E.C.M.; Vieira De Melo, S.A.B.; Costa, G.M.N.; Dias, A.M.A.; de Sousa, H.C.; Braga, M.E.M. Effect of ScCO2 Sorption Capacity on the Total Amount of Borage Oil Loaded by ScCO2 Impregnation/Deposition into a Polyurethane-Based Wound Dressing. J. Supercrit. Fluids 2016, 115, 1–9. [Google Scholar] [CrossRef]
- Pajnik, J.; Stamenić, M.; Radetić, M.; Tomanović, S.; Sukara, R.; Mihaljica, D.; Zizovic, I. Impregnation of Cotton Fabric with Pyrethrum Extract in Supercritical Carbon Dioxide. J. Supercrit. Fluids 2017, 128, 66–72. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, J.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; de la Ossa, E.J.M. Impregnation of Mango Leaf Extract into a Polyester Textile Using Supercritical Carbon Dioxide. J. Supercrit. Fluids 2017, 128, 208–217. [Google Scholar] [CrossRef]
- Banerjee, S.; Bagchi, B.; Pal, K.; Bhandary, S.; Kool, A.; Hoque, N.A.; Biswas, P.; Thakur, P.; Das, K.; Karmakar, P.; et al. Essential Oil Impregnated Luminescent Hydroxyapatite: Antibacterial and Cytotoxicity Studies. Mater. Sci. Eng. C 2020, 116, 111190. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Duan, S.; Zhou, X.; Sun, L.; Qin, C.; Li, M.; Ge, F. Long-Term Antibacterial Film Nanocomposite Incorporated with Patchouli Essential Oil Prepared by Supercritical CO2 Cyclic Impregnation for Wound Dressing. Molecules 2021, 26, 5005. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Baek, K.-H. Biological Efficacy and Application of Essential Oils in Foods—A Review. J. Essent. Oil Bear. Plants 2016, 19, 1–19. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antimicrobial Mechanism of Clove Oil on Listeria Monocytogenes. Food Control 2018, 94, 140–146. [Google Scholar] [CrossRef]
- Fu, Y.; Zu, Y.; Chen, L.; Shi, X.; Wang, Z.; Sun, S.; Efferth, T. Antimicrobial Activity of Clove and Rosemary Essential Oils Alone and in Combination. Phytother. Res. 2007, 21, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Briozzo, J.; Núncez, L.; Chirife, J.; Herszage, L.; D’aquino, M. Antimicrobial Activity of Clove Oil Dispersed in a Concentrated Sugar Solution. J. Appl. Bacteriol. 1989, 66, 69–75. [Google Scholar] [CrossRef]
- Ahmad, N.; Alam, M.K.; Shehbaz, A.; Khan, A.; Mannan, A.; Hakim, S.R.; Bisht, D.; Owais, M. Antimicrobial Activity of Clove Oil and Its Potential in the Treatment of Vaginal Candidiasis. J. Drug Target 2005, 13, 555–561. [Google Scholar] [CrossRef]
- Medeiros, G.R.; Ferreira, S.R.S.; Carciofi, B.A.M. High Pressure Carbon Dioxide for Impregnation of Clove Essential Oil in LLDPE Films. Innov. Food Sci. Emerg. Technol. 2017, 41, 206–215. [Google Scholar] [CrossRef]
- Medeiros, G.R.; Guimarães, C.; Ferreira, S.R.S.; Carciofi, B.A.M. Thermomechanical and Transport Properties of LLDPE Films Impregnated with Clove Essential Oil by High-Pressure CO2. J. Supercrit. Fluids 2018, 139, 8–18. [Google Scholar] [CrossRef]
- Goñi, M.L.; Gañán, N.A.; Strumia, M.C.; Martini, R.E. Eugenol-Loaded LLDPE Films with Antioxidant Activity by Supercritical Carbon Dioxide Impregnation. J. Supercrit. Fluids 2016, 111, 28–35. [Google Scholar] [CrossRef]
- Mosquera, J.E.; Goñi, M.L.; Martini, R.E.; Gañán, N.A. Supercritical Carbon Dioxide Assisted Impregnation of Eugenol into Polyamide Fibers for Application as a Dental Floss. J. CO2 Util. 2019, 32, 259–268. [Google Scholar] [CrossRef]
- Mosquera, J.E.; Goñi, M.L.; Martini, R.E.; Gañán, N.A. Mass Transfer Kinetics of CO2 and Eugenol in the Supercritical Impregnation of Polyamide Fibers: Experimental Data and Modeling. J. Supercrit. Fluids 2020, 166, 105030. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.-C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of Citrus Pectin Films Integrated with Clove Bud Essential Oil: Physical, Thermal, Barrier, Antioxidant and Antibacterial Properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Junka, A.; Żywicka, A.; Chodaczek, G.; Dziadas, M.; Czajkowska, J.; Duda-Madej, A.; Bartoszewicz, M.; Mikołajewicz, K.; Krasowski, G.; Szymczyk, P.; et al. Potential of Biocellulose Carrier Impregnated with Essential Oils to Fight Against Biofilms Formed on Hydroxyapatite. Sci. Rep. 2019, 9, 1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radovic, M.; Adamovic, T.; Pavlovic, J.; Rusmirovic, J.; Tadic, V.; Brankovic, Z.; Ivanovic, J. Supercritical Co2 Impregnation of Gelatin-Chitosan Films with Clove Essential Oil and Characterization Thereof. Chem. Ind. Chem. Eng. Q. 2019, 25, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhang, F.; Liu, Z.; Guo, X.; Wu, Q.; Qiao, L. Controlled Release System by Active Gelatin Film Incorporated with β-Cyclodextrin-Thymol Inclusion Complexes. Food Bioproc. Tech. 2018, 11, 1695–1702. [Google Scholar] [CrossRef]
- Varghese, S.A.; Siengchin, S.; Parameswaranpillai, J. Essential Oils as Antimicrobial Agents in Biopolymer-Based Food Packaging—A Comprehensive Review. Food Biosci. 2020, 38, 100785. [Google Scholar] [CrossRef]
- Ocak, B. Properties and Characterization of Thyme Essential Oil Incorporated Collagen Hydrolysate Films Extracted from Hide Fleshing Wastes for Active Packaging. Environ. Sci. Pollut. Res. 2020, 27, 29019–29030. [Google Scholar] [CrossRef]
- Felix de Andrade, M.; Diego de Lima Silva, I.; Alves da Silva, G.; David Cavalcante, P.V.; Thayse da Silva, F.; Bastos de Almeida, Y.M.; Vinhas, G.M.; Hecker de Carvalho, L. A Study of Poly (Butylene Adipate-Co-Terephthalate)/Orange Essential Oil Films for Application in Active Antimicrobial Packaging. LWT 2020, 125, 109148. [Google Scholar] [CrossRef]
- Perale, G.; Casalini, T.; Barri, V.; Müller, M.; Maccagnan, S.; Masi, M. Lidocaine Release from Polycaprolactone Threads. J. Appl. Polym. Sci. 2010, 117, 3610–3614. [Google Scholar] [CrossRef]
- Tian, Y.; Jacobs, E.; Jones, D.S.; McCoy, C.P.; Wu, H.; Andrews, G.P. The Design and Development of High Drug Loading Amorphous Solid Dispersion for Hot-Melt Extrusion Platform. Int. J. Pharm. 2020, 586, 119545. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Shah, M.A.; Dar, B.N.; Wani, A.A.; Ganai, S.A.; Nishad, J. Supercritical Impregnation of Active Components into Polymers for Food Packaging Applications. Food Bioproc. Tech. 2017, 10, 1749–1754. [Google Scholar] [CrossRef]
- Champeau, M.; Thomassin, J.M.; Tassaing, T.; Jérôme, C. Drug Loading of Polymer Implants by Supercritical CO2 Assisted Impregnation: A Review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Kazarian, S.G.; Brantley, N.H.; West, B.L.; Vincent, M.F.; Eckert, C.A. In Situ Spectroscopy of Polymers Subjected to Supercritical CO2: Plasticization and Dye Impregnation. Appl. Spectrosc. 1997, 51. [Google Scholar] [CrossRef]
- Belizón, M.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Martínez De La Ossa-Fernández, E.J. Supercritical Impregnation of Antioxidant Mango Polyphenols into a Multilayer PET/PP Food-Grade Film. J. CO2 Util. 2018, 25, 56–67. [Google Scholar] [CrossRef]
- Ameri, A.; Sodeifian, G.; Sajadian, S.A. Lansoprazole Loading of Polymers by Supercritical Carbon Dioxide Impregnation: Impacts of Process Parameters. J. Supercrit. Fluids 2020, 164, 104892. [Google Scholar] [CrossRef]
- de Souza, A.C.; Dias, A.M.A.; Sousa, H.C.; Tadini, C.C. Impregnation of Cinnamaldehyde into Cassava Starch Biocomposite Films Using Supercritical Fluid Technology for the Development of Food Active Packaging. Carbohydr. Polym. 2014, 102, 830–837. [Google Scholar] [CrossRef] [Green Version]
- Delpech, M.C.; Coutinho, F.M.B.; Habibe, M.E.S. Bisphenol A-Based Polycarbonates: Characterization of Commercial Samples. Polym. Test 2002, 21, 155–161. [Google Scholar] [CrossRef]
- Jadhav, V.D.; Patil, A.J.; Kandasubramanian, B. Polycarbonate Nanocomposites for High Impact Applications. In Handbook of Consumer Nanoproducts; Springer Nature Singapore: Singapore, 2022; pp. 257–281. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food Packaging—Roles, Materials, and Environmental Issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef]
- Domiński, A.; Konieczny, T.; Duale, K.; Krawczyk, M.; Pastuch-Gawołek, G.; Kurcok, P. Stimuli-Responsive Aliphatic Polycarbonate Nanocarriers for Tumor-Targeted Drug Delivery. Polymers 2020, 12, 2890. [Google Scholar] [CrossRef]
- Ganivada, M.N.; Kumar, P.; Kanjilal, P.; Dinda, H.; das Sarma, J.; Shunmugam, R. Polycarbonate-Based Biodegradable Copolymers for Stimuli Responsive Targeted Drug Delivery. Polym. Chem. 2016, 7, 4237–4245. [Google Scholar] [CrossRef]
- Mölders, N.; Renner, M.; Errenst, C.; Weidner, E. Incorporation of Antibacterial Active Additives inside Polycarbonate Surfaces by Using Compressed Carbon Dioxide as Transport Aid. J. Supercrit. Fluids 2018, 132, 83–90. [Google Scholar] [CrossRef]
- Varga, D.; Alkin, S.; Gluschitz, P.; Péter-Szabó, B.; Székely, E.; Gamse, T. Supercritical Fluid Dyeing of Polycarbonate in Carbon Dioxide. J. Supercrit. Fluids 2016, 116, 111–116. [Google Scholar] [CrossRef]
- de Almeida Fin de Lima, F.; dos Santos, O.A.A.; Pinheiro, N.; de Lima, F.D.A.F.; Santos, O.A.A.; Pinheiro, N. Impregnação de Filmes Do Compósito de Policarbonato (PC) e Sílica-Gel (Si) Com Corante Fotocrômico Em Fluido Supercrítico. Acta Scientiarum. Technol. 2010, 32, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.; Wang, J.; Li, G.; He, J. Effect of Supercritical Carbon Dioxide on the Crystallization and Melting Behavior of Linear Bisphenol A Polycarbonate. J. Polym. Sci. B Polym. Phys. 2004, 42, 280–285. [Google Scholar] [CrossRef]
- Monnereau, L.; Urbanczyk, L.; Thomassin, J.-M.M.; Alexandre, M.; Jérôme, C.; Huynen, I.; Bailly, C.; Detrembleur, C. Supercritical CO2 and Polycarbonate Based Nanocomposites: A Critical Issue for Foaming. Polymer 2014, 55, 2422–2431. [Google Scholar] [CrossRef]
- Adorjan, B.; Buchbauer, G. Biological Properties of Essential Oils: An Updated Review. Flavour Fragr. J. 2010, 25, 407–426. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential Oils: From Extraction to Encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Buchbauer, G. (Eds.) Handbook of Essential Oils; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential Oils, A New Horizon in Combating Bacterial Antibiotic Resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández Ponce, M.T.; Mantell Serrano, C.; Martínez de la Ossa-Fernández, E.J. Characterization of Olive Leaf Extract Polyphenols Loaded by Supercritical Solvent Impregnation into PET/PP Food Packaging Films. J. Supercrit. Fluids 2018, 140, 196–206. [Google Scholar] [CrossRef]
- Prado, J.M.; Prado, G.H.C.; Meireles, M.A.A. Scale-up Study of Supercritical Fluid Extraction Process for Clove and Sugarcane Residue. J. Supercrit. Fluids 2011, 56, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Prado, J.M.; Meireles, M.A.A. Comparative Study on Efficiency of Extraction and Separation Steps of Supercritical Fluid Extraction. In Proceedings of the VIII Iberoamerican Conference on Phase Equilibria and Fluid Properties For Process Design, Praia da Rocha, Portugal, 17–21 October 2009. [Google Scholar]
- Khan, F.; Khanna, S.; Hor, A.-M.; Sundararajan, P.R. The Role of Molecular Volume and the Shape of the Hole Transport Molecule in the Morphology of Model Charge Transport Composites. Can J. Chem. 2010, 88, 247–259. [Google Scholar] [CrossRef]
- Mercier, J.P.; Legras, R. Correlation between the Enthalpy of Fusion and the Specific Volume of Crystallized Polycarbonate of Bisphenol A. J. Polym. Sci. Part B Polym. Lett. 1970, 8, 645–650. [Google Scholar] [CrossRef]
- Tang, M.; Huang, Y.C.; Chen, Y.P. Sorption and Diffusion of Supercritical Carbon Dioxide into Polysulfone. J. Appl. Polym. Sci. 2004, 94, 474–482. [Google Scholar] [CrossRef]
- Zhao, J.J.; Zhao, Y.P.; Yang, B. Investigation of Sorption and Diffusion of Supercritical Carbon Dioxide in Polycarbonate. J. Appl. Polym. Sci. 2008, 109, 1661–1666. [Google Scholar] [CrossRef]
- Tang, M.; Du, T.B.; Chen, Y.P. Sorption and Diffusion of Supercritical Carbon Dioxide in Polycarbonate. J. Supercrit. Fluids 2004, 28, 207–218. [Google Scholar] [CrossRef]
- Sugiura, K.; Ogawa, S.; Tabata, I.; Hori, T. Impregnation of Tranilast to the Poly(Lactic Acid) Fiber with Supercritical Carbon Dioxide and the Release Behavior of Tranilast. J. Fiber Sci. Technol. 2005, 61, 159–165. [Google Scholar] [CrossRef]
- Wei, M.C.; Lin, P.H.; Hong, S.J.; Chen, J.M.; Yang, Y.C. Development of a Green Alternative Procedure for the Simultaneous Separation and Quantification of Clove Oil and Its Major Bioactive Constituents. ACS Sustain. Chem. Eng. 2016, 4, 6491–6499. [Google Scholar] [CrossRef]
- Rodrigues, V.M.; Sousa, E.M.B.D.; Monteiro, A.R.; Chiavone-Filho, O.; Marques, M.O.M.; Angela, M.; Meireles, A. Determination of the Solubility of Extracts from Vegetable Raw Material in Pressurized CO2: A Pseudo-Ternary Mixture Formed by Cellulosic Structure+solute+solvent. J. Supercrit. Fluids 2002, 22, 21–36. [Google Scholar] [CrossRef]
- Guan, W.Q.; Li, S.F.; Hou, C.X.; Yan, R.; Ma, J. Determination and Correlation of Solubilities of Clove Oil Components in Supercritical Carbon Dioxide. J. Chem. Ind. Eng. (China) 2007, 58, 1077–1081. [Google Scholar]
- Varona, S.; Rodríguez-Rojo, S.; Martín, Á.; Cocero, M.J.; Duarte, C.M.M. Supercritical Impregnation of Lavandin (Lavandula Hybrida) Essential Oil in Modified Starch. J. Supercrit. Fluids 2011, 58, 313–319. [Google Scholar] [CrossRef]
- Sun, Y.; Matsumoto, M.; Kitashima, K.; Haruki, M.; Kihara, S.I.; Takishima, S. Solubility and Diffusion Coefficient of Supercritical-CO2 in Polycarbonate and CO2 Induced Crystallization of Polycarbonate. J. Supercrit. Fluids 2014, 95, 35–43. [Google Scholar] [CrossRef]
- Beckman, E.; Porter, R.S. Crystallization of Bisphenol a Polycarbonate Induced by Supercritical Carbon Dioxide. J. Polym. Sci. 1987, 25, 1511–1517. [Google Scholar] [CrossRef]
- Li, G.; Park, C.B. A New Crystallization Kinetics Study of Polycarbonate under High-Pressure Carbon Dioxide and Various Crystallinization Temperatures by Using Magnetic Suspension Balance. J. Appl. Polym. Sci. 2010, 118, 2898–2903. [Google Scholar] [CrossRef]
- von Schnitzler, J.; Eggers, R. Mass Transfer in Polymers in a Supercritical CO2-Atmosphere. J. Supercrit. Fluids 1999, 16, 81–92. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Rodríguez, M.; Martínez De La Ossa, E. Extraction of Antioxidant Compounds from Different Varieties of Mangifera Indica Leaves Using Green Technologies. J. Supercrit. Fluids 2012, 72, 168–175. [Google Scholar] [CrossRef]
- Ushiki, I.; Hayashi, S.; Kihara, S.; Takishima, S. Solubilities and Diffusion Coefficients of Carbon Dioxide and Nitrogen in Poly(Methyl Methacrylate) at High Temperatures and Pressures. J. Supercrit. Fluids 2019, 152, 104565. [Google Scholar] [CrossRef]
- Bentayeb, K.; Rubio, C.; Batlle, R.; Nerín, C. Direct Determination of Carnosic Acid in a New Active Packaging Based on Natural Extract of Rosemary. Anal. Bioanal. Chem. 2007, 389, 1989–1996. [Google Scholar] [CrossRef]
- Cejudo-Bastante, C.; Arjona-Mudarra, P.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J.; Pereyra, C. Application of a Natural Antioxidant from Grape Pomace Extract in the Development of Bioactive Jute Fibers for Food Packaging. Antioxidants 2021, 10, 216. [Google Scholar] [CrossRef]
- Champeau, M.; Thomassin, J.M.; Tassaing, T.; Jerome, C. Drug Loading of Sutures by Supercritical CO2 Impregnation: Effect of Polymer/Drug Interactions and Thermal Transitions. Macromol. Mater. Eng. 2015, 300, 596–610. [Google Scholar] [CrossRef]
- Ma, S.L.; Lu, Z.W.; Wu, Y.T.; Zhang, Z.B. Partitioning of Drug Model Compounds between Poly(Lactic Acid)s and Supercritical CO2 Using Quartz Crystal Microbalance as an in Situ Detector. J. Supercrit. Fluids 2010, 54, 129–136. [Google Scholar] [CrossRef]
- Coutinho, I.; Champeau, M. Synergistic Effects in the Simultaneous Supercritical CO2 Impregnation of Two Compounds into Poly(L- Lactic Acid) and Polyethylene. J. Supercrit. Fluids 2020, 166, 105019. [Google Scholar] [CrossRef]
- Pérez-Conesa, D.; Cao, J.; Chen, L.; McLandsborough, L.; Weiss, J. Inactivation of Listeria Monocytogenes and Escherichia coli O157:H7 Biofilms by Micelle-Encapsulated Eugenol and Carvacrol. J. Food Prot. 2011, 74, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Morán, A.; Gutiérrez, S.; Martínez-Blanco, H.; Ferrero, M.A.; Monteagudo-Mera, A.; Rodríguez-Aparicio, L.B. Non-Toxic Plant Metabolites Regulate Staphylococcus Viability and Biofilm Formation: A Natural Therapeutic Strategy Useful in the Treatment and Prevention of Skin Infections. Biofouling 2014, 30, 1175–1182. [Google Scholar] [CrossRef]
- van Krevelen, D.W.; te Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction Drom Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Nagaraju, P.G.; Sengupta, P.; Chicgovinda, P.P.; Rao, P.J. Nanoencapsulation of Clove Oil and Study of Physicochemical Properties, Cytotoxic, Hemolytic, and Antioxidant Activities. J. Food Process Eng. 2021, 44, e13645. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; J.Wiley and Sons: New York, NY, USA, 2004. [Google Scholar]
- Musto, P.; Wu, L.; Karasz, F.E.; MacKnight, W.J. Fourier Transform Infrared Spectroscopy Investigations of Polybenzimidazole/Poly(Bisphenol-A Carbonate) Blends. Polymer 1991, 32, 3–11. [Google Scholar] [CrossRef]
- Ishaq Lerrick, R. Direct Oxidation of Eugenol Using a Permanganate. Molekul 2009, 4, 48. [Google Scholar] [CrossRef]
- Coulier, L.; Kaal, E.R.; Hankemeier, T. Towards Early Detection of the Hydrolytic Degradation of Poly(Bisphenol A)Carbonate by Hyphenated Liquid Chromatography and Comprehensive Two-Dimensional Liquid Chromatography. Polym. Degrad Stab. 2006, 91, 271–279. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, G.; Yang, Q.; Shi, X.; Shi, A. Fabrication of Microcellular Polycarbonate Foams with Unimodal or Bimodal Cell-Size Distributions Using Supercritical Carbon Dioxide as a Blowing Agent. J. Cell. Plast. 2014, 50, 55–79. [Google Scholar] [CrossRef]
- Jiang, S.; Guan, R.; Chen, H.; Zhao, J.; Zha, S.; Ke, Z. Effect of TBC Plasticization on Thin Polycarbonate Sheet. J. Wuhan Univ. Technol. -Mater. Sci. Ed. 2014, 29, 553–560. [Google Scholar] [CrossRef]
- Shieh, Y.-T.; Liu, K.-H. The Effect of Carbonyl Group on Sorption of CO2 in Glassy Polymers. J. Suprecrit. Fluids 2003, 25, 261–268. [Google Scholar] [CrossRef]
- Wei, M.C.; Xiao, J.; Yang, Y.C. Extraction of α-Humulene-Enriched Oil from Clove Using Ultrasound-Assisted Supercritical Carbon Dioxide Extraction and Studies of Its Fictitious Solubility. Food Chem. 2016, 210, 172–181. [Google Scholar] [CrossRef]


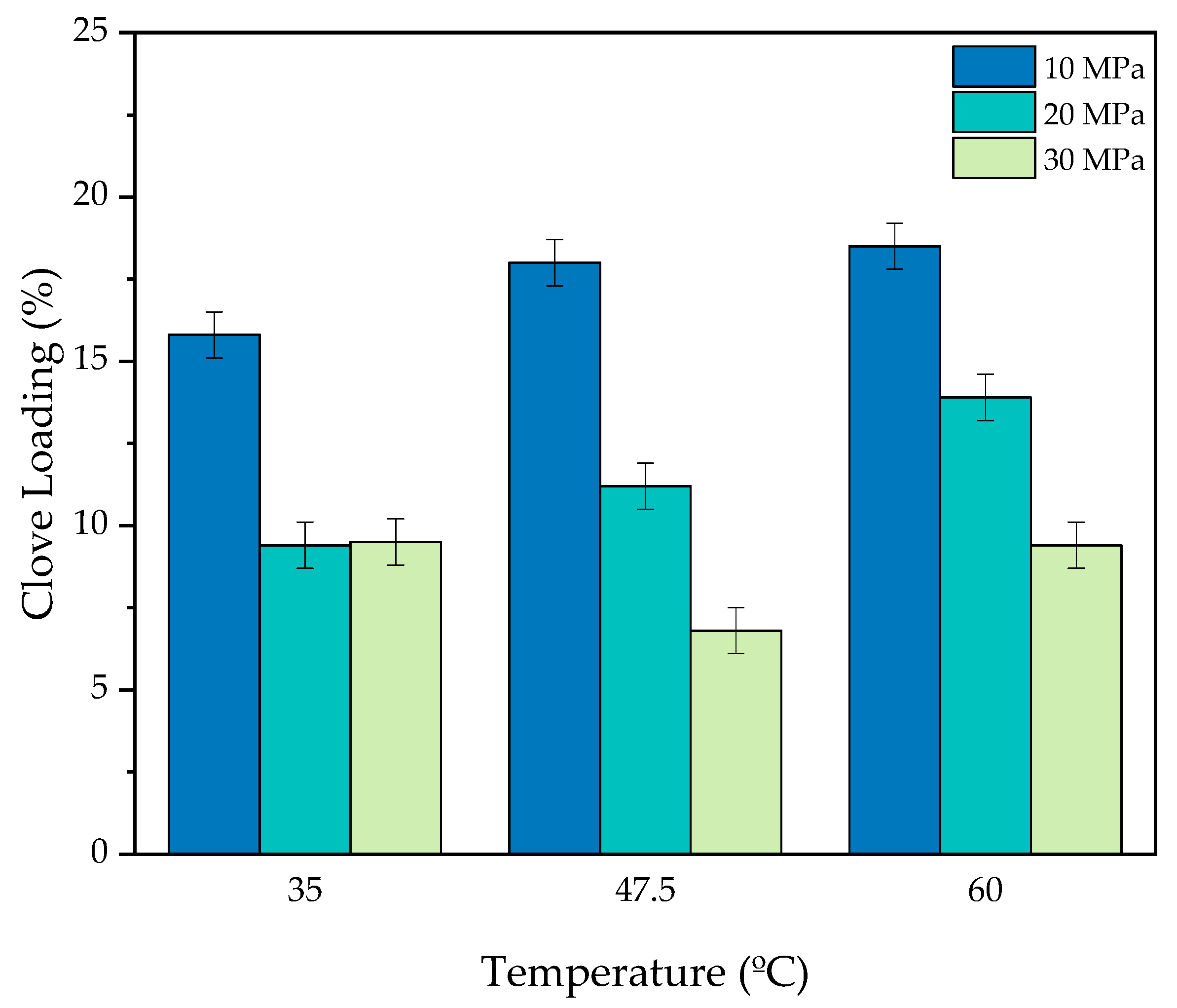


| Assay | Pressure (MPa) 1 | Temperature (°C) 1 | scCO2 Density (kg/m3) | CL (%) |
|---|---|---|---|---|
| 1 | 10 (−1) | 35 (−1) | 712.8 | 15.8 |
| 2 | 30 (+1) | 35 (−1) | 929.1 | 9.5 |
| 3 | 10 (−1) | 60 (+1) | 289.9 | 18.5 |
| 4 | 30 (+1) | 60 (+1) | 829.7 | 9.4 |
| 5 | 10 (−1) | 47.5 (0) | 432.7 | 18.0 |
| 6 | 30 (+1) | 47.5 (0) | 880.4 | 6.8 |
| 7 | 20 (0) | 35 (−1) | 865.7 | 9.4 |
| 8 | 20 (0) | 60 (+1) | 723.7 | 13.9 |
| 9 | 20 (0) | 47.5 (0) | 798.6 | 11.2 |
| 10 | 20 (0) | 47.5 (0) | 798.6 | 9.8 |
| 11 | 20 (0) | 47.5 (0) | 798.6 | 10.2 |
| Variation Source | Sum of Squares | Degrees of Freedom | Mean Square | F 1 | Ftab 2 | p-Value |
|---|---|---|---|---|---|---|
| Regression | 143.2 | 5 | 28.6 | 16.6 | 5.05 | 0.00392 |
| Residuals | 8.6 | 5 | 1.7 | |||
| Lack of fit | 7.6 | 3 | 2.5 | 4.9 | 19.16 | 0.17552 |
| Pure error | 1.0 | 2 | 0.5 | |||
| Total | 151.8 | 10 | ||||
| R 2,3 | 94.33% |
| Assay | Pressure (MPa) | Temperature (°C) 1 | Eugenol (%) | Eugenyl Acetate (%) | β-Caryophyllene (%) | α-Humulene (%) |
|---|---|---|---|---|---|---|
| 1 | 10 (−1) | 35 (−1) | 84.62 ± 0.29 | 12.57 ± 0.13 | 2.33 ± 0.14 | 0.48 ± 0.03 |
| 2 | 30 (+1) | 35 (−1) | 85.25 ± 0.65 | 11.95 ± 0.69 | 2.32 ± 0.05 | 0.48 ± 0.01 |
| 3 | 10 (−1) | 60 (+1) | 81.31 ± 0.73 | 13.86 ± 0.79 | 4.83 ± 0.05 | 0.00 ± 0.00 |
| 4 | 30 (+1) | 60 (+1) | 85.43 ± 1.28 | 12.02 ± 1.39 | 2.13 ± 0.13 | 0.43 ± 0.02 |
| 5 | 10 (−1) | 47.5 (0) | 84.16 ± 0.68 | 12.53 ± 0.35 | 3.31 ± 0.33 | 0.00 ± 0.00 |
| 6 | 30 (+1) | 47.5 (0) | 85.52 ± 0.44 | 11.55 ± 0.32 | 2.43 ± 0.09 | 0.50 ± 0.04 |
| 7 | 20 (0) | 35 (−1) | 85.52 ± 0.03 | 11.67 ± 0.00 | 2.33 ± 0.02 | 0.49 ± 0.01 |
| 8 | 20 (0) | 60 (+1) | 85.39 ± 0.05 | 11.64 ± 0.06 | 2.16 ± 0.01 | 0.51 ± 0.01 |
| 9 | 20 (0) | 47.5 (0) | 86.28 | 11.22 | 2.07 | 0.43 |
| 10 | 20 (0) | 47.5 (0) | 85.80 | 11.59 | 2.19 | 0.43 |
| 11 | 20 (0) | 47.5 (0) | 85.96 | 11.23 | 2.34 | 0.47 |
| Clove extract | 70.06 | 17.06 | 10.67 | 2.20 |
| Model a | Regression/ Residual | Lack of Fit/ Pure Error | R2 | ||
|---|---|---|---|---|---|
| Fcal,1 b | Ftab,1 c | Fcal,2 b | Ftab,2 c | ||
| Eugenol = 86.04 + 1.02 x1 − 1.24 x12 − 0.54 x2 − 0.63 x22 + 0.87 x1 x2 | 15 | 5.05 | 5.8 | 19.16 | 93.76% |
| Eugenyl acetate = 11.29 − 0.57 x1 + 0.83 x12 + 0.22 x2 + 0.45 x22 − 0.30 x1 x2 | 17.4 | 5.05 | 1.6 | 19.16 | 94.56% |
| β-caryophyllene = 2.20 − 0.60 x1 + 0.66 x12 + 0.36 x2 + 0.04 x22 − 0.67 x1 x2 | 8.6 | 5.05 | 12 | 19.16 | 89.53% |
| α-humulene = 0.43 + 0.15 x1 − 0.17 x12 − 0.08 x2 + 0.08 x22 + 0.11 x1 x2 | 5.5 | 5.05 | 34.5 | 19.16 | 84.69% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jordão, A.M.; Coutinho, I.T.; Silva, E.K.; Kato, I.T.; Meireles, M.A.A.; Maia-Obi, L.P.; da Silva, B.G.; Champeau, M. Supercritical CO2 Impregnation of Clove Extract in Polycarbonate: Effects of Operational Conditions on the Loading and Composition. Processes 2022, 10, 2661. https://doi.org/10.3390/pr10122661
Jordão AM, Coutinho IT, Silva EK, Kato IT, Meireles MAA, Maia-Obi LP, da Silva BG, Champeau M. Supercritical CO2 Impregnation of Clove Extract in Polycarbonate: Effects of Operational Conditions on the Loading and Composition. Processes. 2022; 10(12):2661. https://doi.org/10.3390/pr10122661
Chicago/Turabian StyleJordão, Amanda Martins, Isabela Trindade Coutinho, Eric Keven Silva, Ilka Tiemy Kato, Maria Angela A. Meireles, Lígia Passos Maia-Obi, Bruno Guzzo da Silva, and Mathilde Champeau. 2022. "Supercritical CO2 Impregnation of Clove Extract in Polycarbonate: Effects of Operational Conditions on the Loading and Composition" Processes 10, no. 12: 2661. https://doi.org/10.3390/pr10122661
APA StyleJordão, A. M., Coutinho, I. T., Silva, E. K., Kato, I. T., Meireles, M. A. A., Maia-Obi, L. P., da Silva, B. G., & Champeau, M. (2022). Supercritical CO2 Impregnation of Clove Extract in Polycarbonate: Effects of Operational Conditions on the Loading and Composition. Processes, 10(12), 2661. https://doi.org/10.3390/pr10122661