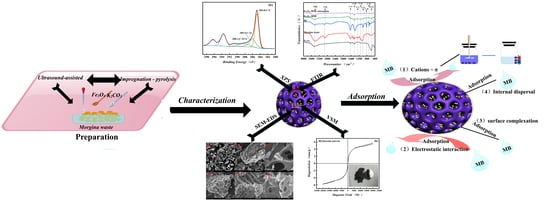
Magnetic Activated Biochar Fe3O4-MOS Made from Moringa Seed Shells for the Adsorption of Methylene Blue
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
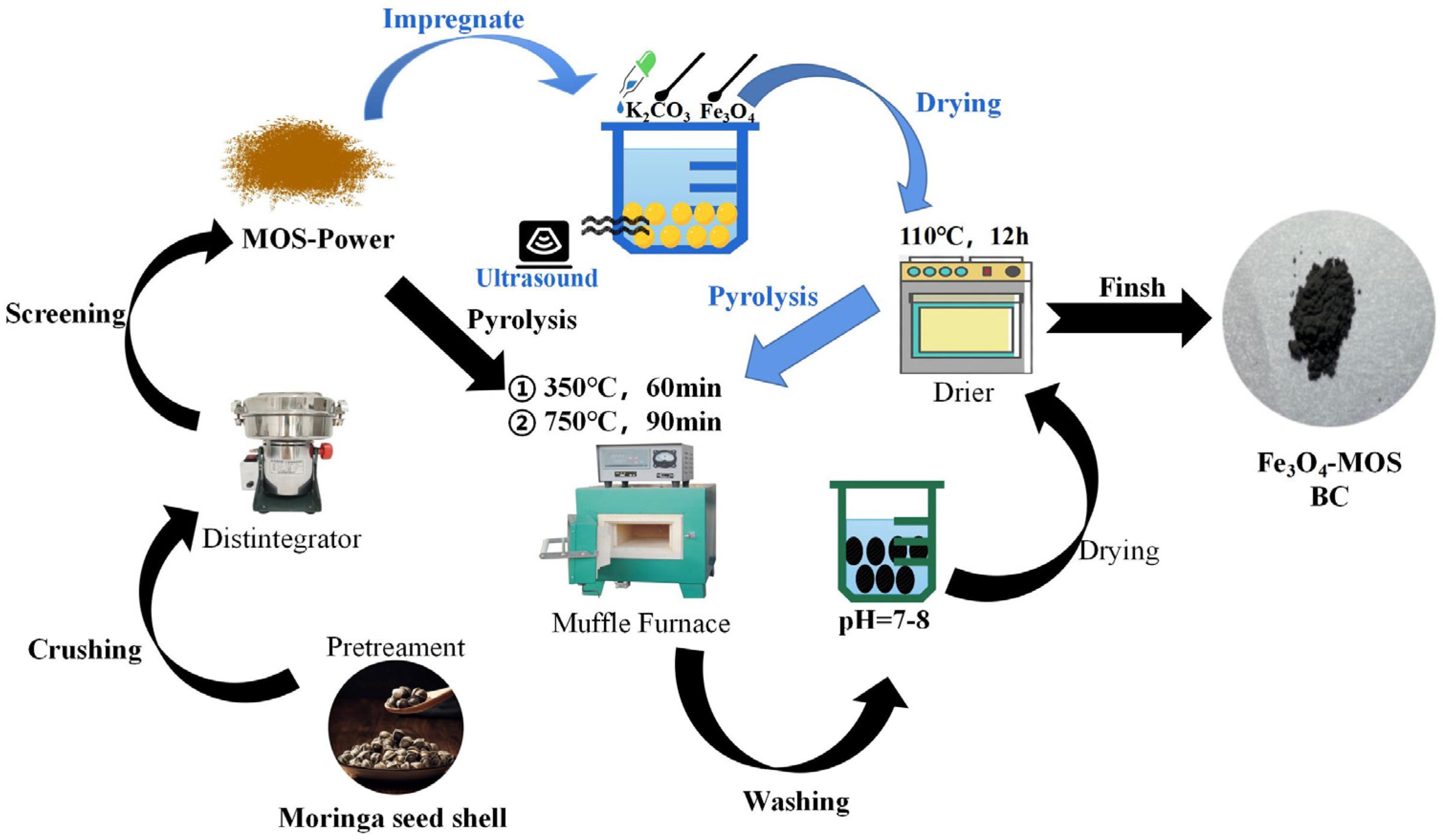
2.2. Preparation of Adsorbents
2.3. Characterization of Biochar
2.4. Adsorption
2.5. Desorption and Regeneration
3. Results and Discussion
3.1. Characterization of BC and Fe3O4-MOS
3.1.1. Surface Morphology and Energy Spectrum Analysis
3.1.2. Crystallization Properties
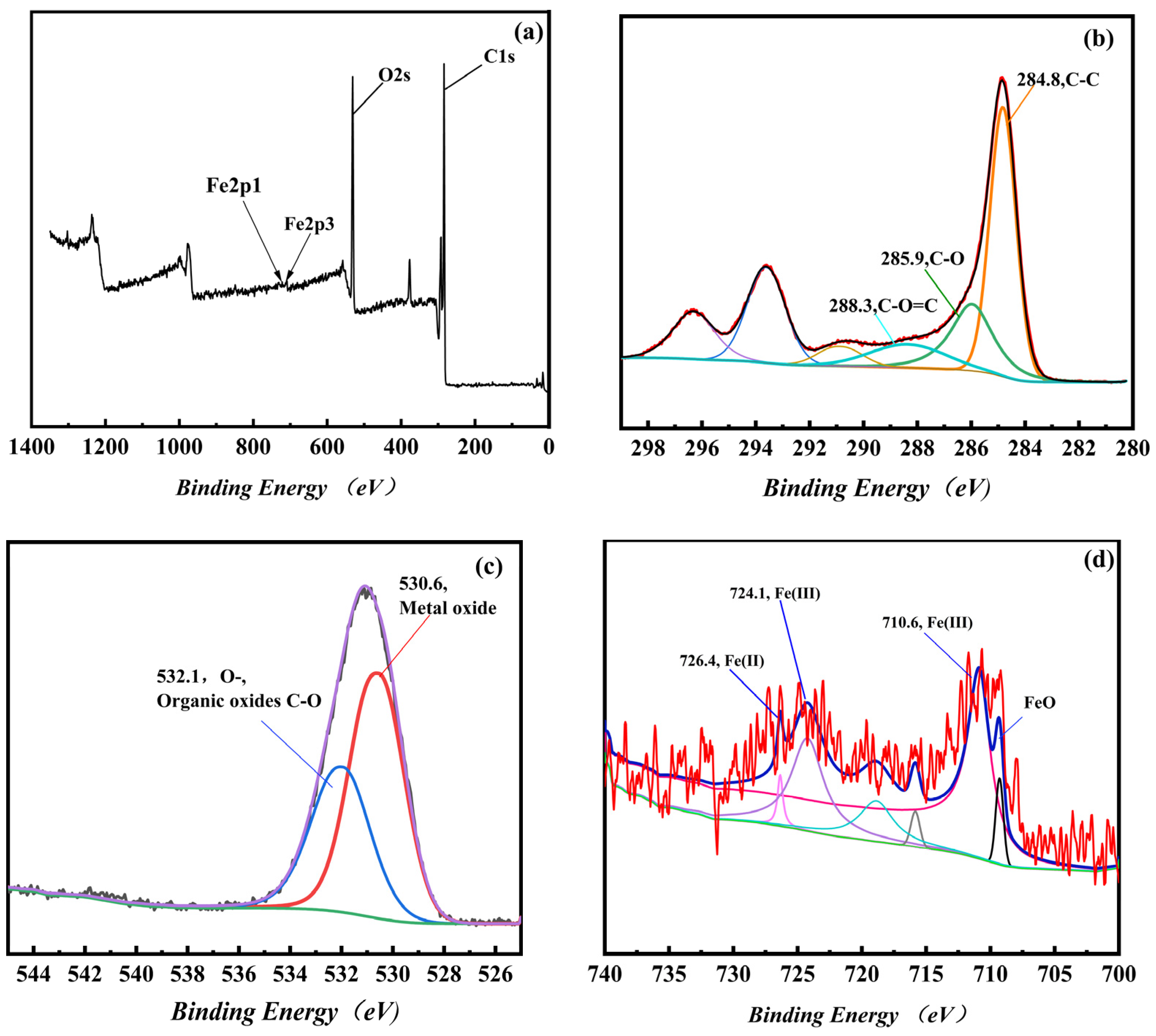
3.1.3. Surface Elements of Fe3O4-MOS Analysis
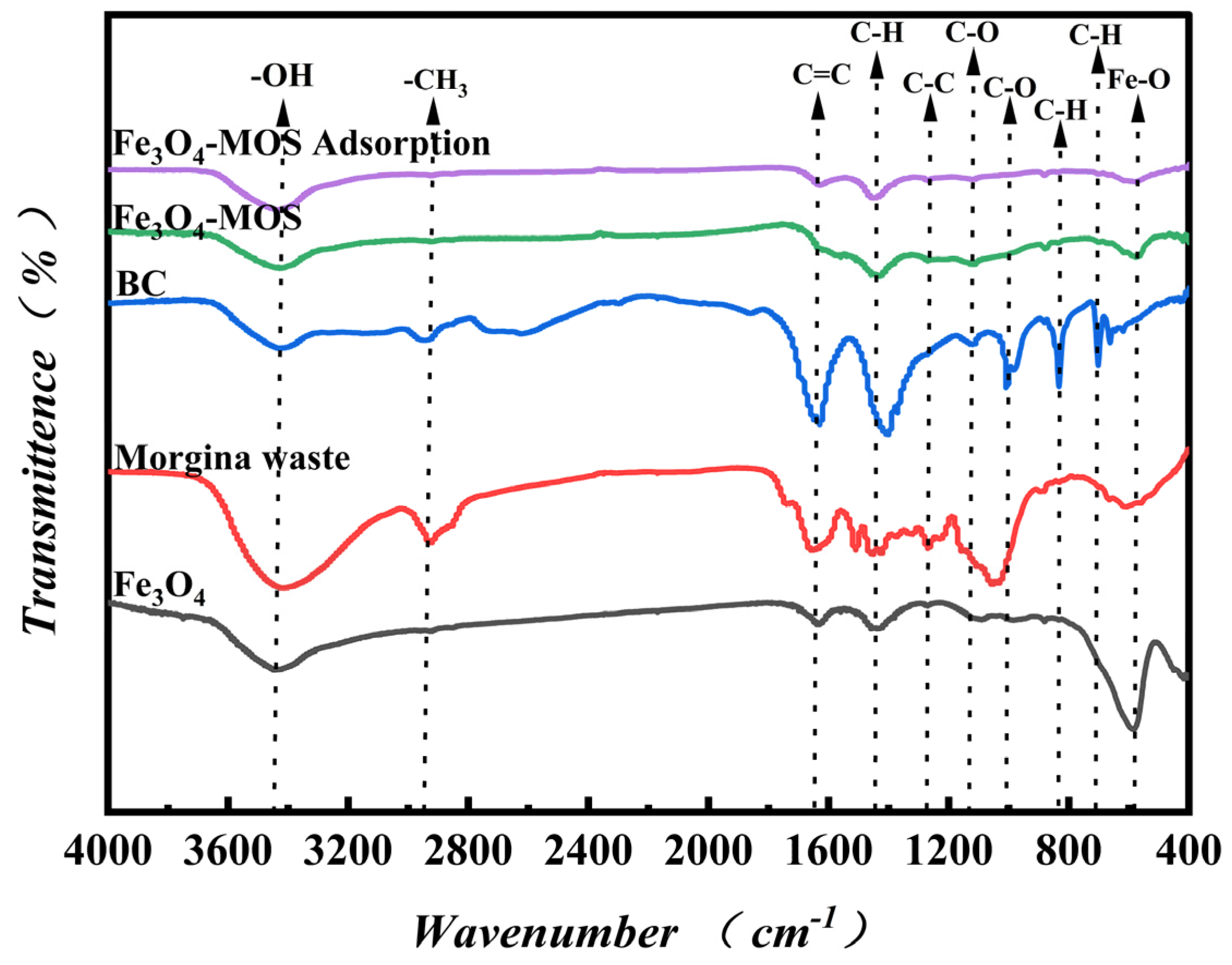
3.1.4. Surface Functional Groups Analysis
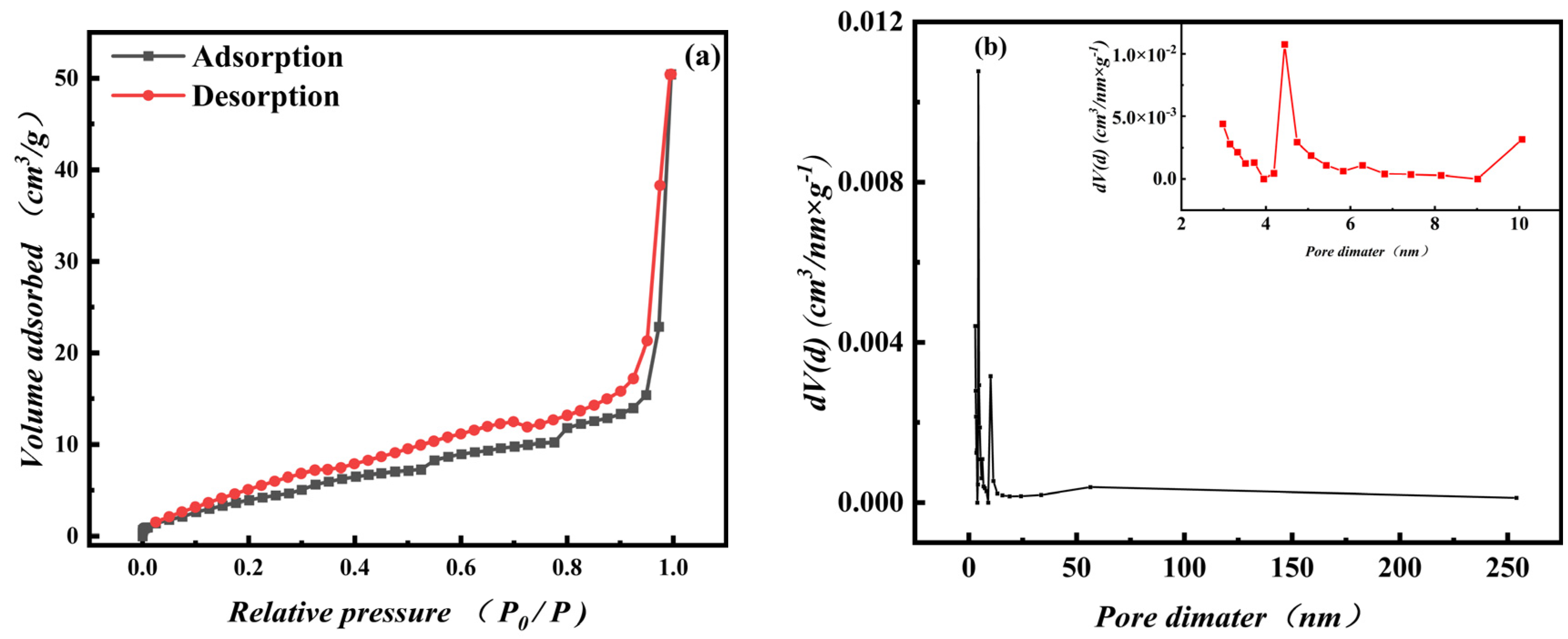
3.1.5. Pore structure Characteristics Analysis
3.2. Adsorption Property Analysis
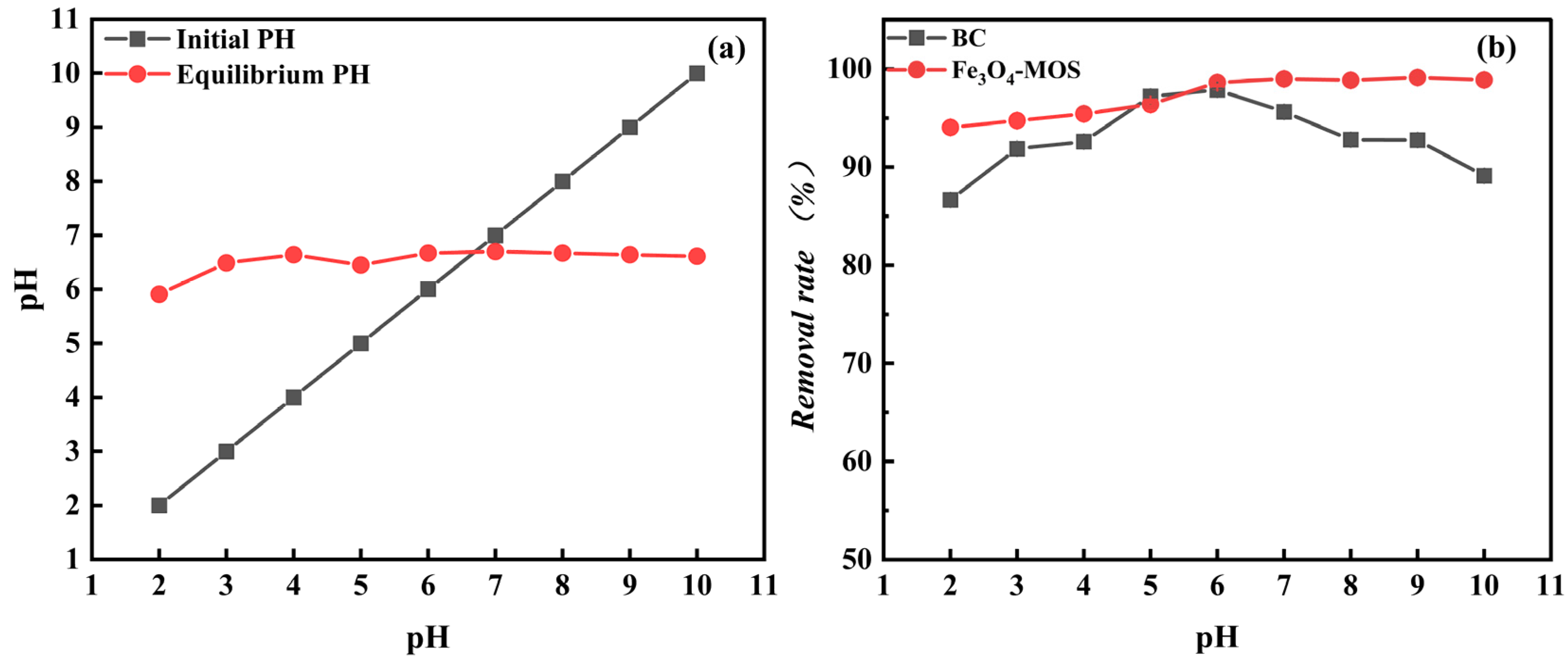
3.2.1. Effect Analysis of Adsorption System pH
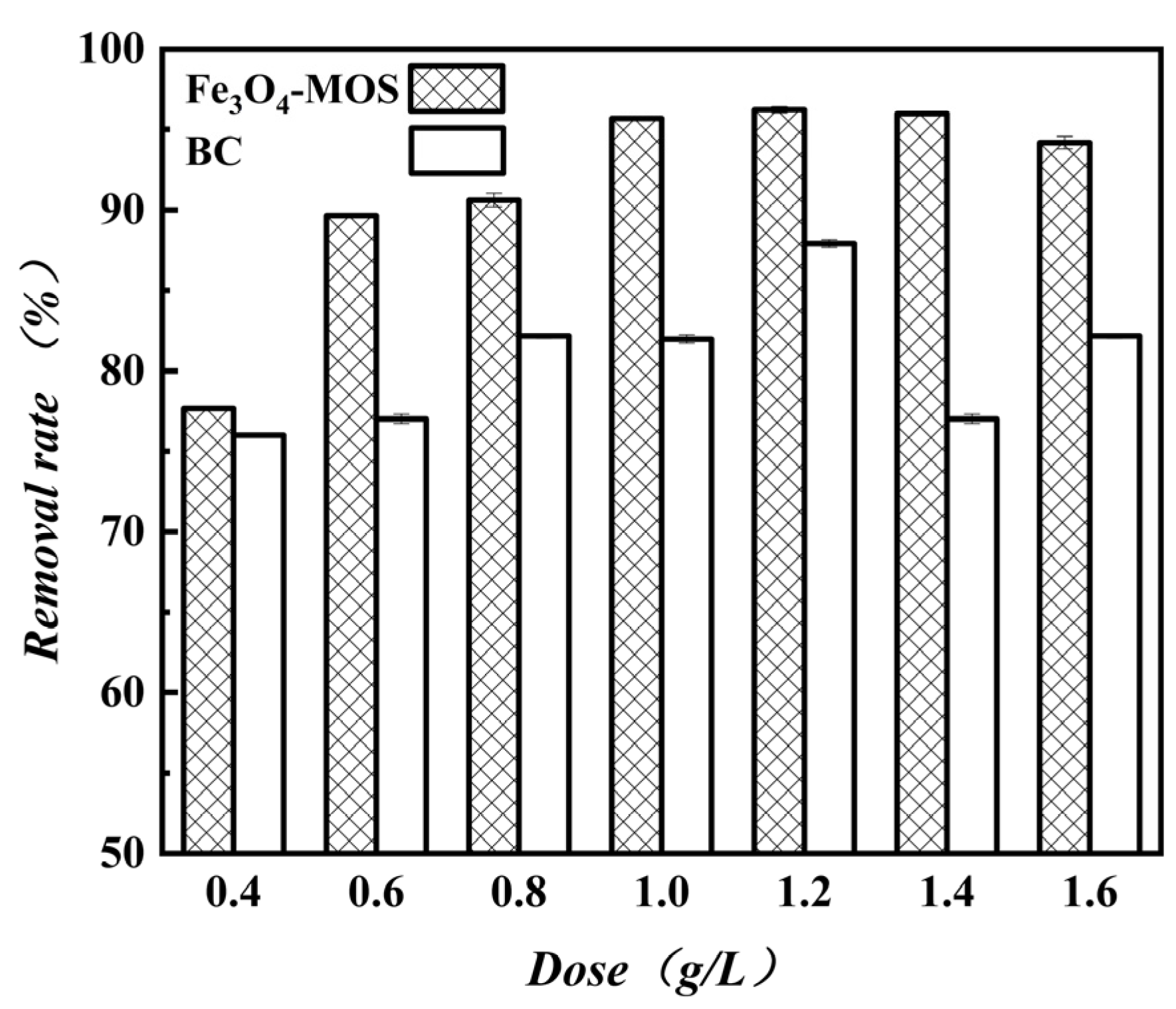
3.2.2. Effect Analysis of Adsorption Material Usage
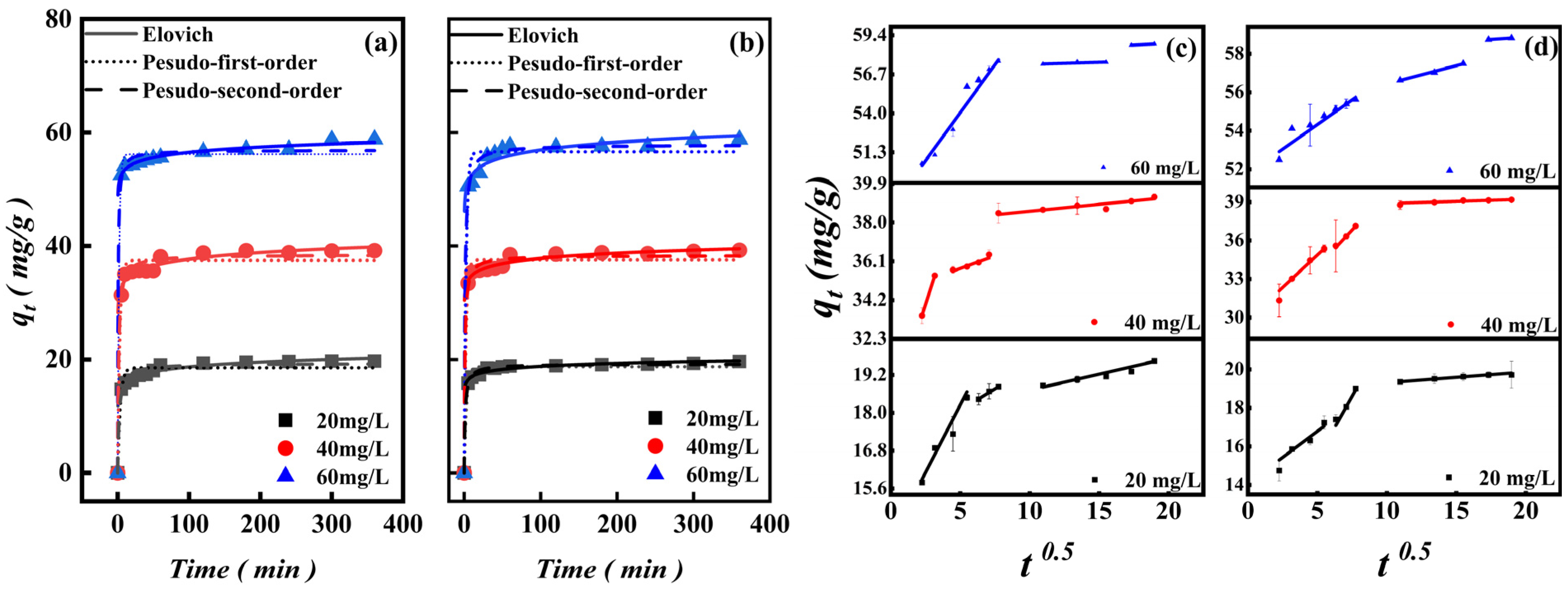
3.2.3. Adsorption Time and Kinetic Analysis
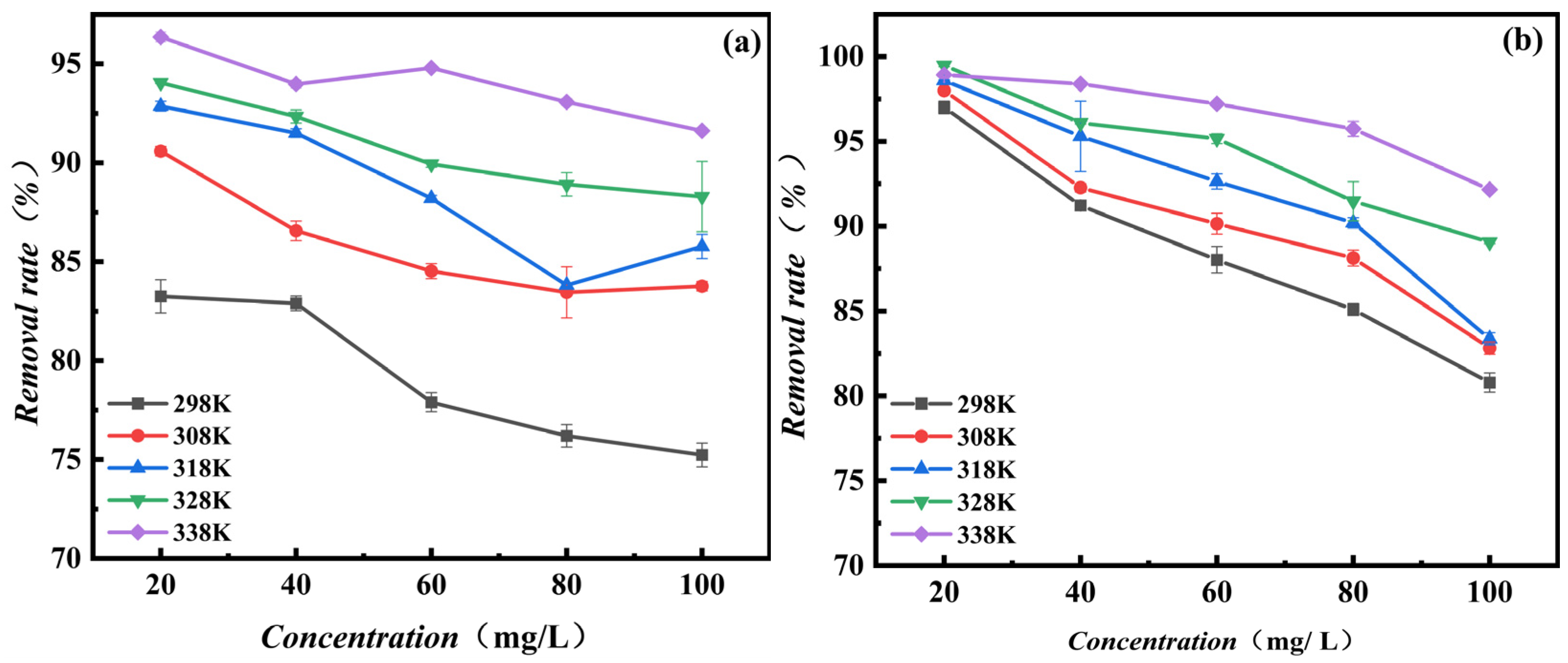
3.2.4. Adsorption Temperature and Thermodynamic Analysis
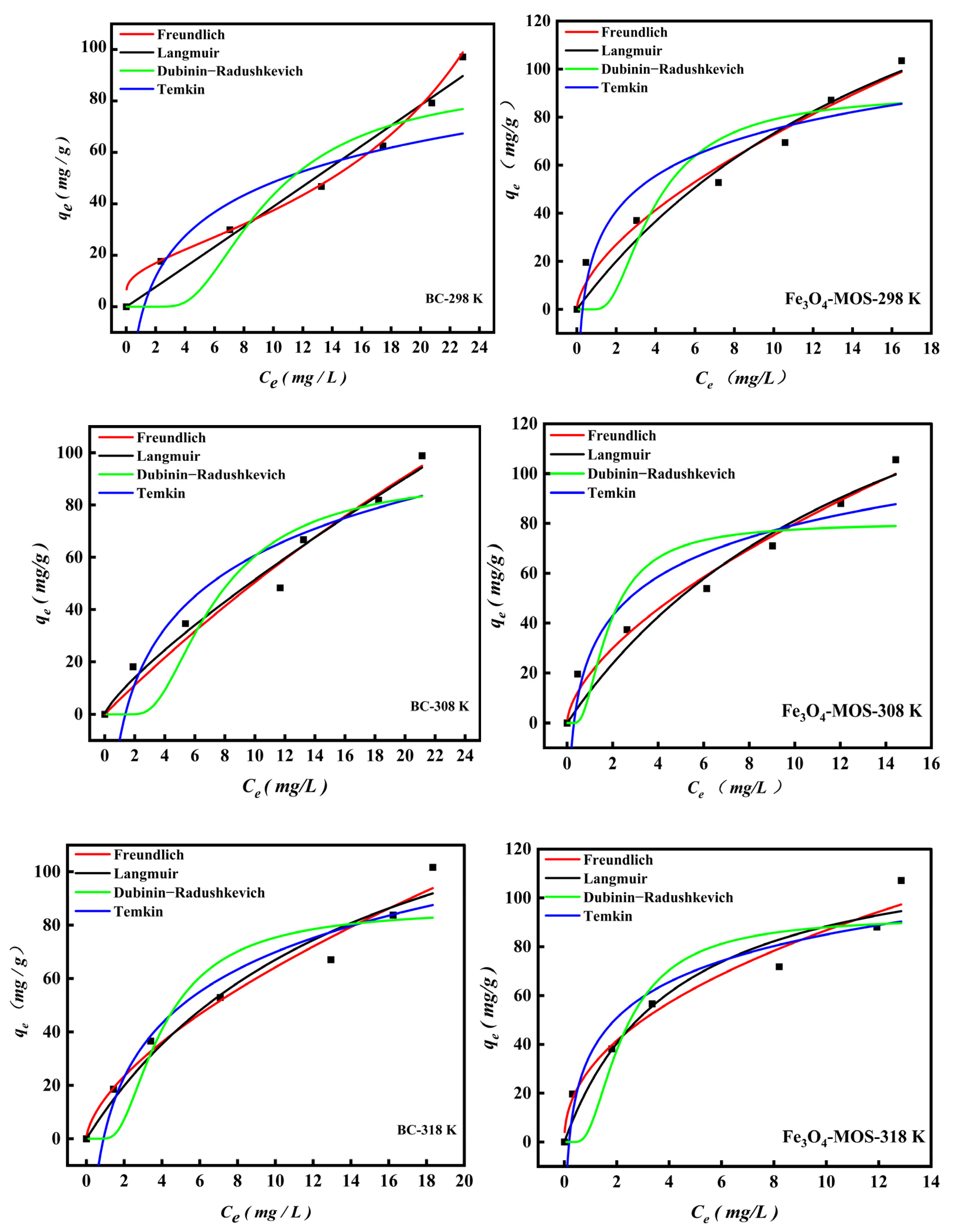
3.2.5. Adsorption Isotherm and Initial MB Concentration
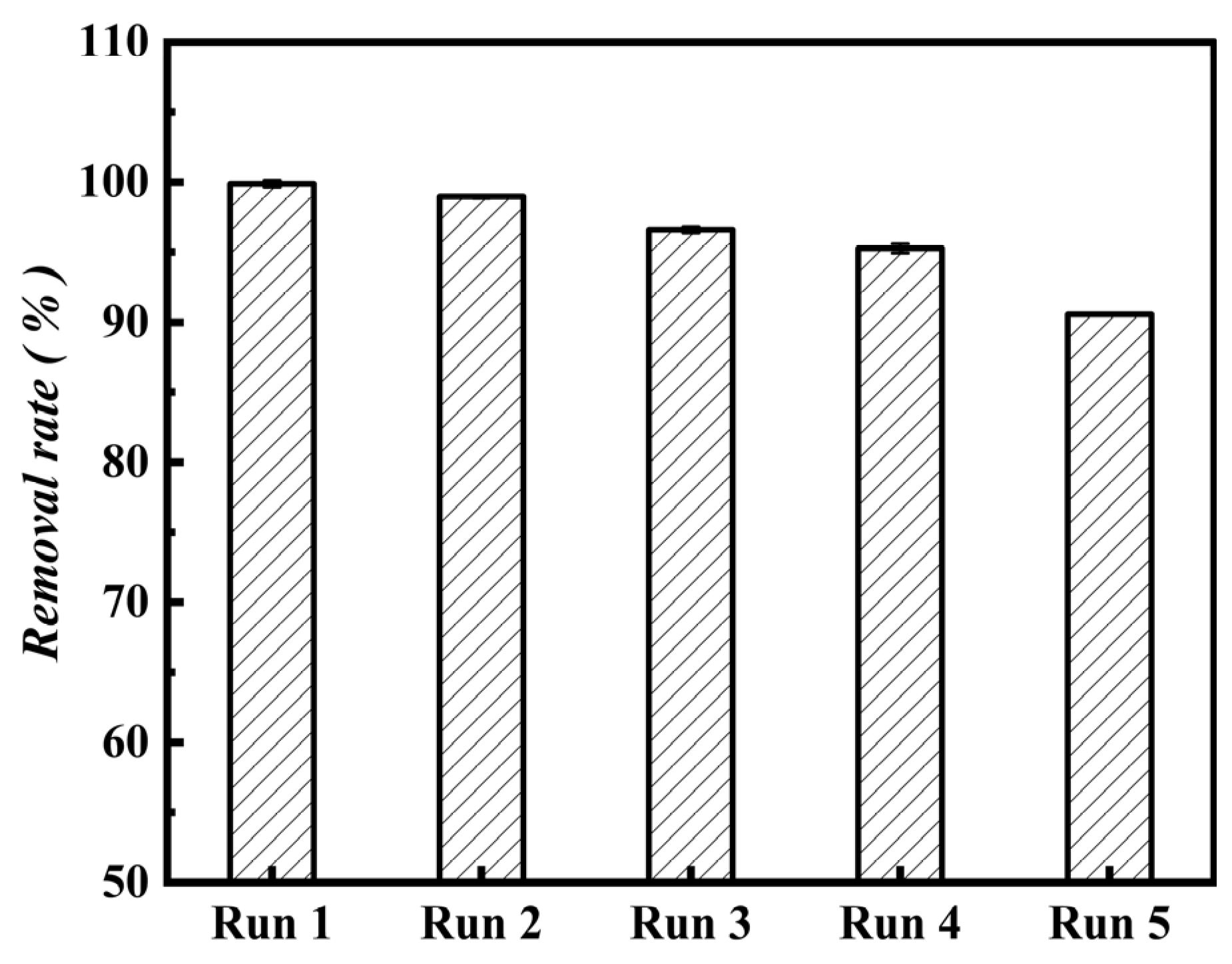
3.3. Regeneration and Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Arora, S. Removal of Synthetic Textile Dyes From Wastewaters: A Critical Review on Present Treatment Technologies. Crit. Rev. Environ. Sci. Technol. 2011, 41, 807–878. [Google Scholar] [CrossRef]
- Kalra, A.; Gupta, A. Recent advances in decolourization of dyes using iron nanoparticles: A mini review. Mater. Today Proc. 2021, 36, 689–696. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Fan, L.; Liu, X.; Cao, W.; Ai, H.; Wang, Z.; Liu, X.; Jia, H. Sorbent Properties of Orange Peel-Based Biochar for Different Pollutants in Water. Processes 2022, 10, 856. [Google Scholar] [CrossRef]
- Li, J.; Wang, R.; Zhang, D.; Su, Z.; Li, H.; Yan, Y. Copper Iodide (CuI) coating as a self-cleaning adsorbent for highly efficient dye removal. J. Alloys Compd. 2019, 774, 191–200. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Serrano-Martínez, A.; Mercader-Ros, M.T.; Martínez-Alcalá, I.; Lucas-Abellán, C.; Gabaldón, J.A.V.M. Degradation and toxicity evaluation of azo dye Direct red 83:1 by an advanced oxidation process driven by pulsed light. J. Water Process Eng. 2020, 37, 101530. [Google Scholar] [CrossRef]
- Alghamdi, W.; el Mannoubi, I. Investigation of Seeds and Peels of Citrullus colocynthis as Efficient Natural Adsorbent for Methylene Blue Dye. Processes 2021, 9, 1279. [Google Scholar] [CrossRef]
- Zeng, H.; Yu, Z.; Shao, L.; Li, X.; Zhu, M.; Liu, Y.; Feng, X.; Zhu, X. A novel strategy for enhancing the performance of membranes for dyes separation: Embedding PAA@UiO-66-NH2 between graphene oxide sheets. Chem. Eng. J. 2021, 403, 126281–126291. [Google Scholar] [CrossRef]
- Vantamuri, A.B.; Shettar, A.K. Biodegradation of Diazo Reactive dye (Green HE4BD) by Marasmius sp. BBKAV79. Chem. Data Collect. 2020, 28, 126281. [Google Scholar] [CrossRef]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Kanakaraju, D.; bin Ya, M.H.; Lim, Y.-C.; Pace, A. Combined Adsorption/Photocatalytic dye removal by copper-titania-fly ash composite. Surf. Interfaces 2020, 19, 100534–100545. [Google Scholar] [CrossRef]
- Low, K.S.; Lee, C.K.; Tan, K.K. Biosorption of basic dyes by water hyacinth roots. Bioresour. Technol. 1995, 52, 79–83. [Google Scholar] [CrossRef]
- M-Ridha, M.J.; Hussein, S.I.; Alismaeel, Z.T.; Atiya, M.A.; Aziz, G.M. Biodegradation of reactive dyes by some bacteria using response surface methodology as an optimization technique. Alex. Eng. J. 2020, 59, 3551–3563. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Gharieb, M.M.; Abou-El-Souod, G.W. Biodegradation of dyes by some green algae and cyanobacteria. Int. Biodeterior. Biodegrad. 2009, 63, 699–704. [Google Scholar] [CrossRef]
- Sadaf, S.; Bhatti, H.N. Batch and fixed bed column studies for the removal of Indosol Yellow BG dye by peanut husk. J. Taiwan Inst. Chem. Eng. 2014, 45, 541–553. [Google Scholar] [CrossRef]
- Wang, L. Application of activated carbon derived from “waste” bamboo culms for the adsorption of azo disperse dye: Kinetic, equilibrium and thermodynamic studies. J. Environ. Manag. 2012, 102, 79–87. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, K.; Gao, M.; Bai, Y.; Chen, L.; Ma, H. Effectively removal of cationic and anionic dyes by pH-sensitive amphoteric adsorbent derived from agricultural waste-wheat straw. J. Taiwan Inst. Chem. Eng. 2017, 76, 65–72. [Google Scholar] [CrossRef]
- Da Rosa, M.P.; Igansi, A.V.; Lütke, S.F.; Sant’Anna Cadaval, T.R.; do Santos, A.C.R.; de Oliveira Lopes Inacio, A.P.; de Almeida Pinto, L.A.; Beck, P.H. A new approach to convert rice husk waste in a quick and efficient adsorbent to remove cationic dye from water. J. Environ. Chem. Eng. 2019, 7, 103504. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K.; Ang, M.; Nishioka, H.J.D.; Treatment, W. Adsorption of methylene blue dye from aqueous solution by novel biomass Eucalyptus sheathiana bark: Equilibrium, kinetics, thermodynamics and mechanism. Desalination Water Treatment. 2016, 57, 5858–5878. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S.; Sasai, R. Arsenate removal from water using Fe3O4-loaded activated carbon prepared from waste biomass. Chem. Eng. J. 2010, 160, 57–62. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Luo, G.; Qian, F.; Zhang, S.; Chen, J. Facile fabrication of magnetic carbon composites from hydrochar via simultaneous activation and magnetization for triclosan adsorption. Env. Sci. Technol. 2014, 48, 5840–5848. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Sawalha, H.; Bader, A.; Sarsour, J.; Al-Jabari, M.; Rene, E. Removal of Dye (Methylene Blue) from Wastewater Using Bio-Char Derived from Agricultural Residues in Palestine: Performance and Isotherm Analysis. Processes 2022, 10, 2039. [Google Scholar] [CrossRef]
- Du, H.; Zhong, Z.; Zhang, B.; Shi, K.; Li, Z. Comparative study on pyrolysis of bamboo in microwave pyrolysis-reforming reaction by binary compound impregnation and chemical liquid deposition modified HZSM-5. J. Environ. Sci. 2020, 94, 186–196. [Google Scholar] [CrossRef]
- Choi, J.; Nam, H.; Capareda, S.C. Effect of metal salts impregnation and microwave-assisted solvent pretreatment on selectivity of levoglucosenone and levoglucosan from vacuum pyrolysis of ashe juniper waste. J. Environ. Chem. Eng. 2019, 7, 102796–102806. [Google Scholar] [CrossRef]
- Guo, F.; Peng, K.; Zhao, X.; Jiang, X.; Qian, L.; Guo, C.; Rao, Z. Influence of impregnated copper and zinc on the pyrolysis of rice husk in a micro-fluidized bed reactor: Characterization and kinetics. Int. J. Hydrogen Energy 2018, 43, 21256–21268. [Google Scholar] [CrossRef]
- Bezerra, C.O.; Cusioli, L.F.; Quesada, H.B.; Nishi, L.; Mantovani, D.; Vieira, M.F.; Bergamasco, R. Assessment of the use of Moringa oleifera seed husks for removal of pesticide diuron from contaminated water. Environ. Technol. 2020, 41, 191–201. [Google Scholar] [CrossRef]
- Homem, N.C.; Vieira, A.M.S.; Bergamasco, R.; Vieira, M.F. Low-cost biosorbent based on Moringa oleifera residues for herbicide atrazine removal in a fixed-bed column. Can. J. Chem. Eng. 2018, 96, 1468–1478. [Google Scholar] [CrossRef]
- Reddy, D.H.; Seshaiah, K.; Reddy, A.V.; Rao, M.M.; Wang, M.C. Biosorption of Pb2+ from aqueous solutions by Moringa oleifera bark: Equilibrium and kinetic studies. J. Hazard. Mater. 2010, 174, 831–838. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, T.; Han, C.; Lv, X.; Bai, L.; Sun, X.; Zhang, P. Facile synthesis of Fe-modified lignin-based biochar for ultra-fast adsorption of methylene blue: Selective adsorption and mechanism studies. Bioresour. Technol. 2022, 344, 126186. [Google Scholar] [CrossRef]
- Li, S.; Yang, F.; Li, J.; Cheng, K. Porous biochar-nanoscale zero-valent iron composites: Synthesis, characterization and application for lead ion removal. Sci. Total Environ. 2020, 746, 141037. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D.R. High resolution XPS of organic polymers. Adv. Mater. 1992, 5, 778. [Google Scholar]
- Zhang, Q.; Chadderdon, X.; Tang, B. Preparation of a magnetically recoverable biocatalyst support on monodisperse Fe3O4 nanoparticles. RSC Adv. 2013, 3, 9924–9931. [Google Scholar] [CrossRef]
- Gurav, R.; Bhatia, S.K.; Choi, T.R.; Park, Y.L.; Park, J.Y.; Han, Y.H.; Vyavahare, G.; Jadhav, J.; Song, H.S.; Yang, P.; et al. Treatment of furazolidone contaminated water using banana pseudostem biochar engineered with facile synthesized magnetic nanocomposites. Bioresour. Technol. 2020, 297, 122472. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, J.; Yi, Y. Biochar and hydrochar derived from freshwater sludge: Characterization and possible applications. Sci. Total Environ. 2021, 763, 144550. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef]
- Zhang, S.; Tao, L.; Zhang, Y.; Wang, Z.; Gou, G.; Jiang, M.; Huang, C.; Zhou, Z. The role and mechanism of K2CO3 and Fe3O4 in the preparation of magnetic peanut shell based activated carbon. Powder Technol. 2016, 295, 152–160. [Google Scholar] [CrossRef]
- Yang, K.; Peng, H.; Wen, Y.; Li, N. Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2010, 256, 3093–3097. [Google Scholar] [CrossRef]
- Ain, Q.U.; Rasheed, U.; Yaseen, M.; Zhang, H.; Tong, Z. Superior dye degradation and adsorption capability of polydopamine modified Fe3O4-pillared bentonite composite. J. Hazard. Mater. 2020, 397, 122758. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Li, M.; Dong, C.W.W.; Shengwan, Z.; Jincai, Z. Study on the adsorption performance of Moringa seed shell biochar on Cu(2+) in solution. Chin. Agric. Sci. Bull. 2020, 36, 84–89. [Google Scholar]
- Chen, W.; Duan, L.; Wang, L.; Zhu, D. Adsorption of Hydroxyl- and Amino-Substituted Aromatics to Carbon Nanotubes. Environ. Sci. Technol. 2008, 42, 6862–6868. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Li, K.; Lu, K.; Chai, Q. Adsorption behavior and mechanism of methylene blue onto biomass-based mesoporous acid activated carbons by microwave heating. Chin. J. Environ. Eng. 2014, 8, 909–916. [Google Scholar]
- Corbett, J.F. Pseudo first-order kinetics. J. Chem. Educ. 1972, 49, 663. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Advances in Water Pollution Research: Removal of Biologically Resistant Pollutant from Waste Water by Adsorption Proceedings of 1st International Conference on Water Pollution Symposium; Pergamon Press: Oxford, UK, 1962; Volume 2, pp. 231–266. [Google Scholar]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. J. Hazard. Mater. 2009, 164, 473–482. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Xu, S.; Wang, M.; Zhang, Y.; Xue, X. A modified method for enhancing adsorption capability of banana pseudostem biochar towards methylene blue at low temperature. Bioresour. Technol. 2019, 282, 48–55. [Google Scholar] [CrossRef]
- Garba, Z.N.; Zhou, W.; Lawan, I.; Xiao, W.; Zhang, M.; Wang, L.; Chen, L.; Yuan, Z. An overview of chlorophenols as contaminants and their removal from wastewater by adsorption: A review. J. Environ. Manag. 2019, 241, 59–75. [Google Scholar] [CrossRef]
- Olu-Owolabi, B.I.; Alabi, A.H.; Diagboya, P.N.; Unuabonah, E.I.; During, R.A. Adsorptive removal of 2,4,6-trichlorophenol in aqueous solution using calcined kaolinite-biomass composites. J. Environ. Manag. 2017, 192, 94–99. [Google Scholar] [CrossRef]
- Agarry, S.E.; Owabor, C.N.; Ajani, A.O. Modified Plantain Peel as Cellulose-Based Low-Cost Adsorbent For The Removal of 2,6-Dichlorophenol From Aqueous Solution: Adsorption Isotherms, Kinetic Modeling, And Thermodynamic Studies. Chem. Eng. Commun. 2013, 200, 1121–1147. [Google Scholar] [CrossRef]
- Karthikeyan, T.; Rajgopal, S.; Miranda, L.R. Chromium(VI) adsorption from aqueous solution by Hevea Brasilinesis sawdust activated carbon. J. Hazard. Mater. 2005, 124, 192–199. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.-J. Biosorption isotherms, kinetics and thermodynamics. Sep. Purif. Technol. 2008, 61, 229–242. [Google Scholar] [CrossRef]
- Geng, X. Study on the fractions of thermodynamic function changes for both adsorption and desorption from a liquid-solid system. Thermochim. Acta 1998, 308, 131–138. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M. Over the Adsorption in Solution. J. Phys. Chem. A 1906, 57, 385–470. [Google Scholar]
- Temkin, M.I. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physicochim. 1940, 12, 327–356. [Google Scholar]
- Dubinin, M.M.; Kadlec, O. Novel ideas in the theory of the physical adsorption of vapors on micropore adsorbents. Carbon 1987, 25, 321–324. [Google Scholar] [CrossRef]
- Zhang, P.; O’Connor, D.; Wang, Y.; Jiang, L.; Xia, T.; Wang, L.; Tsang, D.C.W.; Ok, Y.S.; Hou, D. A green biochar/iron oxide composite for methylene blue removal. J. Hazard. Mater. 2020, 384, 121286. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.H.; Chen, S.M. Biosorption of azo dyes from aqueous solution by glutaraldehyde-crosslinked chitosans. J. Hazard. Mater. 2009, 172, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Peng, N.; Sun, J.; Lu, G.; Chen, M.; Deng, F.; Dou, R.; Nie, L.; Zhong, Y. Adsorptive removal of cationic methylene blue and anionic Congo red dyes using wet-torrefied microalgal biochar: Equilibrium, kinetic and mechanism modeling. Environ. Pollut. 2021, 272, 115986. [Google Scholar] [CrossRef]
- Manoko, M.C.; Chirwa, E.M.M.; Makgopa, K. Non-demineralized paper waste sludge derived magnetic biochar as sorbs for removal of methylene blue, phosphorus, and selenate in wastewater. Clean. Chem. Eng. 2022, 3, 100048. [Google Scholar] [CrossRef]
- Wang, K.; Peng, N.; Sun, J.; Lu, G.; Chen, M.; Deng, F.; Dou, R.; Nie, L.; Zhong, Y. Synthesis of silica-composited biochars from alkali-fused fly ash and agricultural wastes for enhanced adsorption of methylene blue. Sci. Total Environ. 2020, 729, 139055. [Google Scholar] [CrossRef]





| Model | Fe3O4-MOS | BC | ||||
|---|---|---|---|---|---|---|
| 20 mg/L | 40 mg/L | 60 mg/L | 20 mg/L | 40 mg/L | 60 mg/L | |
| qe,exp (mg/g) | 19.72 | 39.18 | 58.81 | 19.65 | 39.27 | 58.81 |
| Pseudo-first order | ||||||
| K1 (min−1) | 0.27 | 0.35 | 0.53 | 0.34 | 0.42 | 0.41 |
| qe,cal (mg/g) | 18.53 | 37.47 | 56.19 | 18.70 | 37.56 | 56.63 |
| R2 | 0.95 | 0.98 | 0.99 | 0.98 | 0.98 | 0.98 |
| Pseudo-second order | ||||||
| K2 (g/mg∙min) | 0.02 | 0.03 | 0.03 | 0.04 | 0.03 | 0.02 |
| qe,cal (mg/g) | 19.50 | 38.45 | 56.90 | 19.18 | 38.33 | 57.83 |
| R2 | 0.996 | 0.98 | 0.996 | 0.997 | 0.992 | 0.994 |
| Intra-particle diffusion | ||||||
| C1 | 14.08 | 29.80 | 51.76 | 13.97 | 28.67 | 47.41 |
| Ki,1 | 0.05 | 1.01 | 0.51 | 0.86 | 2.13 | 1.33 |
| R2 | 0.884 | 0.993 | 0.894 | 0.98 | 1 | 0.985 |
| C2 | 8.91 | 28.04 | 54.53 | 16.72 | 34.50 | 57.12 |
| Ki,2 | 1.29 | 1.17 | 0.19 | 0.27 | 0.25 | 0.62 |
| R2 | 0.972 | 0.894 | 0.992 | 0.995 | 0.90 | 0.81 |
| Ki,3 | 0.05 | 38.54 | 57.89 | 0.10 | 37.87 | 57.77 |
| C3 | 18.74 | 0.03 | 0.04 | 17.71 | 0.07 | 0.05 |
| R2 | 0.993 | 0.994 | 0.994 | 0.960 | 0.960 | 1 |
| Elovich | ||||||
| α | 5.44 × 104 | 1.04 × 108 | 3.31 × 1016 | 2.58 × 108 | 3.87 × 1010 | 1.43 × 1011 |
| β | 1.22 | 1.67 | 1.33 | 1.29 | 1.33 | 1.92 |
| R2 | 0.995 | 0.994 | 0.999 | 0.994 | 0.997 | 0.996 |
| Sample | C0 (mg/L) | ∆H (KJ/mol) | ∆S | ∆G | ||||
|---|---|---|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | 328 K | 338 K | ||||
| Fe3O4-MOS | 20 | 104.51 | 374.71 | −9.32 | −9.60 | −10.97 | −19.12 | −24.08 |
| 40 | 106.17 | 364.57 | −2.05 | −10.61 | −10.28 | −7.09 | −6.19 | |
| 60 | 104.84 | 372.30 | −18.91 | −15.43 | −9.97 | −6.67 | −4.98 | |
| BC | 20 | 29.76 | 114.23 | −3.96 | −5.79 | −6.79 | −7.53 | −8.80 |
| 40 | 35.00 | 126.95 | −2.70 | −4.76 | −6.29 | −5.02 | −7.73 | |
| 60 | 27.77 | 104.09 | −3.12 | −3.64 | −5.31 | −6.49 | −8.15 | |
| Model | Fe3O4-MOS | BC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | 328 K | 338 K | 298 K | 308 K | 318 K | 328 K | 338 K | |
| qe,exp (mg/g) | 103.51 | 105.57 | 107.14 | 108.94 | 109.81 | 97.13 | 98.87 | 101.67 | 104.89 | 107.44 |
| Langmuir | ||||||||||
| kL | 0.05 | 0.07 | 0.24 | 0.40 | 1.30 | 1.28 | 0.01 | 0.07 | 0.01 | 0.11 |
| qe,cal (mg/g) | 219.60 | 205.64 | 125.60 | 120.10 | 103.04 | 88.74 | 460.18 | 165.61 | 496.95 | 196.54 |
| R2 | 0.96 | 0.96 | 0.95 | 0.95 | 0.93 | 0.92 | 0.97 | 0.97 | 0.97 | 0.93 |
| Freundlich | ||||||||||
| KF | 17.58 | 19.57 | 30.22 | 39.03 | 52.03 | 7.81 | 7.97 | 15.17 | 10.72 | 24.22 |
| 1/n | 0.62 | 0.61 | 0.46 | 0.40 | 0.30 | 1.01 | 0.80 | 0.63 | 0.83 | 0.62 |
| R2 | 0.96 | 0.97 | 0.96 | 0.98 | 0.99 | 0.96 | 0.96 | 0.97 | 0.96 | 0.84 |
| Temkin | ||||||||||
| aT | 3.42 | 3.31 | 5.47 | 13.62 | 58.45 | 38.03 | 30.55 | 29.10 | 31.66 | 47.97 |
| bT | 111.55 | 112.91 | 124.48 | 146.53 | 186.60 | 65.15 | 83.82 | 90.85 | 86.14 | 58.58 |
| R2 | 0.90 | 0.90 | 0.93 | 0.94 | 0.96 | 0.87 | 0.91 | 0.95 | 0.89 | 0.96 |
| Dubinin-Redushckevich | ||||||||||
| qm (mg/g) | 90.24 | 80.33 | 92.45 | 93.32 | 89.19 | 123.10 | 291.95 | 86.40 | 112.59 | 111.39 |
| kD | 2.37 × 106 | 5.93 × 107 | 7.84 × 107 | 3.44 × 107 | 6.46 × 108 | 2.92 × 104 | 7.06 × 106 | 2.15 × 106 | 5.13 × 106 | 1.28 × 106 |
| E | 459.32 | 918.24 | 798.60 | 1205.60 | 2782.07 | 41.38 | 266.12 | 482.24 | 312.20 | 625 |
| R2 | 0.86 | 0.90 | 0.89 | 0.89 | 0.86 | 0.94 | 0.86 | 0.87 | 0.93 | 0.98 |
| Biosorbent | Adsorption Capacity | Reference |
|---|---|---|
| Fe-modified lignin-based biochar | 200 mg/g | [31] |
| Paper waste sludge biochar | 5.92 mg/g | [65] |
| Modified swine manure biochar | 143.76 mg/g | [66] |
| Bio-Char | 38 mg/g | [24] |
| Seeds and Peels of Citrullus colocynthis | 18.832 mg/g, 4.480 mg/g | [8] |
| Orange Peel-Based Biochar | 0.984 mg/g | [4] |
| Novel biomass Eucalyptus sheathiana bark | 204.3 mg/g | [20] |
| Fe3O4-MOS | 219.09 mg/g | This Study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Dong, C.; Guo, C.; Yu, L. Magnetic Activated Biochar Fe3O4-MOS Made from Moringa Seed Shells for the Adsorption of Methylene Blue. Processes 2022, 10, 2720. https://doi.org/10.3390/pr10122720
Li M, Dong C, Guo C, Yu L. Magnetic Activated Biochar Fe3O4-MOS Made from Moringa Seed Shells for the Adsorption of Methylene Blue. Processes. 2022; 10(12):2720. https://doi.org/10.3390/pr10122720
Chicago/Turabian StyleLi, Meiping, Cheng Dong, Caixia Guo, and Ligang Yu. 2022. "Magnetic Activated Biochar Fe3O4-MOS Made from Moringa Seed Shells for the Adsorption of Methylene Blue" Processes 10, no. 12: 2720. https://doi.org/10.3390/pr10122720
APA StyleLi, M., Dong, C., Guo, C., & Yu, L. (2022). Magnetic Activated Biochar Fe3O4-MOS Made from Moringa Seed Shells for the Adsorption of Methylene Blue. Processes, 10(12), 2720. https://doi.org/10.3390/pr10122720