Abstract
The rapid advancement of genetically modified (GM) technology over the years has raised concerns about the safety of GM crops and foods for human health and the environment. Gene flow from GM crops may be a threat to the environment. Therefore, it is critical to develop reliable, rapid, and low-cost technologies for detecting and monitoring the presence of GM crops and crop products. Here, we used visible near-infrared (Vis-NIR) spectroscopy to distinguish between GM and non-GM Brassica napus, B. juncea, and F1 hybrids (B. juncea X GM B. napus). The Vis-NIR spectra were preprocessed with different preprocessing methods, namely normalization, standard normal variate, and Savitzky–Golay. Both raw and preprocessed spectra were used in combination with eight different chemometric methods for the effective discrimination of GM and non-GM plants. The standard normal variate and support vector machine combination was determined to be the most accurate model in the discrimination of GM, non-GM, and hybrid plants among the many combinations (99.4%). The use of deep learning in combination with Savitzky–Golay resulted in 99.1% classification accuracy. According to the findings, it is concluded that handheld Vis-NIR spectroscopy combined with chemometric analyses could be used to distinguish between GM and non-GM B. napus, B. juncea, and F1 hybrids.
1. Introduction
Brassica juncea L. Czern (Brown Mustard) is an important annual crop and is an outcome of hybridization between the diploid Brassica species B. rapa (AA, 2n = 20) and B. nigra (BB, 2n = 16) followed by spontaneous hybridization with chromosome doubling [1]. In China and Korea, wild B. juncea is a natural weedy species widely found along roadsides or empty lands [2,3]. It is known to have the highest potential for gene transfer from B. napus after B. rapa [4]. It has previously been reported that conventional and transgenic B. napus hybridize with B. juncea spontaneously or by hand pollination [5,6,7,8]. Recently, Tang et al. [9] found the estimated frequencies of natural gene flow from the genetically modified (GM) B. napus to 10 different B. juncea cultivars in the field experiment varied from 0.08 to 0.93%. The transgenic hybrids’ ability to persist is determined by their fitness as crop–wild hybrids [10]. Little is known about the fitness of the F1 hybrid between B. juncea and B. napus in the environment. According to Lim et al. [2], seeds from a hybrid of B. juncea and GM B. napus have shown an increase in dormancy and overwintering traits, suggesting that they could become soil seed banks. Seeds in such a seed bank can germinate again if they meet a favorable environment, leading to the formation of a feral population. As a result, the transgene may spread across the ecosystem. If the flowering period of B. juncea overlaps with that of B. napus, there is a possibility of forming hybrids with GM B. napus and releasing them into the environment. If GM B. napus and hybrids (B. juncea X GM B. napus) can be quickly identified and removed, it will be useful to avoid the unintentional environmental release of transgenes and promote the safe management of GM B. napus.
Various methods have been used to detect genetically modified organisms (GMOs), including enzyme-linked immunosorbent assays (ELISA), lateral flow strips, biosensors, Western blots, real-time PCR, qualitative polymerase chain reaction (qPCR), microarrays, electrophoresis, Southern blots, liquid chromatography, and gas chromatography [11]. Nowadays, spectroscopy is one of the rapid, accurate, and nondestructive methods for distinguishing between GM and non-GM crops that does not require complex sample processing [11]. Spectroscopy-based GMO identification is not to detect changes in DNA or single proteins but to detect unknown structural changes due to genotype alterations generated by the introduction of transgenes for specific traits [12]. Generally, a vast number of spectroscopy methods are available for detecting structural changes in different samples, including absorption spectroscopy, photoacoustic spectroscopy, light-induced thermoelastic spectroscopy, and photothermal spectroscopy [13,14,15]. Among them, near-infrared (NIR) spectroscopy working with the principle of absorption spectroscopy is the most common for the detection of GMOs [11]. NIR spectroscopy coupled with chemometric analyses was found to be effective in discriminating various types of GM and non-GM crops with very high accuracy [11,16,17]. To distinguish transgenic soybean oils from non-transgenic ones, Luna et al. [18] used NIR and support vector machine discriminant analysis (SVM-DA). Later, Garcia-Molina et al. [19] used NIR spectroscopy in combination with partial least square (PLS) analysis to successfully distinguish low gliadin wheat grain from non-transgenic wheat lines with 96% of classification accuracy. It has been shown that using spectroscopic and machine learning algorithms makes it possible to distinguish not only GM and non-GM plants, but also plant species [11,20] and even varieties [21]. However, there is no study that discriminates the GM and non-GM plants with their interspecific hybrids. Therefore, in this study, we used visible near-infrared (Vis-NIR) spectroscopy coupled with different preprocessing and machine learning methods for effective discrimination of B. juncea, GM B. napus, and their hybrids (B. juncea X GM B. napus).
2. Results and Discussion
2.1. Spectral Analysis and Preprocessing
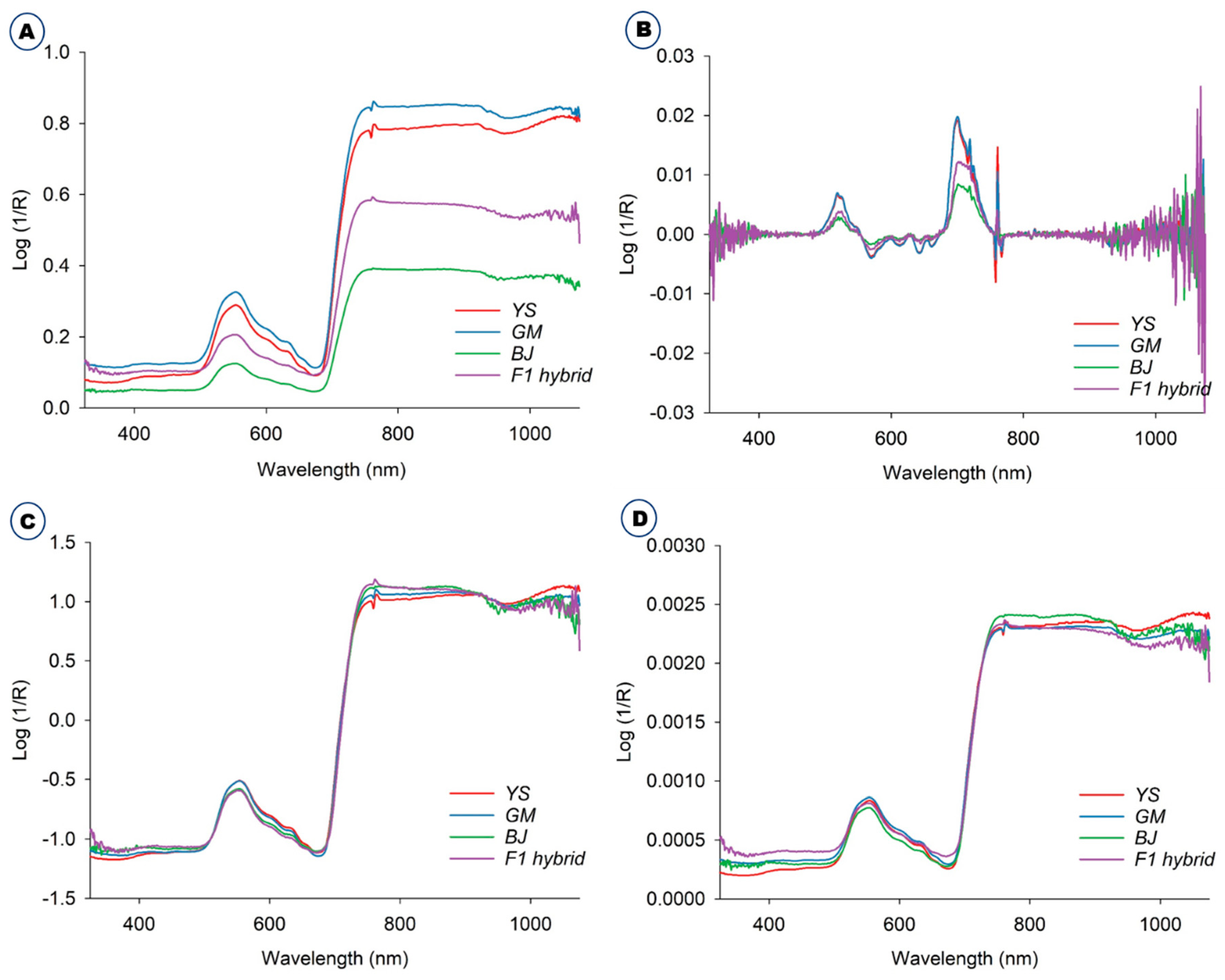
The averaged raw spectra of the B. napus, GM B. napus, B. juncea, and F1 hybrids collected in the green house are depicted in Figure 1A. The original unprocessed raw spectra were ones that had not been altered in any manner. The Savitzky–Golay preprocessed spectra are shown in Figure 1B. Standard normal variate (SNV) (Figure 1C) and normalization (Figure 1D) procedures were used to preprocess the spectra acquired from these plants. The spectra were preprocessed to remove systemic noise and highlight the variations across the samples [17]. The majority of the spectra acquired from four plants followed a similar pattern, despite variances in spectral reflectance. The difference in average reflectance between GM and non-GM B. napus, B. rapa, and F1 hybrids is thought to reflect the changes in hundreds of physicochemical constituents in the plant leaves. In general, NIR spectra disclose the information about a material’s chemical composition and physical state. This provides structural data on the chemical functional groups of the elements that constitute the molecular fingerprints of the sample [22,23]. The spectral data were preprocessed to remove systemic noise and emphasize variations across samples. Using a variety of preprocessing methods at the same time will help us achieve a higher level of classification accuracy and provide us with the opportunity to choose the optimal preprocessing method for a specific sample [11]. The average spectra for all the plants, raw and preprocessed, were effectively visualized using three different methods: the Savitzky–Golay smoothing filter (21 points), normalization, and standard normal variate (Figure 1). In general, the normalization is the process of regularizing the data with respect to variations in sample preparation, sample thickness, absorber concentration, etc. Derivatives are mainly used to resolve peak overlap and eliminate constant and linear baseline shifts between samples. SNV is often used on spectra where baseline and path length changes cause differences between otherwise identical spectra [11,24].
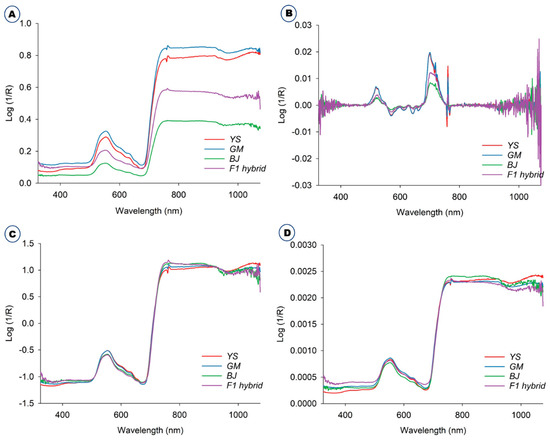
Figure 1.
Average raw and preprocessed spectra of B. napus (YS), GM B. napus (GM), B. juncea (BJ), and F1 hybrids (B. juncea X GM B. napus). (A) Average raw and preprocessed spectra with different methods, including (B) Savitzky–Golay, (C) standard normal variate, and (D) normalization.
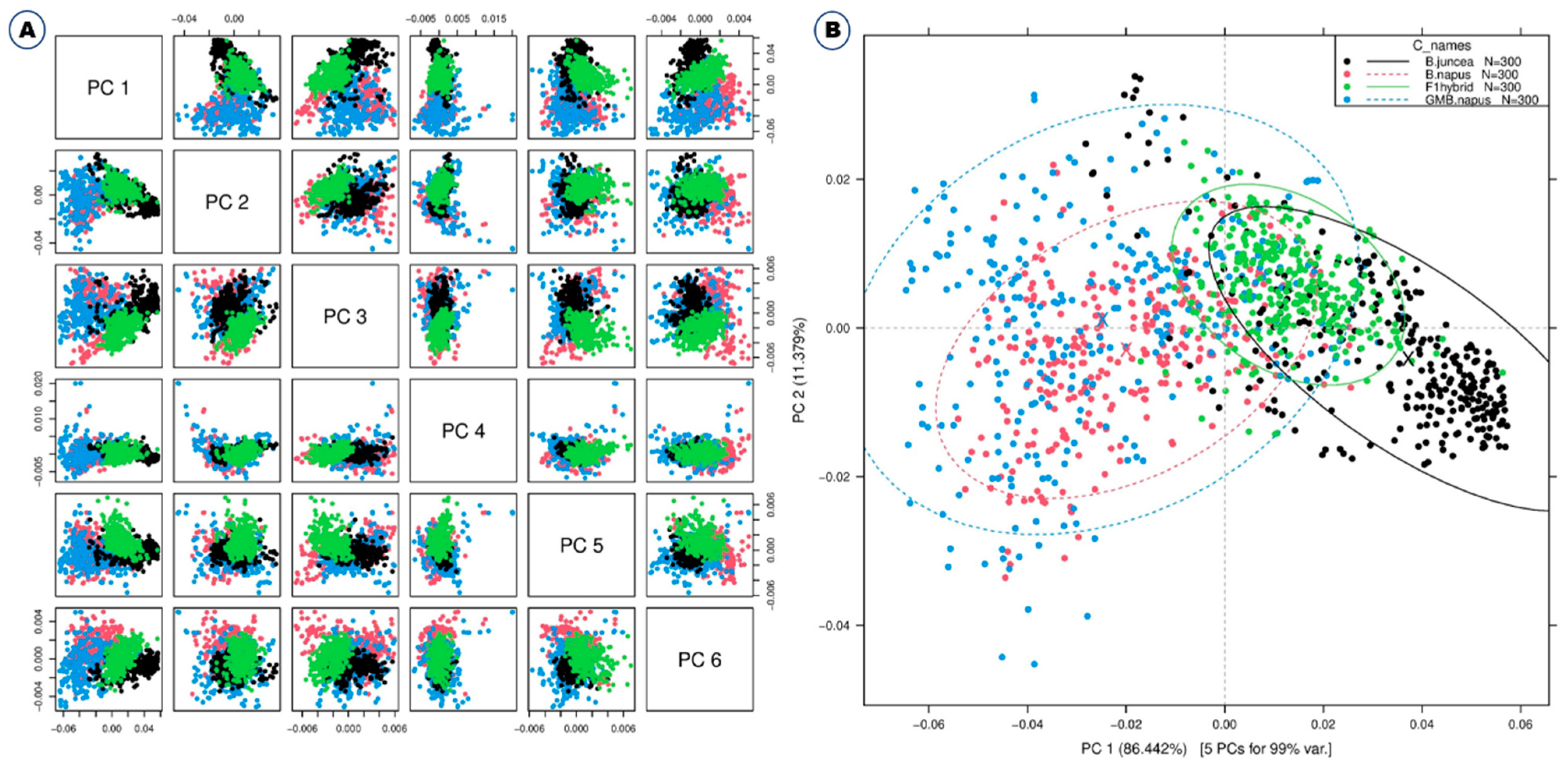
Some typical peaks can be seen in this figure, especially around 500–600 nm, which is the spectral range for chlorophyll [25], and also around 800 nm. However, it is difficult to differentiate these samples solely on the basis of spectral reflectance. Thus, Vis-NIR spectroscopy coupled with various models and machine learning methods such as discriminant analysis and principal component analysis (PCA) was used for effective discrimination [11]. All of the different PCs showed the same slight pattern of separation for the different samples in the PCA paired plot from PC1 to PC6 (Figure 2A), but PC1 vs. PC2 showed the most visual differences, as shown in Figure 2B, so outlier detection was performed using these two PCs before starting preprocessing for the machine learning methods.
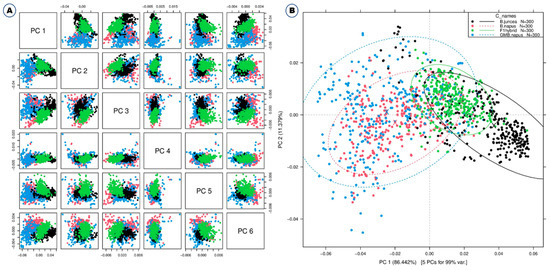
Figure 2.
Principal component analyses based on the Vis-NIR spectra of B. napus, GM B. napus, B. juncea, and F1 hybrids. Raw spectra have been used. (A) Paired blot; (B) axes are first and second principal components.
2.2. Chemometric Analysis for Discrimination of B. napus, GM B. napus, B. juncea, and F1 Hybrids
The classification accuracy of different chemometric methods combined with various preprocessing methods was calculated in order to determine the most exact way of distinguishing between GM and non-GM B. napus, B. juncea, and F1 hybrids. A summary of the classification accuracy for the different methods can be found in Table 1. Both original raw spectra and preprocessed spectra assessed with chemometric analyses resulted in effective discrimination with different classification accuracies. However, preprocessed spectra were found to have comparatively higher classification accuracy than raw spectra in most of the chemometric analyses. The classification accuracies of the different methods generally ranged from 62.6 to 99.4% (Table 1).

Table 1.
Classification accuracy of the combinations of preprocessing and model for reflectance spectra from B. napus, GM B. napus, B. juncea, and F1 hybrids.
From Table 1, the Savitzky–Golay pretreatment proved to be the most efficient preprocessing method for classifying the different plant species with all the tested classification methods except for the support vector machine (SVM) classification technique, where SNV proved to be more effective. Using the Savitzky–Golay, classification accuracies were always higher than when only raw spectra were used. Classification accuracies for the Savitzky–Golay ranged from 80.1 to 99.1%.
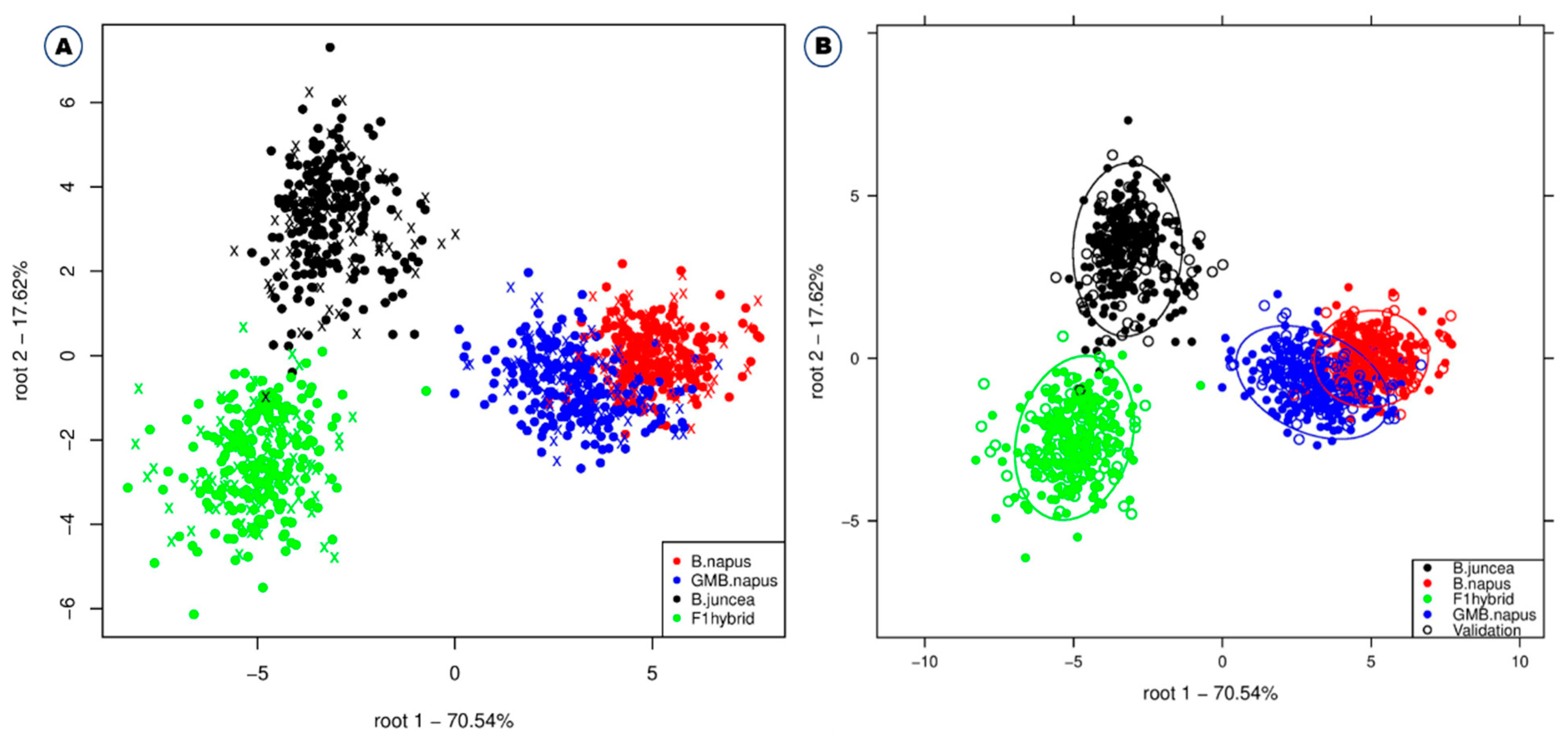
Among the different classification methods, support vector machine, linear discriminant analysis, deep learning, and fast large margin were found to have higher classification accuracies in combination with different preprocessing methods (SNV/SVM, 99.4%; Savitzky–Golay/Deep Learning, 99.1%; Savitzky–Golay/SVM, 98.8%) (Table 1). The support vector machine model showed a high accuracy of 97.1% even when using the raw spectrum without preprocessing the data. The support vector machine is especially suitable for high-dimensional data, and the value of each attribute has no limit [26]. When comparing the average value of accuracy according to each model application for each of the four preprocessing methods, Savitzky–Golay showed the highest accuracy, followed by standard normal variate, raw spectrum, and normalization (Table 1). Similar studies have already been performed by various researchers on various crops. Feng et al. [17] used NIR in combination with support vector machine and partial least squares discriminant analysis (PLS–DA) for the effective discrimination of GM and non-GM maize. Similarly, VNIR multispectral imaging and PLS–DA were used for discrimination of GM and non-GM rice using least squares support vector machines (LS-SVM) and PCA backpropagation neural network (PCA-BPNN) [27]; Fourier transform Infrared (FT-IR) was also used for discrimination of GM and non-GM soybeans with Kth nearest neighbors (KNN) [28]. NIR and support vector machine discriminant analysis (SVM-DA) and PLS–DA were used for discrimination of GM and non-GM soybean [18], and NIR and PLS–DA were used for identification of herbicide-resistant GM soybean seeds [16]. The use of Vis-NIR for discrimination of transgenic tomato using DA and PLS–DA [23] and the use of Vis-NIR for discrimination of RNAi transgenic wheat using NIR and PLS [19] are examples of effective discrimination of GM and non-GM crops using spectroscopy and chemometric analyses. Linear discriminant analysis also yields higher accuracy of 96.5% even when no preprocessing is performed. Figure 3 also shows the linear discriminant analysis plot for discriminating the four different plant varieties. GM B. napus slightly overlapped with B. napus, but B. juncea and F1 hybrids were completely separated from each other and all the other plant varieties. This suggests that GM B. napus and non-GM B. napus may share similar biological composition compared to B. juncea and F1 hybrids. Similar studies also reported higher classification accuracy using NIR spectroscopy and linear discriminant analysis to monitor mung bean sprouts [29], classify different melon varieties [30], and detect pea protein powder containing adulterants [31].
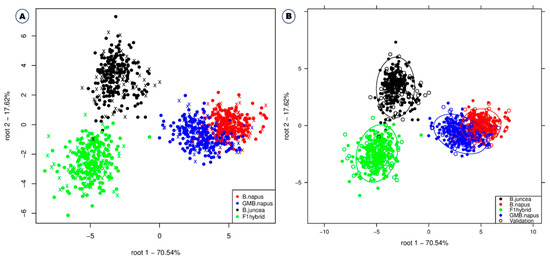
Figure 3.
Linear discriminant analysis for the effective discrimination of B. napus, GM B. napus, B. juncea, and F1 hybrids without confidence circles (A) and with confidence circles (B).
2.3. Significance of Preprocessing and Selection of Optimal Classification Model
The efficiency of preprocessing and machine learning methods used in the study was statistically analyzed (Table 2). After cross-validation, the mean percentage of classification accuracy of each chemometric method combined with various preprocessing methods indicated significant modeling for the discrimination of GM and non-GM B. napus, B. juncea, and F1 hybrids (Table 2).

Table 2.
Means of percentage of B. napus, GM B. napus, B. juncea, and F1 hybrids correctly classified using four different preprocessing methods and four different classification models using reflectance spectra.
The statistical analysis with analysis of variance (ANOVA) (Table 3) showed the sum of square and mean sum of square values of various preprocessing and machine learning approaches used with statistical significance at p ≤ 0.005. However, there was no significance with p ≥ 0.005 when using a combination of preprocessing and different machine learning methods together (p value of 0.0005).

Table 3.
Analysis of variance of percentage of correctly classified B. napus, GM B. napus, B. juncea, and F1 hybrids from four different preprocessing methods and four different classification models using reflectance spectra.
The confusion matrix depicts the degree of error in the classification of the evaluated plants, indicating that Savitzky–Golay smoothing in combination with support vector machine was the most effective classification approach (Table 4).

Table 4.
Confusion matrix from the execution with the best accuracy (Savitzky–Golay and support vector machine).
3. Materials and Methods
3.1. Plant Materials
The seeds used in the study, namely B. napus L. ‘Youngsan’ and B. juncea var. integrifolia and GM B. napus seeds with CAMV 35S-regulated bar and early flowering gene (BrAGL20), were procured from the National Agrobiodiversity Center, Jeonju, Republic of Korea. For the hybrid preparation, artificial hand pollination was performed with B. juncea and GM B. napus, and the seeds of F1 hybrids (B. juncea X GM B. napus) were used for further research. The hybrids were confirmed through the survival assay after 0.3% Basta treatment; the phenotype of the hybrids; and polymerase chain reaction with 35S ribosomal DNA, BrAGL20 gene partial region, bar gene, and chloroplast marker. All of the seeds were grown in soil pots (Figure 4) and kept in a controlled environment. This research was carried out in the greenhouse of the National Institute of Agricultural Sciences, Jeonju, Republic of Korea, during May–July 2019.

Figure 4.
Representative figures for the plants selected for the spectral analysis. (A) B. napus; (B) GM B. napus; (C) B. juncea; (D) F1 hybrids.
3.2. Spectral Data Collection
A handheld integrated portable spectrum analyzer (FieldSpec HandHeld 2, ASD Inc., Longmont, CO, USA) was used to collect Vis-NIR diffuse reflectance spectra in the range of 325–1075 nm with a stepping of 1.5 nm in reflectance mode (log/R). The spectra were collected on the adaxial surface of the fully expanded leaves, which can easily capture light. Three spectra were obtained from various parts of the leaf blade of 100 plants in each group. A total of 300 (3 × 100 = 300) spectra were collected from each group and used for further analysis. To avoid unnecessary noise, the optical window of the Vis-NIR device was placed in direct contact with the leaf’s surface throughout each spectrum capture, ensuring that the sensor window was completely covered [32,33].
3.3. Preprocessing and Machine Learning Methods
Due to system parameters and environmental noise, background signals appeared in the raw spectra of samples. Different preprocessing methods, such as raw spectra assessment, normalization (area), standard normal variate (SNV), and derivatives (Savitzky–Golay (first differentiation)) were used, which can reduce the spectral noise and improve the accuracy of modeling approaches. The computations on preprocessing were performed with Unscrambler X software, version 10.5.1 (CAMO ASA, Oslo, Norway).
For the effective visualization and discrimination of spectral data, several machine learning methods were used and compared. The modeling was performed with the software package RapidMiner studios Version 9.0.002 (Rapidminer, Inc., Boston, MA, USA). In the study, eight classification methods were used to find the best modeling approach with the highest classification accuracy, namely deep learning, decision tree, support vector machine, random forest, generalized linear model, fast large margin, naive Bayes, and linear discriminant analysis. Linear discriminant analysis was performed in R-studio using the Aquap2 package developed by Kovacs and Pollner [34]. For each of the algorithms, the inputs were provided as the data points of the spectra and the classes were the identification labels of B. napus, GM B. napus, B. juncea, and F1 hybrids (B. juncea X GM B. napus). Cross-validation was performed to assess the robustness of the models in predicting the different sample types. For this, the data were divided into a training set and a validation set. The training set was made up of two-thirds of the data; thus, the spectra from the first and second replicates of each sample were included, while the validation set was made up of spectra from the third replicate. The data splitting was done three times, such that each sample was used at least once in the calibration and validation set. The classification results are displayed as score plots or confusion matrix, which illustrates the percentages of classification accuracy. One-way analysis of variance (ANOVA) was used to compare means for determining the influence of (1) the scatter correction method, (2) the eight machine learning methods, and (3) the interaction of preprocessing and machine learning methods. As a mean comparison method, Tukey’s range test was used at a significance level of p ≤ 0.05.
4. Conclusions
In conclusion, Vis-NIR spectroscopy in combination with machine learning methods could effectively discriminate between GM and non-GM B. napus, B. juncea, and the F1 hybrids (B. juncea X GM B. napus). The utilization of Vis-NIR spectroscopy and chemometric analyses for the discrimination of GM and non-GM crops is quick and accurate. It can also deliver information for monitoring and safety management of agro-food market products in which GMOs are introduced. Among the different combinations of preprocessing and machine learning methods, the combination of standard normal variate and support vector machine was found to be the most effective method, with 99.6% classification accuracy, but Savitzky–Golay smoothing also yields good classification accuracy when other classification methods are used. Thus, it is proposed that this nondestructive method be employed in the field for the rapid detection and management of unintended releases of GM Brassicaceae crops into the environment. It is suggested to create a database with broad-spectrum results on GM and non-GM Brassicaceae crops for the effective utilization of the technology in the field.
Author Contributions
Conceptualization: S.-I.S., Y.-J.O. and C.-S.N.; methodology: S.-I.S., S.P., Y.-J.O., Y.-H.L., E.-K.S., H.-J.K., T.-H.R. and Y.-S.C.; formal analysis: S.-I.S., S.P., Y.-J.O. and W.-S.C.; data curation: S.-I.S., Y.-J.O. and C.-S.N.; writing—original draft preparation: S.-I.S., S.P. and J.-L.Z.Z.; visualization: S.P. and J.-L.Z.Z.; project administration: S.-I.S.; funding acquisition: S.-I.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out with the support of “Research Program for Agricultural Science & Technology Development and 2022 Post-doctoral Fellowship Program (Project No. PJ014943012022)”, National Institute of Agricultural Sciences, Rural Development Administration, Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, J.; Liu, D.; Wang, X.; Ji, C.; Cheng, F.; Liu, B.; Hu, Z.; Chen, S.; Pental, D.; Ju, Y.; et al. The genome sequence of allopolyploid Brassica juncea and analysis of differential homoeolog gene expression influencing selection. Nat. Genet. 2016, 48, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Yook, M.J.; Zhang, C.J.; Nah, G.; Park, S.; Kim, D.S. Dormancy associated weedy risk of the F1 hybrid resulted from gene flow from oilseed rape to mustard. Weed Turfgrass Sci. 2015, 4, 35–43. [Google Scholar] [CrossRef]
- Zhang, C.J.; Yook, M.J.; Park, H.R.; Lim, S.H.; Kim, J.W.; Song, J.S.; Nah, G.; Song, H.R.; Jo, B.H.; Roh, K.H.; et al. Evaluation of maximum potential gene flow from herbicide resistant Brassica napus to its male sterile relatives under open and wind pollination conditions. Sci. Total Environ. 2018, 634, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Devos, Y.; De Schrijver, A.; Reheul, D. Quantifying the introgressive hybridisation propensity between transgenic oilseed rape and its wild/weedy relatives. Environ. Monit. Assess. 2009, 149, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, J.A.; Dale, P.J. Opportunities for gene transfer from transgenic oilseed rape (Brassica napus) to related species. Transgenic Res. 1994, 3, 263–278. [Google Scholar] [CrossRef]
- Song, X.L.; Huangfu, C.H.; Qiang, S. Gene flow from transgenic glufosinate-or glyphosate-tolerant oilseed rape to wild rape. Chin. J. Plant Ecol. 2007, 31, 729–737. [Google Scholar]
- Cao, D.; Stewart Jr, C.N.; Zheng, M.; Guan, Z.; Tang, Z.X.; Wei, W.; Ma, K.P. Stable Bacillus thuringiensis transgene introgression from Brassica napus to wild mustard B. juncea. Plant Sci. 2014, 227, 45–50. [Google Scholar] [CrossRef]
- Liu, Y.; Neal Stewart Jr, C.; Li, J.; Wei, W. One species to another: Sympatric Bt transgene gene flow from Brassica napus alters the reproductive strategy of wild relative Brassica juncea under herbivore treatment. Ann. Bot. 2018, 122, 617–625. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.; Chen, G.; Bu, C.; Liu, F.; Liu, L.; Zhao, X. Transgene introgression from Brassica napus to different varieties of Brassica juncea. Plant Breed. 2018, 137, 171–180. [Google Scholar] [CrossRef]
- Di, K.; Stewart Jr, C.N.; Wei, W.; Shen, B.C.; Tang, Z.X.; Ma, K.P. Fitness and maternal effects in hybrids formed between transgenic oilseed rape (Brassica napus L.) and wild brown mustard [B. juncea (L.) Czern et Coss.] in the field. Pest Manag. Sci. 2009, 65, 753–760. [Google Scholar] [CrossRef]
- Sohn, S.-I.; Pandian, S.; Oh, Y.-J.; Zaukuu, J.-L.Z.; Kang, H.-J.; Ryu, T.-H.; Cho, W.-S.; Cho, Y.-S.; Shin, E.-K.; Cho, B.-K. An Overview of Near Infrared Spectroscopy and Its Applications in the Detection of Genetically Modified Organisms. Int. J. Mol. Sci. 2021, 22, 9940. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.E. Detection of genetically modified organisms in foods. Trends Biotechnol. 2002, 20, 215–223. [Google Scholar] [CrossRef]
- Ma, Y.; Lewicki, R.; Razeghi, M.; Tittel, F.K. QEPAS based ppb-level detection of CO and N2O using a high power CW DFB-QCL. Opt. Express 2013, 21, 1008–1019. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; He, Y.; Tong, Y.; Yu, X.; Tittel, F.K. Quartz-tuning-fork enhanced photothermal spectroscopy for ultra-high sensitive trace gas detection. Opt. Express 2018, 26, 32103–32110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, S.; Ma, Y.; He, Y.; Patimisco, P.; Sampaolo, A.; Spagnolo, V. Ppt level carbon monoxide detection based on light-induced thermoelastic spectroscopy exploring custom quartz tuning forks and a mid-infrared QCL. Opt. Express 2021, 9, 25100–25108. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choung, M.G. Nondestructive determination of herbicide-resistant genetically modified soybean seeds using near-infrared reflectance spectroscopy. Food Chem. 2011, 126, 368–373. [Google Scholar] [CrossRef]
- Feng, X.; Peng, C.; Chen, Y.; Liu, X.; Feng, X.; He, Y. Discrimination of CRISPR/Cas9-induced mutants of rice seeds using near-infrared hyperspectral imaging. Sci. Rep. 2017, 7, 15934. [Google Scholar] [CrossRef]
- Luna, A.S.; da Silva, A.P.; Pinho, J.S.; Ferré, J.; Boqué, R. Rapid characterization of transgenic and non-transgenic soybean oils by chemometric methods using NIR spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 100, 115–119. [Google Scholar] [CrossRef]
- Garcia-Molina, M.D.; Garcia-Olmo, J.; Barro, F. Effective identification of low-gliadin wheat lines by near infrared spectroscopy (NIRS): Implications for the development and analysis of foodstuffs suitable for celiac patients. PLoS ONE 2016, 11, e0152292. [Google Scholar] [CrossRef]
- Li, T.; Su, C. Authenticity identification and classification of Rhodiola species in traditional Tibetan medicine based on Fourier transform near-infrared spectroscopy and chemometrics analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 204, 131–140. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Morais, C.L.; Lima, K.M.; Leite, G.W.; Oliveira, G.S.; Casagrande, I.P.; Neto, J.P.S.; Teixeira, G.H. Using intact nuts and near infrared spectroscopy to classify Macadamia cultivars. Food Anal. Met. 2018, 11, 1857–1866. [Google Scholar] [CrossRef] [Green Version]
- Cordella, C.; Moussa, I.; Martel, A.C.; Sbirrazzuoli, N.; Lizzani-Cuvelier, L. Recent developments in food characterization and adulteration detection: Technique-oriented perspectives. J. Agric. Food Chem. 2002, 50, 1751–1764. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Ying, Y.; Ying, T.; Yu, H.; Fu, X. Discrimination of transgenic tomatoes based on visible/near-infrared spectra. Anal. Chim. Acta 2007, 584, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Rinnan, Å.; Van Den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Gaye, B.; Zhang, D.; Wulamu, A. Improvement of support vector machine algorithm in big data background. Mat. Prob. Eng. 2021, 2021, 5594899. [Google Scholar] [CrossRef]
- Liu, C.; Liu, W.; Lu, X.; Chen, W.; Yang, J.; Zheng, L. Nondestructive determination of transgenic Bacillus thuringiensis rice seeds (Oryza sativa L.) using multispectral imaging and chemometric methods. Food Chem. 2014, 153, 87–93. [Google Scholar] [CrossRef]
- Alcantara, G.B.; Barison, A.; Santos, M.D.S.; Santos, L.P.; de Toledo, J.F.; Ferreira, A.G. Assessment of genetically modified soybean crops and different cultivars by Fourier transform infrared spectroscopy and chemometric analysis. Orbital Electr. J. Chem. 2010, 2, 41–52. [Google Scholar]
- Nugraha, D.T.; Zaukuu, J.L.Z.; Bósquez, J.P.A.; Bodor, Z.; Vitalis, F.; Kovacs, Z. Near-infrared spectroscopy and aquaphotomics for monitoring mung bean (Vigna radiata) sprout growth and validation of ascorbic acid content. Sensors 2021, 21, 611. [Google Scholar] [CrossRef]
- Zaukuu, J.L.Z.; Gillay, Z.; Kovacs, Z. Standardized extraction techniques for meat analysis with the electronic tongue: A case study of poultry and red meat adulteration. Sensors 2021, 21, 481. [Google Scholar] [CrossRef]
- Zaukuu, J.L.Z.; Aouadi, B.; Lukács, M.; Bodor, Z.; Vitális, F.; Gillay, B.; Gillay, Z.; Friedrich, L.; Kovacs, Z. Detecting low concentrations of nitrogen-based adulterants in whey protein powder using benchtop and handheld NIR spectrometers and the feasibility of scanning through plastic bag. Molecules 2020, 25, 2522. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.I.; Oh, Y.J.; Pandian, S.; Lee, Y.H.; Zaukuu, J.L.Z.; Kang, H.J.; Ryu, T.H.; Cho, W.S.; Cho, Y.S.; Shin, E.K. Identification of Amaranthus Species using Visible-Near-Infrared (Vis-NIR) spectroscopy and machine learning methods. Remote Sens. 2021, 13, 4149. [Google Scholar] [CrossRef]
- Sohn, S.I.; Pandian, S.; Zaukuu, J.L.Z.; Oh, Y.J.; Park, S.-Y.; Na, C.S.; Shin, E.K.; Kang, H.J.; Ryu, T.H.; Cho, W.S.; et al. Discrimination of transgenic canola (Brassica napus L.) and their hybrids with B. rapa using Vis-NIR spectroscopy and machine learning methods. Int. J. Mol. Sci. 2022, 23, 220. [Google Scholar] [CrossRef] [PubMed]
- Pollner, B.; Kovacs, Z. Dedicated Aquaphotomics-Software R-Package „aquap2“General Introduction and Workshop. Aquaphotomics: Understanding Water in the Biological World. In Proceedings of the 5th Kobe University Brussels European Centre Symposium Innovation, Environment and Globalization—Latest EU-Japan Research Collaboration, Bruxelles, Belgium, 14 October 2014. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).