Synthesis of a Ternary Polysulfonate Dispersant and Its Suspension Performance
Abstract
:1. Introduction
2. Materials and Experiments
2.1. Materials
2.2. Experiments
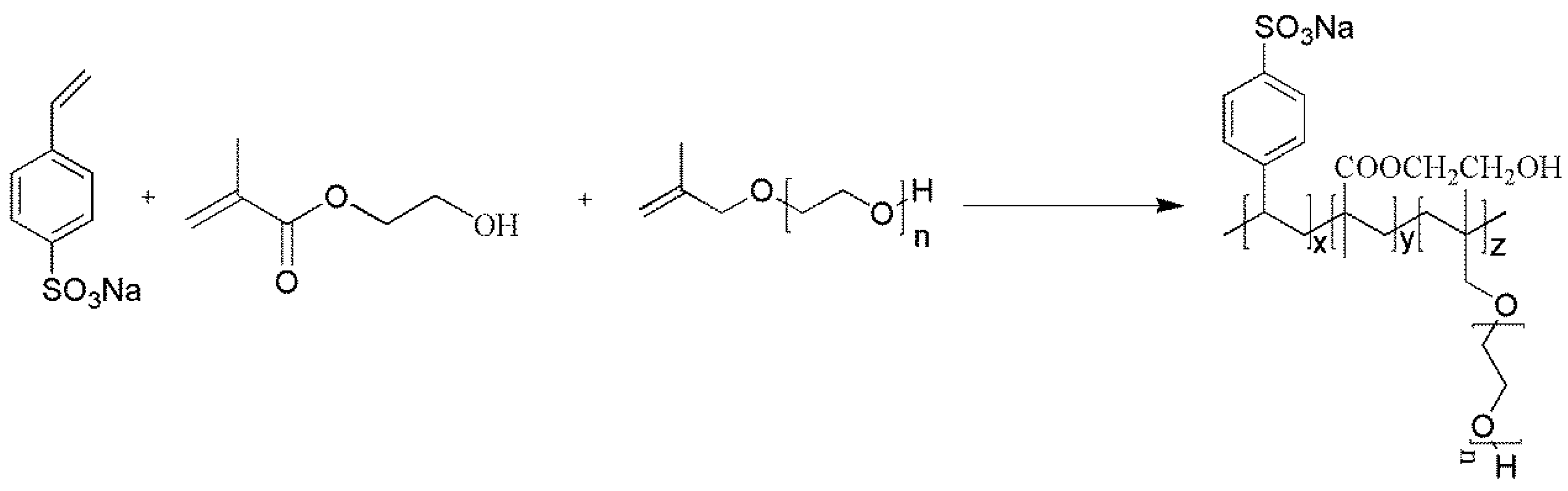
2.2.1. Synthesis of Polysulfonate Dispersant
2.2.2. Characterization
3. Results and Discussion
3.1. Optimization of Synthesis Conditions
3.2. Characterization and Performance
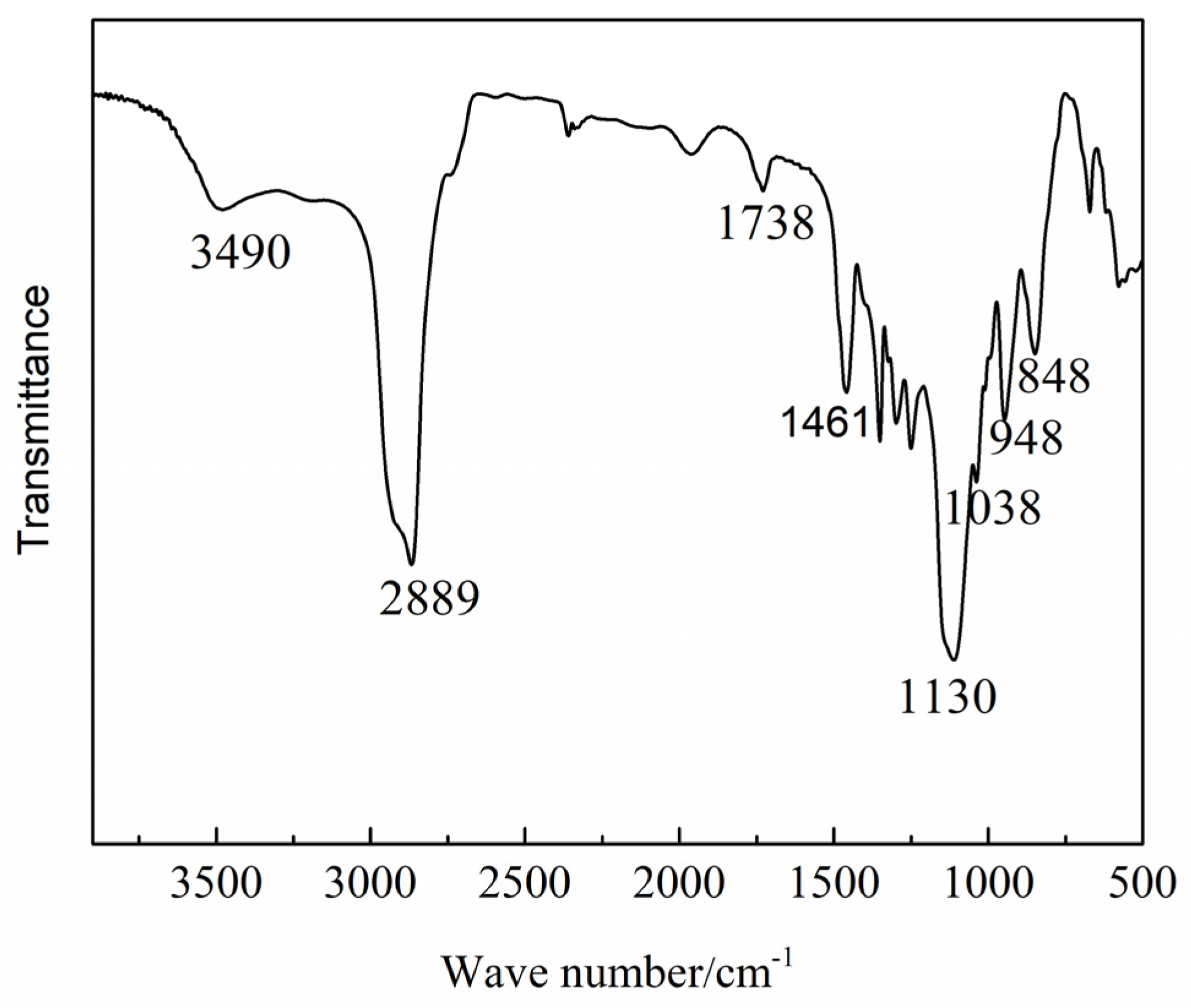
3.2.1. Fourier Transform Infrared (FTIR) Analysis
3.2.2. H Nuclear Magnetic Resonance (NMR) Analysis
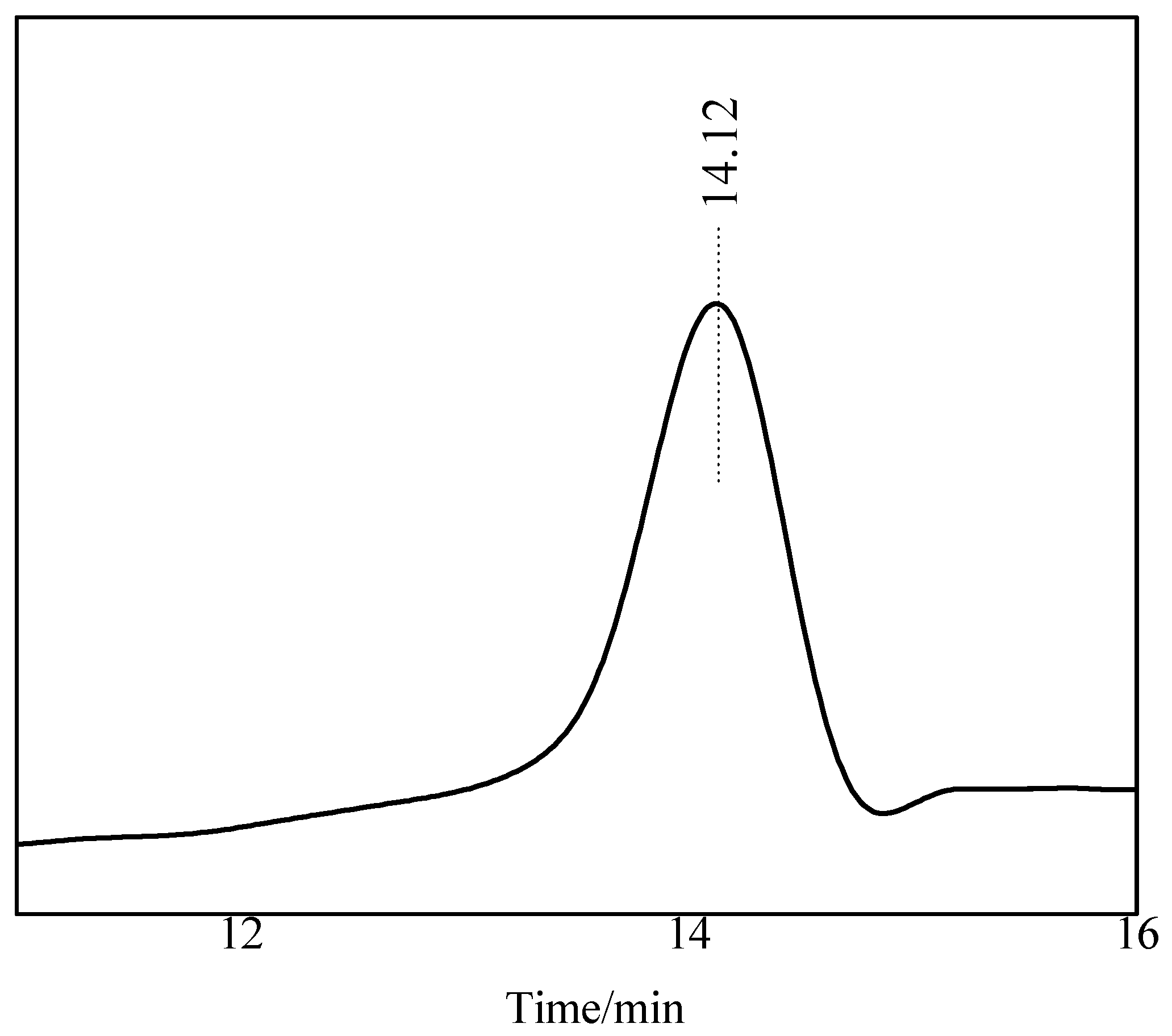
3.2.3. Gel Permeation Chromatography (GPC) Analysis
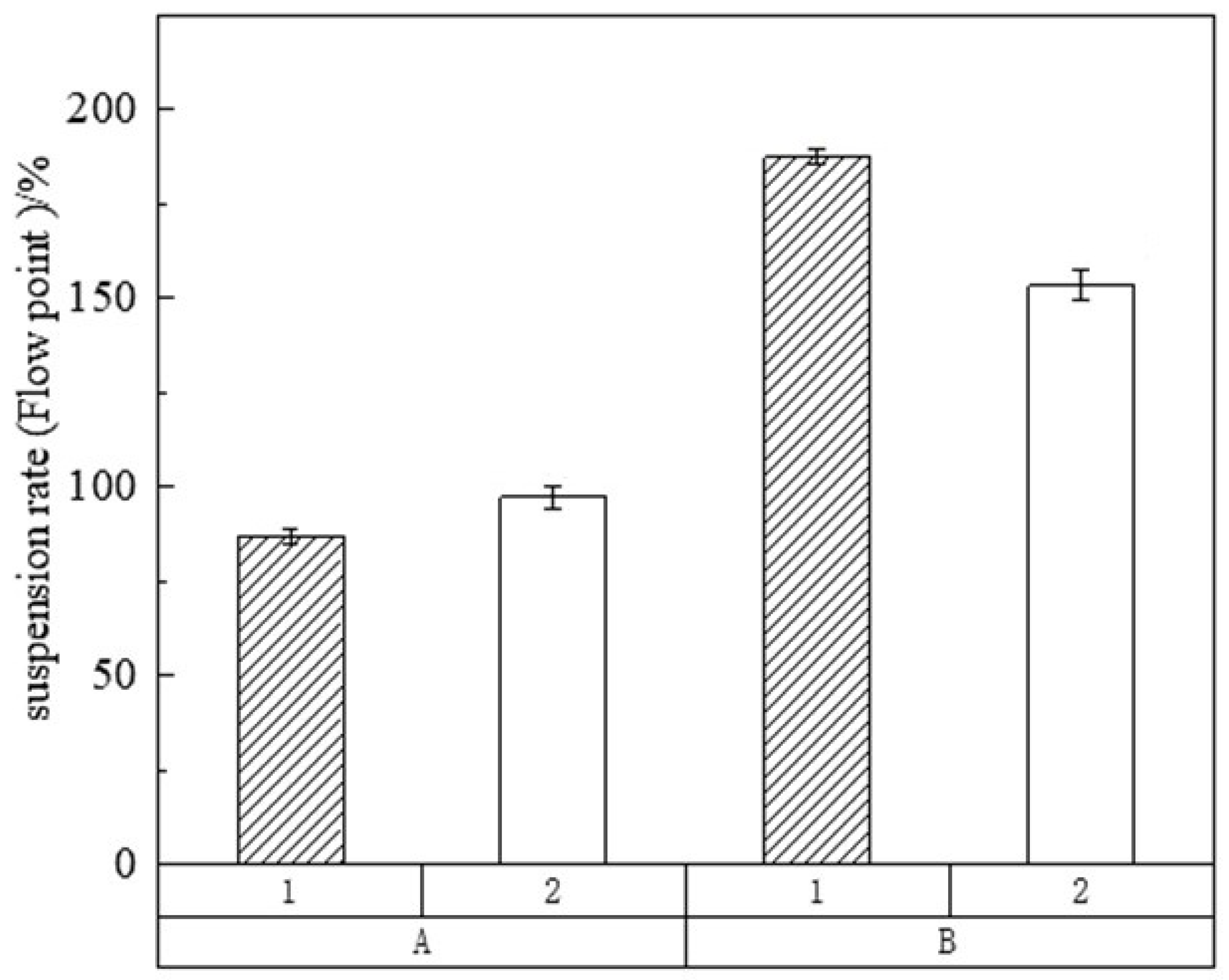
3.2.4. Suspension Performance
3.2.5. Surface Tension Analysis
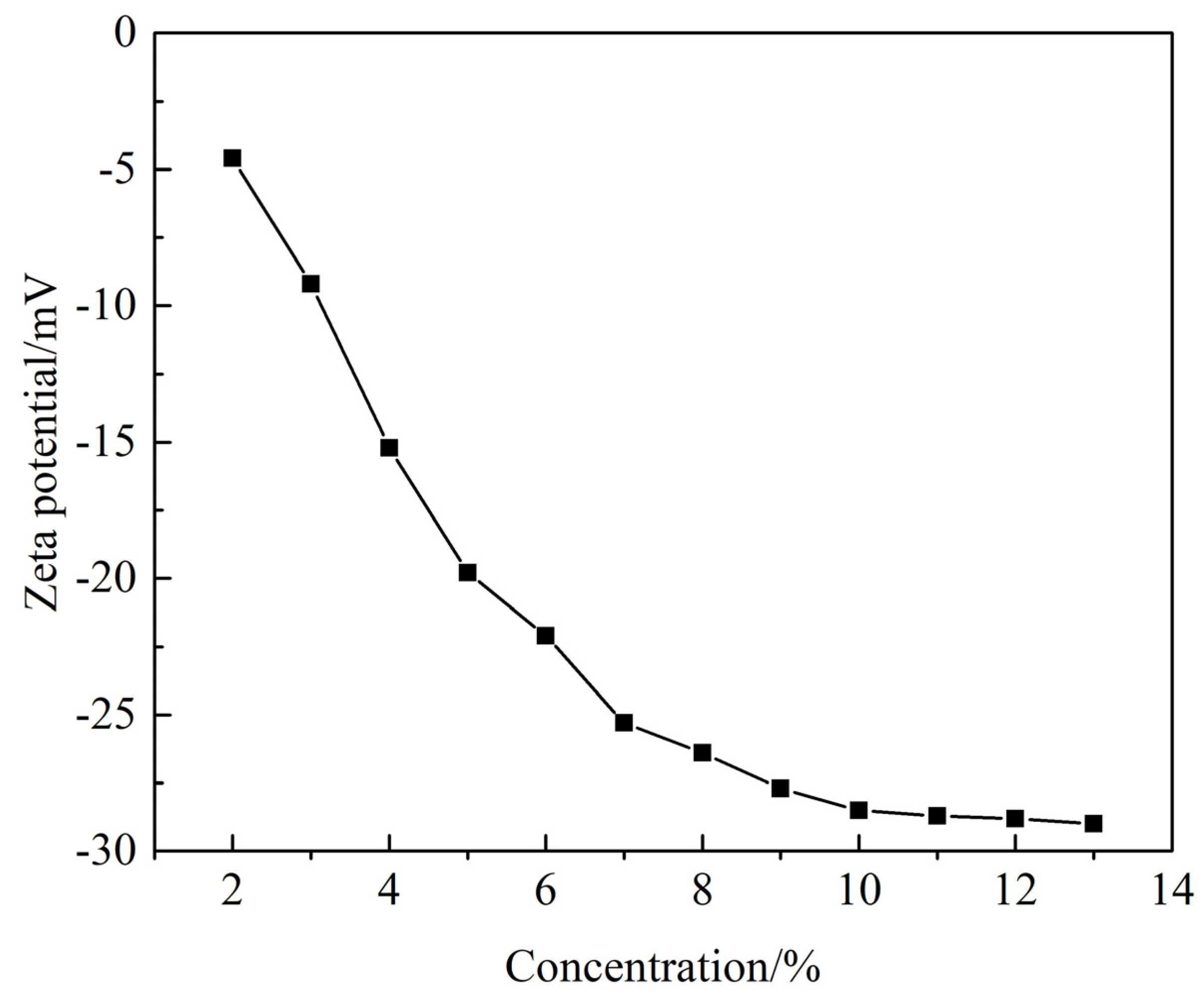
3.2.6. Zeta Potential Analysis
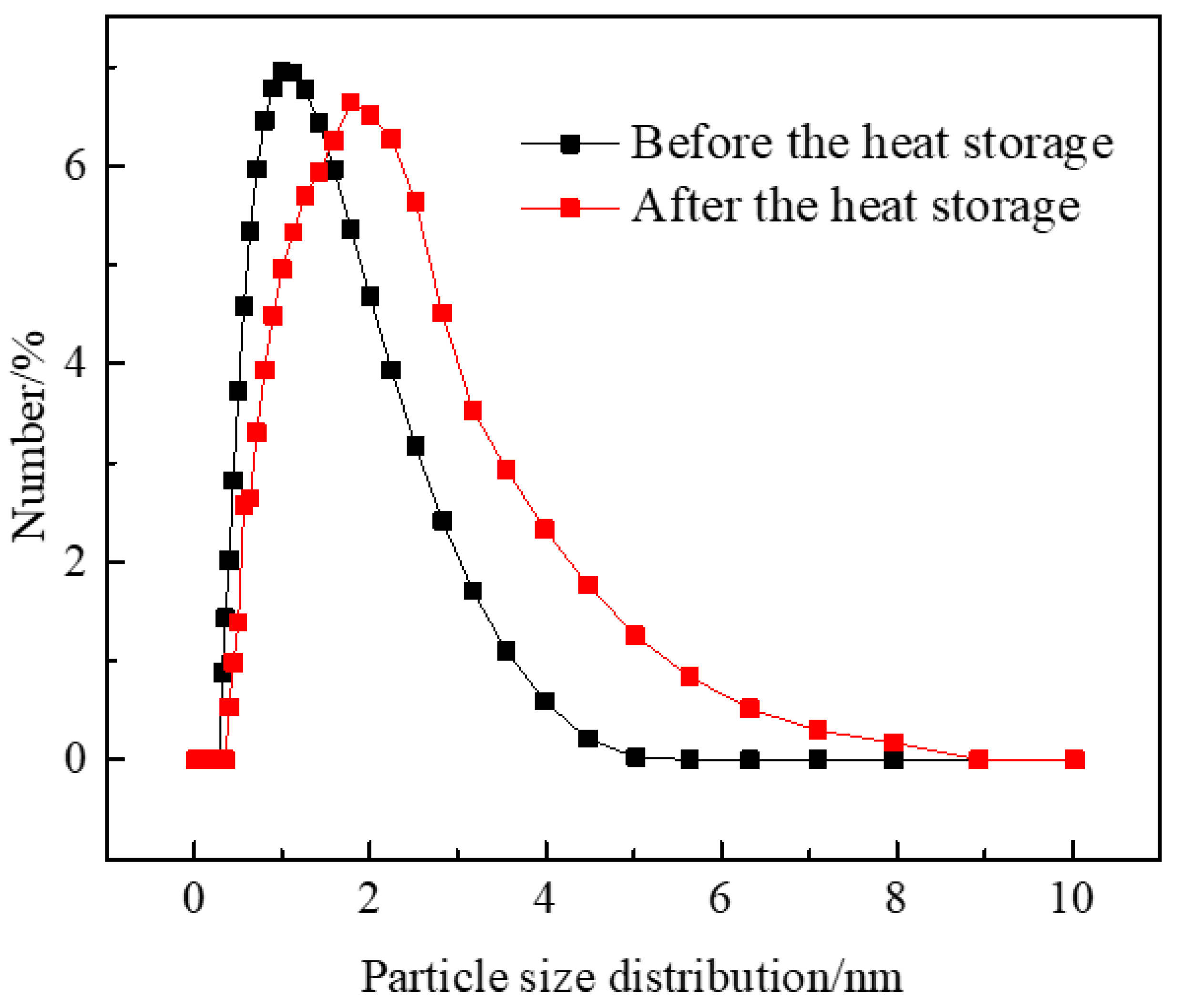
3.2.7. Particle Size Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Naizhen, H. Application of polycarboxylate dispersants in pesticide formulations. Agrochemical 2016, 55, 1–8. [Google Scholar]
- Chen, Z.; Tian-rui, R. Synthesis and application of a copolymer dispersant of sodium styrene acrylate and hydroxyethyl methacrylate. J. Process. Eng. 2008, 8, 240–247. [Google Scholar]
- Soheili, M.; Mohamadnia, Z.; Karimi, B. Switching from Ethylene Trimerization to Ethylene Polymerization by Chromium Catalysts Bearing SNS Tridentate Ligands: Process Optimization Using Response Surface Methodology. Catal. Lett. 2018, 148, 3685–3700. [Google Scholar] [CrossRef]
- Nai-zhen, H. Factors affecting pesticide suspension formulation physical stability and countermeasures. Agrochemical 2012, 51, 90–94. [Google Scholar]
- Zhang, G.H.; Zhu, N.; Li, Y.B.; Zhu, J.F.; Jia, Y.R.; Ge, L. Influence of side-chain structure of polycarboxylate dispersant on the performance of coal water slurry. Fuel Processing Technol. 2017, 161, 1–7. [Google Scholar] [CrossRef]
- Zhu, Y.; Fang, Y.; Min, Y.; Huang, X.; Li, W.; Yuan, J.; Wang, S.; Wu, Z. Comb-like polymer with sulfo groups and its dispersion and rheological properties in aqueous ceramic suspensions. J. Appl. Polym. Sci. 2017, 134, 44563. [Google Scholar] [CrossRef]
- Wang, G.; Bai, Y.; Ma, X.; Wang, W.; Yin, Q.; Du, Z. Effects of the PEG length of polycarboxylate-based terpolymers on their dispersion properties. J. Mol. Liq. 2017, 225, 333–338. [Google Scholar] [CrossRef]
- Ran, Q.; Qiao, M.; Liu, J.; Miao, C. SMA-g-MPEG comb-like polymer as a dispersant for Al2O3 suspensions. Appl. Surf. Sci. 2012, 258, 2447–2453. [Google Scholar] [CrossRef]
- Akhlaghi, O.; Akbulut, O.; Menceloglu, Y.Z. Extensional rheology and stability behavior of alumina suspensions in the presence of AMPS-modified polycarboxylate ether-based copolymers. Colloid Polym. Sci. 2015, 293, 2867–2876. [Google Scholar] [CrossRef] [Green Version]
- Xing, W.; Xian, Z. Synthesis of comb polycarboxylate dispersants and their application in imidacloprid water suspension. J Process. Eng. 2014, 14, 345–349. [Google Scholar]
- Shujin, S.; Shuqin, Z. Anhydride copolymer influence on the dispersion stability of 20% deworming urea suspension. Chin. J. Appl. Chem. 2012, 29, 1046–1051. [Google Scholar]
- Lidong, W.; Tianrui, R. Synthesis and dispersion properties of carboxylate sulfonate copolymer. J. Process. Eng. 2010, 10, 1193–1199. [Google Scholar]
- Lee, W.F.; Chen, Y. Synthesis and swelling properties of 2-hydroxyethyl methacrylate-co-1-vinyl-3-(3-sulfopropyl) imidazolium betaine hydrogels. J. Appl. Polym. Sci. 2001, 81, 2888–2900. [Google Scholar] [CrossRef]
- Chiappisi, L. Polyoxyethylene alkyl ether carboxylic acids: An overview of a neglected class of surfactants with multiresponsive properties. Adv. Colloid Interface Sci. 2017, 250, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Balbuena, L.; Arteaga-Jiménez, A.; Hernández-Zapata, E.; Márquez-Beltrán, C. Applicability of the Gibbs Adsorption Isotherm to the analysis of experimental surface-tension data for ionic and nonionic surfactants. Adv. Colloid Interface Sci. 2017, 247, 178–184. [Google Scholar] [CrossRef]
- Zhang, N.; Nguyen, A.V.; Zhou, C. A review of the surface features and properties, surfactant adsorption and floatability of four key minerals of diasporic bauxite resources. Adv. Colloid Interface Sci. 2018, 254, 56–75. [Google Scholar] [CrossRef]
- Tan, L.; Yu, C.; Wang, M.; Zhang, S.; Sun, J.; Dong, S.; Sun, J. Synergistic effect of adsorption and photocatalysis of 3D g-C3N4-agar hybrid aerogels. Appl. Surf. Sci. 2019, 467, 286–292. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; Chemical Industry Press: Beijing, China, 2021. [Google Scholar]
- Zhang, G.; Zhu, N.; Zhu, X. Influence of Polycarboxylate Dispersants with Different Molecular Structures on the Performance of Coal Water Slurry. J. Dispers. Sci. Technol. 2016, 37, 1799–1805. [Google Scholar] [CrossRef]
- Peng, X.; Li, X.; Li, Z. Evaluation of the Dispersing Properties of Polycarboxylate-Type Superplasticizers with Different Molecular Weight in Cement Systems. J. Dispers. Sci. Technol. 2013, 34, 1265–1272. [Google Scholar] [CrossRef]



| HEMA:SSS | APEG:SSS | Initiator/% | Temp/°C | Time/h | |
|---|---|---|---|---|---|
| 1 | 3.2 | 1.2 | 4 | 70 | 2 |
| 2 | 6.3 | 1.0 | 8 | 80 | 3 |
| 3 | 9.5 | 0.8 | 10 | 90 | 4 |
| 4 | 12.7 | 0.6 | 12 | 100 | 5 |
| Factors | 1 | 2 | 3 | 4 | 5 | Flow Point |
|---|---|---|---|---|---|---|
| HEMA:SSS | APEG:SSS | Initiator | Temp | Time | ||
| 1 | 1 | 1 | 1 | 1 | 1 | 1.9510 |
| 2 | 1 | 2 | 2 | 2 | 2 | 2.1256 |
| 3 | 1 | 3 | 3 | 3 | 3 | 2.0836 |
| 4 | 1 | 4 | 4 | 4 | 4 | 1.9761 |
| 5 | 2 | 1 | 2 | 3 | 4 | 2.0732 |
| 6 | 2 | 2 | 1 | 4 | 3 | 2.1373 |
| 7 | 2 | 3 | 4 | 1 | 2 | 1.7619 |
| 8 | 2 | 4 | 3 | 2 | 1 | 1.9325 |
| 9 | 3 | 1 | 3 | 4 | 2 | 2.0212 |
| 10 | 3 | 2 | 4 | 3 | 1 | 1.5818 |
| 11 | 3 | 3 | 1 | 2 | 4 | 1.7815 |
| 12 | 3 | 4 | 2 | 1 | 3 | 1.5413 |
| 13 | 4 | 1 | 4 | 2 | 3 | 1.953 |
| 14 | 4 | 2 | 3 | 1 | 4 | 1.7843 |
| 15 | 4 | 3 | 2 | 4 | 1 | 2.1013 |
| 16 | 4 | 4 | 1 | 3 | 2 | 2.0932 |
| I | 2.034 | 2.000 | 1.991 | 1.760 | 1.892 | |
| II | 1.976 | 1.907 | 1.960 | 1.948 | 2.000 | |
| III | 1.731 | 1.932 | 1.955 | 1.958 | 1.929 | |
| IV | 1.983 | 1.886 | 1.818 | 2.059 | 1.904 | |
| R | 0.303 | 0.114 | 0.173 | 0.299 | 0.108 |
| Retention Time (min) | Mn (g/mol) | Mw (g/mol) | Mz (g/mol) | Mz+1 (g/mol) | Polydispersity Index |
|---|---|---|---|---|---|
| 14.12 | 10617 | 13404 | 13982 | 15277 | 1.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, K.; Huang, Y.; Yu, X.; Wang, D. Synthesis of a Ternary Polysulfonate Dispersant and Its Suspension Performance. Processes 2022, 10, 195. https://doi.org/10.3390/pr10020195
Pei K, Huang Y, Yu X, Wang D. Synthesis of a Ternary Polysulfonate Dispersant and Its Suspension Performance. Processes. 2022; 10(2):195. https://doi.org/10.3390/pr10020195
Chicago/Turabian StylePei, Kemei, Yongjie Huang, Xiao Yu, and Dekai Wang. 2022. "Synthesis of a Ternary Polysulfonate Dispersant and Its Suspension Performance" Processes 10, no. 2: 195. https://doi.org/10.3390/pr10020195
APA StylePei, K., Huang, Y., Yu, X., & Wang, D. (2022). Synthesis of a Ternary Polysulfonate Dispersant and Its Suspension Performance. Processes, 10(2), 195. https://doi.org/10.3390/pr10020195





