Abstract
Beer production has over a thousand-year tradition, but its development in the present continues with the introduction of new technological and technical solutions. The methods for modeling and optimization in beer production through an applied analytical approach have been discussed in the present paper. For this purpose, the parameters that are essential for the main processes in beer production have been considered—development of malt blends, guaranteeing the main brewing characteristics; obtaining wort through the processes of mashing, lautering and boiling of wort; fermentation and maturation of beer. Data on the mathematical dependences used to describe the different stages of beer production (one-factor experiments, modeling of mixtures, experiment planning, description of the kinetics of microbial growth, etc.) and their limits have been presented, and specific research results of various authors teams working in this field have been cited. The independent variables as well as the objective functions for each stage have been defined. Some new trends in the field of beer production have been considered and possible approaches for their modeling and optimization have been highlighted. The paper suggests a generalized approach to describe the main methods of modeling and optimization, which does not depend on the beer type produced. The proposed approaches can be used to model and optimize the production of different beer types, and the conditions for their application should be consistent with the technological regimes used in each case. The approaches for modeling and optimization of the individual processes have been supported by mathematical dependencies most typical for these stages. Depending on the specific regimes and objectives of the study, these dependencies can be adapted and/or combined into more general mathematical models. Some new trends in the field of beer production have been considered and possible approaches for their modeling and optimization have been highlighted.
1. Introduction
Beer production dates back centuries and although its technology has been known for many years, there are still a number of tasks that can be performed in scientific and practical terms [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. In recent years, the beverage market has seen a boom in craft beer production. Craft breweries provide the production of a number of new assortments of beer, while large brewers rely on established beer brands and styles. In addition, the tendency is for the consumer to be offered a set of beverage indicators, which, in addition to quenching the thirst, also offer a certain set of functional benefits for human health. This in turn leads to the desire of producers to optimize the brewing process in such a way as to ensure the quality of the beverage in every aspect [15,16,17,18,19,20,21,22].
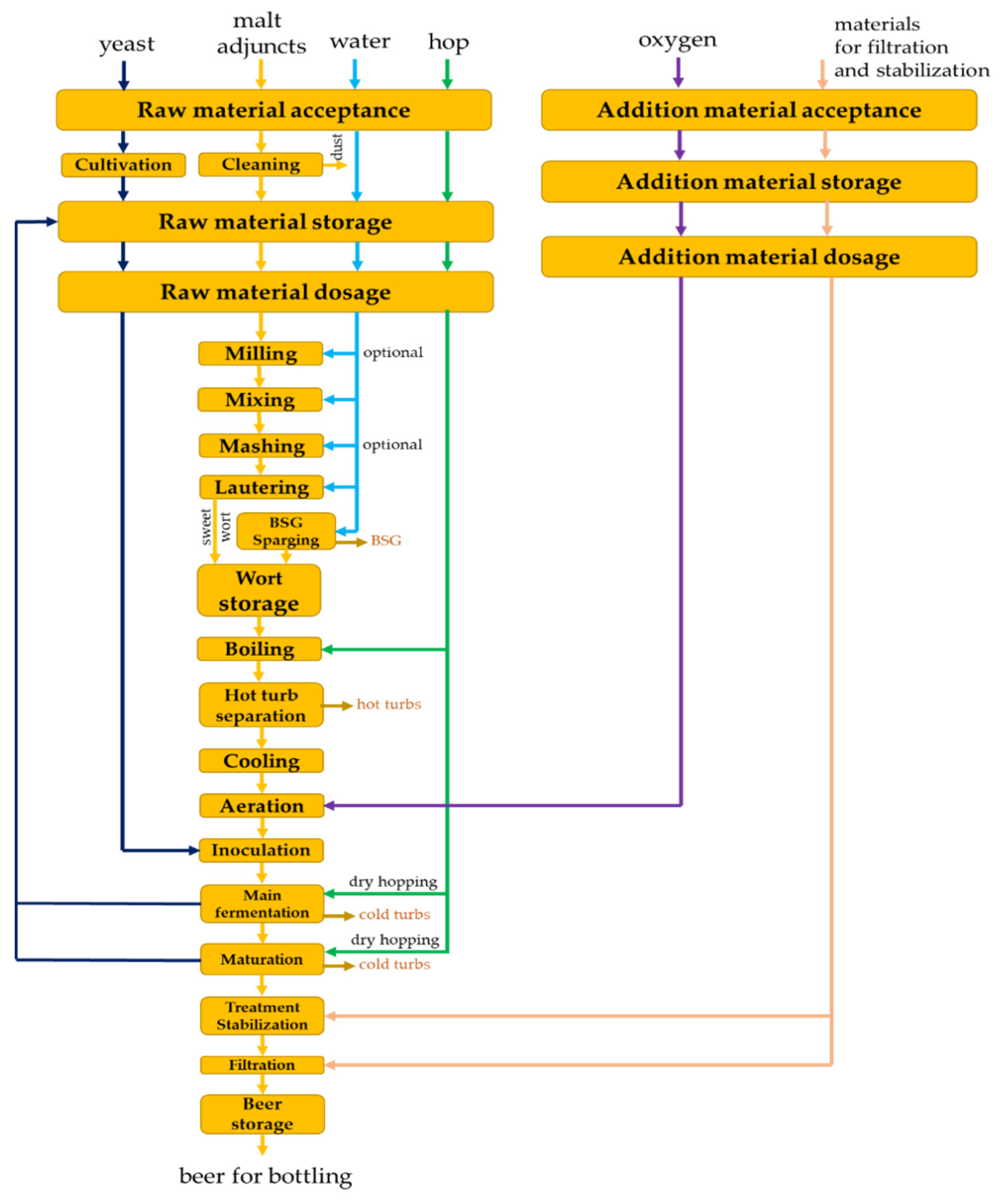
Beer production is a complex technological process that combines physical, chemical and biological (microbiological and biochemical) processes. The appropriate choice of parameters at each stage of production guarantees the required quality and the possibility to manage the process in the most correct way. The set of processes itself can be modeled and optimized through different approaches, which in turn leads to difficulties in decision making [23,24]. Brewing can be divided into three major technological stages: wort obtaining; alcoholic fermentation and maturation; stabilization and processing of the beer. Each of these stages contains a number of technological operations, often with intertwining parameters that affect to varying degrees the quality of the finished drink. An operational scheme for the production of beer is presented in Figure 1, and the main parameters that have been subjected to modeling, optimization, control and management are given in Table 1.
Figure 1.
Basic flow chart for beer production. The presented scheme is author’s and has been developed according to the general rules of beer production, described in [1,2,3,23].

Table 1.
Parameters for modeling and optimization of some basic processes in beer production.
The purpose of the present paper is to present some of the most important methods for mathematical modeling and optimization of some of the most important processes in beer production through the so-called generalized approach. This approach will enable researchers in the field of beer and wort-based beverage production to identify the most important models they can apply in the process of developing new technologies. We believe that this publication will contribute to a better understanding of the need to apply a scientific approach to the development of new beer assortments, especially with the entry of craft breweries on the global beer market.
We also hope to fill in some gaps in the modeling and optimization of the beer production process that are found in publications examining this process. Very often these gaps are related to the application of only one method of modeling and optimization, without looking for opportunities to combine approaches in order to better understand the research process. Last but not least, it should be noted that this approach allows the models to be applied to a wide variety of brewing assortments, as it is known that there are different beer styles worldwide.
Some new trends in the modeling and optimization of beer production processes have been considered at the end of the paper. They are related to the processes of dry hopping and the modeling of the fermentation process with analytical dependences. Although these processes are well known, there is still a lack of solid scientific basis on which to make relevant discussions and summaries. Some possibilities for obtaining drinks with a functional effect have also been commented. Such research is increasingly beginning to appear in the scientific literature, but not enough experience has yet been gained to be the subject of analysis and inclusion in the discussion of the current research in this field.
2. Modeling, Optimization and Control of Processes in Obtaining Wort
2.1. Modeling of the Malt Mixture
The production of different types of beer and wort-based beverages is based on a combination of different malt types. From a practical point of view, malts can be divided into three main groups—basic malts, special malts and functional malts.
The basic malt types are those malt types that provide the main amount of extract in the wort. They are characterized by significant starch content, which provides high yield of extract and fermentable sugars in the wort. This malt group usually includes light malt types, as well as some malts with a higher degree of kilning. Special malts are malts that provide certain color, taste and aroma in the mixture, but do not contribute significantly to the amount of wort extract. These malts have usually undergone special germination and a higher degree of heat treatment. In many cases, they undergo additional roasting. Functional malt types usually provide some function in the wort—pH adjustment, smoking of the wort and others [11].
The question of how to combine malts is crucial. Usually malts are combined based on the experience of the brewer or adjustments are made in already known malt blends. From a scientific point of view, however, the question of how to combine malts is a little different. One must approach it purely in practical terms, namely what beer one aims to obtain, on the one hand, but on the other hand—in purely scientific terms—what exact target functions for optimization to choose.
When modeling mixtures, the proportions of the various components in the mixture are chosen as independent factors and their influence on one or several target functions is studied. The total amount of all components in the mixture gives 100%, i.e., the whole amount of malt meal. In this case, various methods are possible to study the influence of the individual components in the mixture. Various parameters can be set as target functions at this point—extract yield, wort color, antioxidant capacity, content of phenolic compounds, diastatic strength and others [11]. It is important to note that the target function depends only on the proportion of the individual component in the mixture and not on the amount of the mixture. The amount of the mixture can be the subject of further research [25,26].
А. Simplex-Lattice designs [25,26].
In this modeling method, a lattice of the form {q, m} is constructed, where q is the number of components of the mixture, and the coordinates of the experimental points are defined as follows: the proportion of the individual component occupies m + 1 equal intervals from 0 to 1, i.e.,:
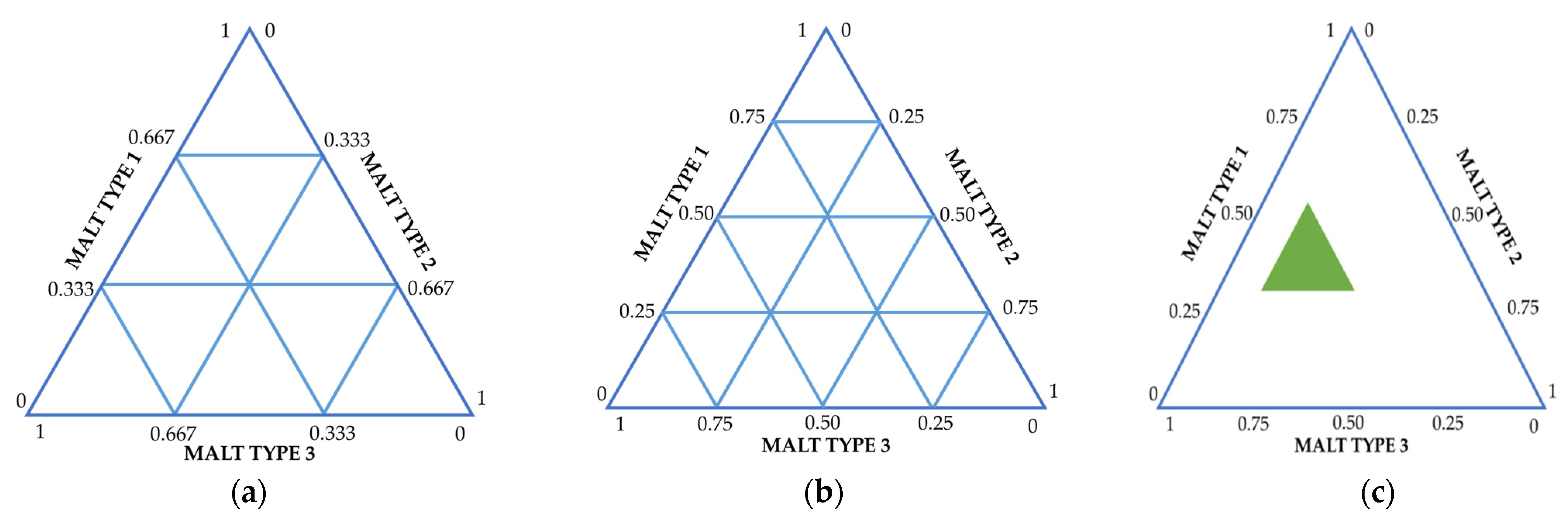
thus describing all possible combinations in the mixture. Through this plan, all points except the central one are located on the boundaries of the simplex. Figure 2a shows a schematic diagram of a simplex lattice for a three-component mixture.
xi = 0, 1/m, 2/m, …, 1 за i = 1, 2, 3, …, q
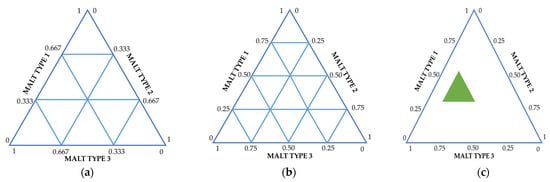
Figure 2.
Basic types of simplex plans for modeling mixtures. The presented schemes are adapted according to [25,26], taking into account the main characteristics of malt/unmalted raw material [11,24,27]. (a) {3,3} Simplex-Lattice designs, (b) {3,3} Simplex-Centroid designs, (c) Constrained mixture designs.
As a result of the implementation of the simplex-lattice with respect to the objective function, the so-called canonical equations, which have the following form, are obtained:
B. Simplex-Centroid designs [25,26].
In this method, a plan for the experiment with 2q − 1 number of samples, where q is the number of components of the mixture, is developed. The points in the plan correspond to q permutations (1, 0, 0,… 0) or q single components of the mixture, q/2 permutations in (0.5, 0.5, 0,…,) or all two-component mixtures, q/3 permutations (1/3, 1/3, 1/3, 0, …, 0) etc. to the end, depending on q. The implementation of the plan leads to obtaining a canonical equation of the type:
Figure 2b shows a schematic diagram of the simplex lattice for the centroid plan.
Both of the presented mixture modeling plans are constructed in a specific way so that the points are located along the simplex boundaries with the exception of the common centroid [25,26].
From the point of view of brewing technology, these two plans can be useful in the modeling of malt blends, as the number of components does not exceed 4 and for some of the components there are no restrictions on the proportions of the component. In general, the optimization of the malt mixture is not an easy task, as not only target functions with a clear numerical meaning (extract, phenolic content, antioxidant capacity, color, etc.) should be included, but also those that do not have a numerical expression. Such functions are the organoleptic description of the wort obtained from the mixture. They are usually the result of the processes selected for wort production and therefore it is difficult to influence them at this stage.
C. Constrained mixture designs [25,26].
In some cases, it is necessary to impose restrictions on the amount of a component. In this case, it is possible to use a plan of the experiment with the introduction of constraints (Figure 2c). These constraints can be in both the upper and lower limits of the component. Mathematically, this is expressed by the following form—Li ≤ xi ≤ Ui, i = 1, 2, …, q, where Li is the lower limit for the i-th component and Ui is the upper limit for the i-th component.
This general type of the equations allows the introduction of constraints from both the bottom and the top. In the general case, the constraint plan is again a simplex, so for easier work it is possible to introduce a new simplex with coordinates of the points, which are calculated according to the following rule:
This simplification allows the use of the already described two methods for modeling in confined space.
From the point of view of modeling the composition of the malt mixture, the constrained method is suitable for the use of some special malts, whose proportions in the mixture are relatively small (in this case we mean mainly roasted malts with color above 5000 EBC units). Usually these malts participate in the total blend by no more than 2–3% and aim to give some specific characteristics—color, taste and aroma. From this point of view, it is recommended that these malts do not participate in the formation of the mixture, and their influence to be studied by classical single-factor experiments after modeling the mixture by one of two main methods—Simplex-Centroid designs or Simplex-Lattice designs [11].
The constrained method and its modification (through so-called pseudo-components) can be used to model the participation of non-malted raw materials in the mixture. The use of non-malted raw materials leads to a reduction in the price of the final product and is rather typical for industrial beer production. However, craft breweries use a number of non-traditional raw materials that do not always undergo malting. Usually the amount of unmalted raw materials is limited by the available enzyme systems in malts and the ability to conduct quality and complete mashing process. However, Constrained mixture designs are suitable for modeling the content of unmalted raw materials as their amount can theoretically reach up to 50% of the total amount of raw material [28,29,30].
2.2. Modeling and Optimization of the Malt Milling Processes
When milling malt and unmalted raw materials, the components of the endosperm are released from the husk, favorable conditions for the mashing processes and obtaining the maximum extract amount are created [1,2,3]. The choice of method for milling malt and unmalted raw materials depends on the chosen method for lautering of the mash. In one case, when using classical lautering tun, roller mills are used, in which one of the purposes of milling is to preserve the integrity of the husk. In the other case, when using mash-filters, hammer mills are used, which allow fine milling of the raw material. Preserving the integrity of the husk has led to the development of four groups of milling methods—dry, wet, dry milling with conditioning and wet milling with conditioning [1,2,3,23].
Here the modeling and optimization of the process are related to the knowledge of the physical nature of the milling, on the one hand, and the biological nature of the mashing process that follows, on the other hand. Fine milling of the grain raw materials is necessary from the point of view of fast and complete extraction of the extractive substances. Fine milling is not always appropriate, given that malt grains are not homogeneous, but are composed of different parts that have different effects on the wort composition and properties. In practice, the so-called “optimal degree of milling” is used, which gives the ratio between the individual fractions of the malt meal. This degree is usually determined by empirical dependencies, as it depends on many non-numerical factors. Another important feature in the modeling of the milling process is the fact that different raw materials are ground to different degrees. In general, malted raw materials are ground “coarser”, while non-malted raw materials are ground “finer” due to the low amount of enzyme systems [1,23].
Another important condition for the milling process is the different physicochemical composition of the different parts of the grain. Usually the scales, due to the high content of phenolic compounds, should not be finely digested, while the endosperm has different structure and needs different treatment. All these differences define this rather empirical approach to modeling the milling process [1,3,23].
The mixing of malt meal with water and the subsequent saccharification of the malt mash are the main stage in which the qualitative and quantitative profile of the wort is formed. In these two stages, called mashing for short, enzymatic hydrolysis of biopolymers takes place, and its degree and the quality of the resulting wort are controlled by the so-called time-temperature pauses. As a result, the soluble substances present in the malt pass into the wort, and the available high molecular weight substances pass into soluble form and are dissolved in the wort mainly through enzymatic reactions [1,2,3,23].
2.3. Modeling and Optimization of the Mashing Process
The modeling process here begins with the choice of a mashing mode. The mashing methods are divided into three groups—infusion methods, decoction methods and combined methods. Usually the choice of a mashing mode is made depending on indicators such as quality of the raw materials, the style of the beer produced, and the available technological equipment. In recent years, due to the improvement of the quality of the produced raw materials and/or the use of enzyme preparations, the infusion method has become very popular in beer production technology. Infusion methods are characterized by the fact that the whole mash is saccharificated simultaneously at a temperature of 60–72° C, while in the decoction methods the individual parts of the mash (1/3–1/2) are saccharificated, then boiled and added to the main amount (1/2–2/3) [1,2,3].
After selecting the mashing mode, one proceeds to its optimization. Here the process includes both conducting single-factor experiments and modeling with the help of design of experiments. The choice of approach largely depends on the objectives of the study.
The use of single-factor experiments usually involves studying the factors time or temperature of a given pause, while fixing a constant level of a given parameter. When studying the influence of the mashing time, the temperature of the mash is fixed at a constant level. Usually the choice of the temperature level corresponds to the known data on the optimum temperature of the given enzyme system, the influence of which one determines (Table 2) [31]. In this case, the target function is the product of the enzymatic reaction one is studying. This type of experiment is usually aimed at determining the optimal duration of the respective pause. This approach is largely appropriate for both laboratory and industrial practice. Usually some of the pauses are selected and the rest are fixed at an appropriate level.

Table 2.
Optimum of action of enzyme systems in malt [31].
Similar practice was applied by Ivanov еt al. 2016 in the optimization of the mashing regime in the production of low-alcohol and non-alcoholic beer. In this case, the optimization of the regime was carried out by eliminating the pause at 63 °C, varying the duration of the pauses at 50 °C and 77 °C and the effect of the pause on the yield of extract and the content of fermentable sugars in wort was determined [32].
Montanari et al., 2005 applied a similar approach (single-factor experiments) to study the effect of two methods—double decoction mashing method and combined mashing method—on the characteristics of the resulting wort. In this case, the optimization parameters were the wort composition and the extract yield. It was found that the choice of mashing method does not affect the final product, but had a significant effect on the composition of the wort fermentable extract. The authors found that changes in the qualitative and quantitative composition of the fermentable extract of the wort, as well as in the quality of the final product can be achieved through modifications in the mashing method [33].
Tschoeke et al., 2019 expanded the possibilities for optimizing the mashing process by using experiment design of the type 34. In this experiment the independent variables were—pH of the mash, temperature, hydro-module (dilution of the mash) and particle size distribution of the malt meal. The reaction kinetics of action of the two main amylases of the amylase complex—α-amylase and β-amylase, as well as the amount of accumulated sugars in the wort were used as a target function. The authors found that a significant factor for the accumulation of fermentable sugars in wort was the used hydro-module (dilution of the malt slurry). It has also been found that dilution was important in combination with other factors studied and that the particle size distribution of malt meal had a certain significance on the yield of sugars, but this effect was smaller than the effect of dilution. The authors confirmed the fact that the particle size distribution was crucial for the process of draining of the malt slurry in the next technological stage [34].
In the study presented by Tschoeke et al., 2019, it was of interest to determine the influence of individual factors on the kinetics of the enzymatic reactions occurring in malt. Determining the enzyme kinetics is a method for optimizing the mashing process, through which the desired results can be most easily achieved. Enzymatic hydrolysis of starch takes place in three phases. Gelatinization of starch is not an instantaneous process and depends on the temperature, time and size of the starch grains. It is described by first-order kinetic equations [35,36,37]:
where: rg—rate of gelatinization of starch, g/(kg·s); [SS]—concentration of ungelatinized starch, g/kg; R—universal gas constant, J/(mol·K); T—temperature, K; Eg—activation energy, J/mol; Kg—rate constant; kg—kinetic factor, U/(kg·s).
The data of Brandam et al., 2003 showed that Equation (8) was a continuous function in the range of the optimal action of β-amylase—60–63 °C [35]. The main reason for this is the inhomogeneous distribution of starch in the grain structure, which also determines differences in the rate of the enzymatic reaction. The more important conclusion from Equation (8) is the fact that the temperature of the process is the most important factor for the rate of the enzymatic reaction [35,36,37].
The second stage of the process is the enzymatic hydrolysis of starch under the action of the amylase complex. According to Table 2 this process is strictly dependent on the temperature, and the pauses should be established at the temperatures at which the corresponding enzyme has maximum activity. The duration of the corresponding pause usually does not exceed 60–90 min, as most often after this time the enzymatic activity begins to decrease. These two effects—enzymatic activation and loss of activity are described by the following equations [35]:
where: rac—total rate of the enzymatic reaction, U/(kg·s); rde—rate of inactivation of the enzyme complex, U/(kg·s); kde—pre-exponential constant for enzyme inactivation, s−1; Ede—activation energy in the inactivation of the enzyme systems, J/mol; R—universal gas constant, J/(mol·K); T—temperature, K; [E]—total enzyme activity in the mash, U/kg; αS(T)—relative specific enzyme activity, -;
Brandam et al., 2003 showed that the relative activity of the corresponding enzyme can be described by a fourth-degree polynomial with respect to the corresponding temperature. Model (9) has been validated for the process of enzymatic hydrolysis of starch, and data on the kinetic parameters for the accumulation of fermentable sugars and non-fermentable dextrin during mashing can be found in the publication [35]. It should be noted that the kinetic parameters that are identified in laboratory conditions can be transferred to industrial volumes, but it is necessary to validate the model to the new conditions. One of the reasons is that in larger volumes the enzyme systems work longer than those established in laboratory conditions [33,34,35].
Tschoeke et al., 2019 suggested that the mashing process be described by first-order chemical reaction kinetics, and based on the known dependencies for this type of kinetics, it is established that the starch concentration during mashing should decrease exponentially [34]:
where: CA—starch concentration; CA0—initial starch concentration; k—kinetic constant; τ—mashing time.
Based on the description of kinetics as a first-order chemical reaction, the concentration of sugars in the wort should be described by the equation:
where: q—stoichiometric coefficient; Cg—concentration of sugars.
Tschoeke et al., 2019 suggested a linear dependence of the rate of starch hydrolysis on the process parameters—temperature, pH, particle size distribution and dilution of the mash. Consequently, an equation for the formation of the extract, which includes the linear dependence and the kinetic constants of the process was proposed [34]:
where: a, Brix/K; b, Brix; c, Brix/mm; d, Brix/% w; e, Brix—coefficients in the regression equation; T—process temperature, K; pH—active acidity of the medium; Gr—particle size distribution, mm; Di—dilution, %; K0—kinetic constant, min−1; Ea—activation energy, J/mol; R—universal gas constant, J/K·mol.
The authors showed that according to this kinetic model, the most important parameter on which the wort extract depends is the dilution (hydro-module) of the mash. It was found that the highest concentration of sugars in wort was achieved in diluted mash. It should also be noted that since a planned experiment was used to model the process, it was found that the combined effects of the studied factors were important for the wort composition. The developed model allows various researchers and breweries to determine the importance of individual factors so as to obtain the optimal composition of the wort. The data also showed certain importance of the particle size distribution, which should be taken into account during the lautering of the mash in the next technological stage [34,38,39,40].
As a result of the conducted statistical analyzes it was established that there is an optimal interval of action of the enzyme systems. The highest activity and therefore yield of extract was observed at temperatures of 60–70 °C and pH between 4.8 and 6.2. The two intervals coincided with the optimums of action of the malt amylase complex. In addition, it has been found that maximum extract yield was observed at lower pH in diluted slurries obtained with finer ground malt [34].
From the point of view of the control of the mashing process, it is necessary to expand the models with models that take into account the dynamics of change of certain parameters and, above all, the process temperature. A similar approach has been applied by [41], who proposed balance equations for temperature change, as a function not only of the process but also of the equipment used. The model consisted of two parts—the kinetic model (8), developed by Brandam et al. 2003 [35] and balance equations for temperature change. The result was a system of equations that is applied to object-oriented control of the mashing process.
Viader et al., 2021 applied a one-factor study and modification of the time-temperature mode of mashing in order to improve the action of the malt amylase complex [42]. As a result of the study, it was proposed to optimize the mashing mode by reducing the total process time by 20 minutes. This was achieved by including pauses at lower temperatures, which activated both enzymes. The proposed optimization did not negatively affect the parameters of the wort and the beer obtained from it: the stability of the foam, the composition of the sugars in the wort and the concentration of ethanol [42].
In [33] the influence of two different mashing methods was studied—decoction (method A) and combined mashing method (method B). A mixture of malt and corn was used in the study (the article did not mention the specific ratio of the two raw materials in the blend). The influence of both methods on the analytical and economic parameters (yield of extract, beer quality and energy costs) of the obtained wort was studied. The authors proved that in terms of quality parameters, both methods led to similar wort conditions. Significant changes were observed in the economic parameters of the mashing process. Method B provided higher yield of extract within 33 min, reduced energy consumption by 20%. It has also been found that the amount of sugars increased by 5.3 g/dm3, which led to an increase in the alcohol by 0.25% v/v [33].
2.4. Lautering Process Modeling and Optimization
The mash separation into its two main phases is achieved by keeping the brewers’ spent grains on a porous barrier—sieve or semi-permeable fabric, depending on the chosen design of the apparatus. The mash is characterized by a high concentration of solid phase, which when retained on the barrier stratifies depending on the relative density of the particles. In this way, a permeable additional filtration bed is formed, through which the wort and then the sparging water are filtered. In the lautering process, the pores of the additional filtration bed become clogged, which requires it to be degreased periodically. The process takes place in two stages: wort draining, which is completely subjected to the laws of hydrodynamics, and brewers spent grains washing, a process completely subjected to the laws of mass transfer [1,2,3].
The lautering process is described by Poiseuille’s law (13), written in a modified form known as the Darcy equation [1,2,3,23]:
where: Q—volume of the filtered liquid for time t through the surface s, m3; p—pressure difference on both sides of the filter barrier, Pa; r—average radius of one capillary, m; s—filtration surface, m2; z—number of capillaries in 1 m2; η—dynamic viscosity, Pa·s; l—average length of one capillary, m.
The law of lautering itself shows which factors have the strongest influence on the filtration process [1,2,3,23]. The first factors are the structure and the thickness of the filtration bed. These two parameters are of major importance in classical filter apparatuses. The optimization is primarily related to changes in the method and mode of milling. Coarse milling provides a loose and highly permeable layer, but the resulting wort is cloudy, and often with incomplete enzymatic processes in the mashing phase. Fine milling is favorable for the extraction of the extract, but makes it difficult to filter due to the formation of a dense bed with small capillaries, which are quickly clogged by fine particles. The filtration rate is determined by the number and size of capillaries. All other things being equal, the capillaries are longer with a thick filtration bed, which leads to deterioration of the filtration rate. The diffusion of the extractives from the particles takes place simultaneously with the mash draining. Efficient extraction and high yield of extractives is obtained by using small particles in which the path taken by the substances is minimized. The fine particles also provide a high surface/diameter ratio, which accelerates diffusion. This process is also accelerated by the increase in temperature, but due to the requirement for mash viscosity, the temperature cannot exceed 78 °С. The existence of a connection between the hydrodynamic lautering process and the diffusion of extractives suggests a compromise in the milling degree and the specific load on the filtration surface [1,2,3,23].
Poiseuille’s law shows significant influence of the malt slurry viscosity, which is highly dependent on the process temperature. The high slurry temperature reduces the liquid phase viscosity and, therefore, increases the filtration rate. However, excessive temperature rise is not beneficial to the process. The reason for this is the saccharification of the undissolved starch and the increase in the liquid viscosity. Since α-amylase is inactivated at temperatures above 80 °C, the resulting adhesive sharply reduces the filtration rate. For this reason, the lautering temperature of the slurry is kept in the range of 75–78 °C [1,2,3,23].
It is worth noting that very often the lautering process is part of the research on the mashing process. This is due to the fact that the mash viscosity (Table 1) can also be a target function of optimizing the mashing mode.
2.5. Modeling and Optimization of the Process of Boiling of Wort with Hops
Wort boiling is one of the main processes in beer production. Over the years and the introduction of new technologies, this process has developed significantly, and the purposes of boiling can be summarized as follows: [1,43,44,45,46,47]: Inactivation of enzymes; Sterilization of wort; Extraction and isomerization of hop substances; Coalgulation of proteins in wort; Formation of proteins/polyphenolic complexes; Formation of aromatic and color complexes; Formation of reducing components, giving the wort oxidation-reduction potential, which at a later stage protects the wort from oxidation processes; pH decreasing; Wort concentration by water evaporation; Evaporation of volatile components formed in the wort during mashing; Evaporation of volatile components added together with hops.
2.5.1. Modeling and Optimization of the Boiling Process from a Technical Point of View
From a technical point of view, the most important element to be modeled is energy consumption. The energy used in the form of water vapor to concentrate the wort should be minimized by taking a number of technical decisions. The process of wort boiling is extremely energy-intensive, and depending on the design of the brewing system used, the energy used varies from 24 MJ/hl to 54 MJ/hl. However, the technical decisions taken must not affect the quality of the wort obtained at the end of the boiling. It is known that the rate of chemical reactions occurring in wort increases with increasing the boiling temperature. It was found that brewing at low pressure and a temperature of about 104–110 °C does not significantly reduce the time for wort processing. Boiling regimes in the temperature range of 118–140 °C significantly reduce the boiling time, but this is mainly related to the increase in energy consumption for wort boiling [1,43,44,45,46].
From a technical point of view, the modeling of the boiling process involves the development of new constructions of the apparatus or the optimization of the existing constructions. The following approaches are applied here: change of the kettle material, study of the heat exchange process in the kettle, application of computational fluid dynamic (CFD) methods for modeling the wort flow inside the apparatus. Typically, the optimization process involves modeling the heat transfer rate, which is described using the Fourier equation when changing the parameters of the apparatus [1,48,49,50]:
where: q—heat transfer rate; Ka—coefficient of thermal conductivity of the material; A—the cross section along which the heat exchange takes place; ΔT—the average temperature difference between steam and heated liquid; X—wall thickness.
However, empirical dependencies were rather required in the modeling process, both due to the complexity of the process and due to the accumulation of heat in the heating surfaces. The beginning of the process of wort boiling leads to a change in its thermophysical parameters and especially its density. A fine film of water vapors is formed on the heating surface in the period of intense boiling, which reduces the efficiency of heat transfer. This effect is most significant in systems with an internal heating body, due to the fact that it reduces the intensity of wort stirring [1,48,51].
2.5.2. Modeling and Optimization of the Boiling Process from a Technological Point of View
From a technological point of view, the optimization of the boiling process is related to the selection of the boiling mode so as to ensure the process objectives to a maximum degree. Since the process is temperature dependent, it can be expected that the changes in the boiling temperature are the ones that have the most significant impact on the boiling quality.
The increase in the boiling temperature, respectively the wort pressure, leads to acceleration of the processes of wort formation, isomerization of α-acids, color compounds formation, DMS evaporation process acceleration, etc. This shows that boiling at high temperatures can reduce energy costs. On the other hand, the accelerated wort boiling disrupts the finished beer foaming. It can be said that increasing the boiling temperature with every 4 °C halves the boiling time. However, the different processes taking place in the wort are characterized by different temperature coefficients and therefore the rates at which they take place differ significantly. In conclusion, it can be said that boiling at atmospheric pressure gives satisfactory results in terms of wort quality [1].
In their work, Scheuren et al., 2016 considered a possible technological approach to optimize the boiling process [52]. This approach involved changes in the individual boiling phases through changes in the rate of the pump (used for wort recirculation) and the rate of evaporation in order to achieve flash evaporation. The second optimization tool even enhanced this combination with mechanical improvement. In this way, a reduction in the wort boiling energy can be achieved. In this case, research was aimed at improving the volatility of dimethyl sulfite (DMS). Here the study aims to compare two boiling methods—flash boiling and classical boiling. The authors cited data that in terms of total evaporation, classical boiling of the entire volume was preferable, while flash boiling improved DMS volatility. To prove the claim and optimize the boiling process, it was divided into two phases [52]:
- Phase 1 enhances the advantage of flash evaporation which is an improved DMS conversion due to higher temperatures. This is done by reducing the pump around velocity of wort through the boiler at a constant evaporation rate. The wort pumped through the boiler and treated at higher temperatures shows a strongly reduced content of DMS precursor (DMSP) when leaving the boiler and entering the kettle.
- Phase 2 is meant to even up the flash evaporation’s disadvantage which is the worse DMS evaporation. For this purpose the temperature difference of the flash evaporation (difference of wort temperature within boiler and within kettle) of the superheated wort has to be reduced. The reduction is realized by increasing the pump around velocity of wort through the boiler while the evaporation rate is kept constant.
Since the process of formation and evaporation of DMS is time-dependent, the following model has been proposed to optimize it (two models are presented—for flash boiling and for classical boiling in the whole volume):
where: dxi—concentration of the i-th aromatic component (in this case DMS); D—ratio of circulation rate/degree of evaporation; L0—wort volume; ωi—correction coefficient for flash evaporation; Ki—volatility; ci,0—precursor concentration in the liquid phase; ni,g—amount of DMS formed.
Through the presented Equations (16) and (17) the authors showed that it is possible to optimize the technological process of evaporation through some technical changes in the evaporation rate [52].
The quest for the ultimate boiling technology is not yet complete. Over the last decade brewing technology has advanced considerably to achieve more efficient use of energy. Further research has to be made to increase this efficiency, without forgetting to improve beer quality, which still remains a major concern to the brewer. Microbiological and colloidal stability of beer are already largely under control, but flavor stability and the effect of process parameters on this stability remain a heavily investigated challenge. A lot of effort has to be made by the brewer to keep the quality of his beer undisputed and a modern boiling kettle can help him to achieve this. Manufacturers respond to this by offering innovative boiling technology, but they must never forget the basic engineering principle: keep it simple [53].
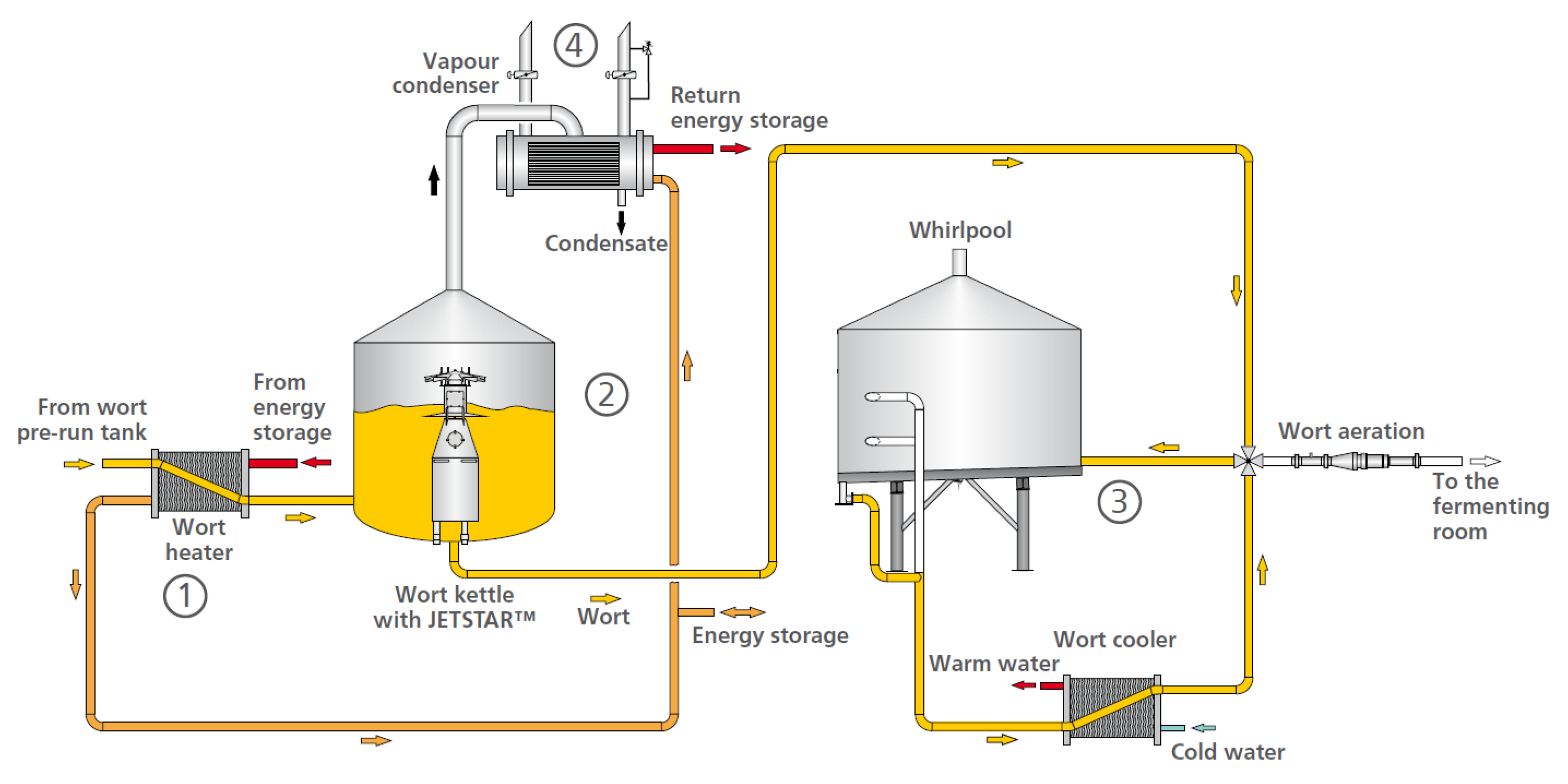
In terms of technology, the achievement of the necessary parameters of wort after boiling can be done by solving some purely technical issues. Figure 3 presents a generally accepted scheme of a boiling system with energy recovery [54].
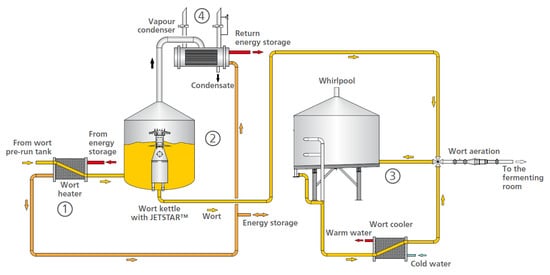
Figure 3.
Jetstar wort brewing system [54].
Wort preheating before the boiling machine preserves the high-molecular protein substances that are involved in foam formation. In addition, the thermal stress on the wort is reduced, which improves the taste stability of the resulting beer. The DMS concentration in the final product decreases with wort pre-boiling during its removal from the boiling machine. This is achieved with a small heat exchanger. The use of Jetstar technology improves the stirring process in the boiling machine, and from there the wort temperature profile in the whole machine improves as well. Homogeneous temperature distribution leads to acceleration of the temperature-dependent boiling processes and reduction of their time [54].
2.5.3. Kinetics of the Wort Hopping Process
The use of hops (Humulus lupulus) is one of the important processes for the formation of the beer bitter taste and aroma. The amount of hops used in wort is defined by the alpha-bitter acids, hop essential oil and the amount of polyphenols extracted. Alpha-acids are the dominant class of compounds in hops and represent about 10% of the hops dry matter. A total 98% of the hops alpha-acids are formed from cohumulone, n-humulone, and adhumulone and only 2% are from posthumulone, prehumulone and adprehumulone. Iso-alpha-acids are isomeric forms of humolone, with 70% of the transformation taking place during wort boiling. It is the isoforms that are responsible for the beer bitter taste and the process of beer foaming. Beer bitterness depends on the degree of isomerization of humolone during brewing [55,56,57].
The main point in modeling the hopping process is the knowledge of the kinetics of extraction, isomerization and degradation of hop bitterness. Since these processes are temperature dependent, their description is important for understanding the hopping process. Iso-alpha-acids are known to hydrolyze or convert to humulin acids and other undesirable compounds. It is believed that the formation of iso-alpha-acids is not equivalent to the loss of alpha-acids, especially at the beginning of the wort boiling. Iso-alpha-acid losses become more significant with prolonged heating times [55,58].
For acquiring knowledge of the processes occurring during hopping Huang et. al. 2013 proposed the following kinetic model for studying the processes of isomerization and degradation of iso-alpha-bitter acids [55]. The model reflects the influence of temperature and boiling time on the amount of iso-alpha-bitter acids [55]:
Huang et al. 2013 suggested that the kinetics of the process of degradation of iso-alpha-acids depends on the temperature and the pH of the process [55]. It has been found that this process can be described by first-order chemical kinetics equations.
3. Modeling and Optimization of the Processes of Main Fermentation, Additional Fermentation and Maturation of Beer
The wort obtained in the previous technological stages is cooled to the fermentation temperature and used for beer production. The taste and aroma profile of the drink, which everyone knows, is formed during the processes of main fermentation and maturation. The fermentation process is biochemical, performed by different types of yeast, that transform the wort into finished beer with certain qualitative and quantitative composition [1,2,3,23,59,60].
The formation of a quality product in organoleptic terms requires knowledge of the fermentation process, which is decisive for the quality of the final product. On the other hand, the current stage of technology requires an increase in productivity, which is associated with reduction in fermentation time, increase in fermentation volume or the production of highly extractive wort. In general, changes in these directions usually negatively affect the quality of the finished beer [1,2,3,23,59,60].
For beer production in practice there are two main types of yeast—top-fermenting or ale yeast and bottom-fermenting or lager yeast. The first group refers to the species Saccharomyces cerevisiae, the second one, according to the last classification, refers to the species Saccharomyces pastorianus [1,2,3,23,59,60]. The main difference between the two types is the fermentation temperature. The top-fermenting strains carry out the fermentation process at 15–25 °C, while the bottom-fermenting strains are characterized by a temperature range between 8 °C and 15 °C. To a large extent, the second main difference—behavior in the fermentation volume—has lost its significance due to the entry of modern technological equipment into technology. Differences between the two yeast types are also observed in their metabolism [59,60,61].
From the point of view of modeling the fermentation process, the choice of mathematical dependencies to describe it is extremely important. The fermentation process for beer production can be divided into two main parts in terms of metabolic products. The first part of the model includes the primary metabolism of the cells (substrate consumption, ethanol accumulation, CO2 formation and biomass accumulation). The second part of the model includes the secondary metabolism of cells and describes the accumulation of the main groups of metabolites—esters, aldehydes, higher alcohols and vicinal diketones. Equation (17) presents this generalized kinetic model of alcoholic fermentation. The model is based on the knowledge of the biochemistry of the alcohol fermentation process. It is worth noting that the secondary metabolism is directly related to the growth and accumulation of biomass, so the development of a complete model of the alcohol fermentation process requires knowledge of the kinetics of the primary metabolism [1,2,3,23,59,60,62].
Naturally, in the development and application of the kinetic model, it is necessary to take into account various elements of yeast metabolism. In the first place, wort fermentation in industrial conditions is carried out mainly by batch fermentation methods. It is characteristic of these methods that the microbial population (yeast cells) grows in the three main phases—lag phase, exponential phase and stationary phase. Data in the literature show that the main groups of metabolites accumulate in the exponential phase, during which the intense alcohol fermentation takes place. Usually the parameters of the model (17) represent an average value for the three phases, but the exponential phase of cell growth is of the greatest importance [1,2,3,23,59,60,62,63,64].
The active fermentation process depends on a number of factors—the composition of the wort used for fermentation, the amount of dissolved oxygen, the amount and age of the inoculum (pure or production cultures of brewer’s yeast), the fermentation temperature. The control of these parameters can direct the fermentation process in one direction or another, and from there to change the taste and aroma profile of the product [1,23,59,60,62,63,64,65].
For the quality of the model used, it is essential to choose an equation that will be used to describe the specific growth rate μ of the yeast cells. Monod-based dependencies are generally used in practice (19). The main equation used is the Monod Equation (19), which is modified in a certain way depending on the objectives of the study and usually bears the name of the author who proposed it—Aiba (20), Ghose and Thyagi (21), Monod equation with product inhibition (22), Monod equation with product and substrate inhibition (23). In [60] a critical analysis of these models was made on the basis of real data from fermentation processes carried out with bottom- and top-fermenting yeast strains.
where: X—biomass concentration, g/dm3; P—ethanol concentration, g/dm3; S—real extract, g/dm3; YP/S, YX/S—yield coefficients; μ—specific growth rate, h−1; q—specific ethanol accumulation rate, g/(g·h); Е—ester concentration, mg/dm3; FA—higher alcohol concentration, mg/dm3; А—aldehyde concentration, mg/dm3; VDK—vicinal diketone concentration, mg/dm3; YFA, YЕ, YА, YVDK—yield coefficients of the corresponding metabolites, mg/(g·h); kА, kVDK—reduction coefficients for aldehydes and vicinal diketones, mg/(g·h).
where: μmax—maximum specific growth rate of yeast, h−1; S—substrate concentration (fermentable extract concentration), g/dm3; qpmax—maximum specific rate of ethanol accumulation, g/(dm3·h); KSX, KSP—saturation constants, g/dm3, KiX, KiP—constants of inhibition of cell growth and ethanol accumulation, g/dm3; Pxmax, Ppmax—maximum ethanol concentration at which complete inhibition of the cell growth and the ethanol accumulation occurs, g/dm3.
Data from [60,66,67,68] show that Equations (23) and (24) are the most suitable for modeling the alcohol fermentation process in brewing, and the accuracy of the model and therefore its choice depends largely on the fermentation conditions. For the production of “standard” beer types the model with product and substrate inhibition is more suitable, while for the production of beer with reduced alcohol content the model with growth inhibition by product is more suitable [68]. Тhe establishment of substrate inhibition is of interest. It can be explained by the phenomenon of catabolic repression [23,60,61,69].
The main wort extract content is carbohydrates (glucose, fructose, sucrose, maltose and matrotriose). Dextrins with varying degrees of polymerization are also found in the wort. Wort composition depends on both the raw materials used and the method of mashing chosen. It can be modeled according to the methods described in Section 2.3. It is important to note that sugars are fermented in a certain order in the process of alcoholic fermentation. Due to the presence of catabolic repression, maltose and maltotriose are fermented after the concentration of monosaccharides in the wort falls below a certain value [1,23,59,60,69]. This effect can be described if a modification of the model is used (17). A similar modification of the model was proposed by Ramirez and Maciejowski, 2007 [60,70], who used a description of the process of substrate consumption (in this case composed of glucose G, maltose M and maltotriose N) and the Monod-based kinetics (Equation (20)). The model presented by Ramirez and Maciejowski regarding the consumption of the substrate reflects the catabolic repression, and this is described with the members and in dependencies (26) and (27) for the specific rate of absorption of maltose and maltotriose. In the paper, the authors suggested that the kinetic constants in Equations (25)–(27) were a function of the fermentation temperature, and this dependence can be described by the Arrhenius’ law (28). In addition, cell growth and alcohol accumulation in beer have been described with dependence (24) as conjugated functions of absorption of the corresponding sugars [60,70].
where: μi—specific rate of absorption of the respective sugars by the yeast cells (i = G, M, N), g/(g·h); X—biomass concentration, g/dm3; G, M, N—concentration of glucose, maltose and maltotriose, g/dm3; μmaxi—maximum specific rate of absorption of the respective sugars by the yeast cells (i = G, M, N), g/(g·h); Ki—saturation constant, g·dm3 (i = G, M, N); K′G, K″M—constants of inhibition of maltose and matrotriose uptake by the uptake of glucose and maltose, g/dm3.
The accumulation of cellular metabolites and the reduction of some of them that determine the taste and aroma of beer are described by the dependencies presented in the system of differential Equation (19). These dependencies show that the processes of formation of the taste and aroma profile of the beverage are coupled with cell growth, which means that the accuracy of the models largely depends on the selected models to describe the primary metabolism.
Naturally, when developing models, it is necessary to know cellular metabolism and how it is related to the growth of the yeast cells. In our work [60] a critical analysis of the metabolism and its relationship with the models used was made, and here we will highlight only some important points:
- The absorption of amino acids by yeast cells occurs in a certain order, and for this purpose the amino acids are divided into 4 main groups—A, B, C and D—according to the order of their absorption (amino acids of group D are not absorbed by yeast cells). The order of absorption has a direct effect on the yeast metabolism [23,60,65,69]. This means that if a kinetic model of amino acid absorption is to be developed, the order of absorption must be reflected in it. A similar model was proposed in the work of Ramirez and Maciejowski, 2007 [70].
- More than 40 higher alcohol types are found in beer. They are produced in two main ways and have significant influence on the beer taste and aroma profile [60,71,72,73]. The first pathway of higher alcohol synthesis is anabolic and involves the synthesis of higher alcohols from carbohydrates, while the second one is catabolic and involves the synthesis of higher alcohols in the process of amino acid metabolism [60,62,71,72]. In both cases, the accumulation of higher alcohols is associated with cell growth and can be described by the YFA yield factor.
- Esters are the second group of metabolites that form beer taste and aroma profile. Their synthesis is directly related to cell growth, with the main amount of them accumulating in beer during the exponential phase of yeast cell growth [60,62,71,73]. The data from our research confirmed this statement [60,67,68]. The type of wort used, as well as the distribution of fermentable sugars in it, are essential for the amount of esters accumulated. The data showed that highly extractive wort accumulated larger amounts of esters, with the ratio of glucose and maltose in the wort having the greatest significance [60,62,63,72,73,74,75,76]. As already mentioned, ester synthesis is directly related to cell growth and is described by the YES coefficient. It is possible to make a model that involves the accumulation of esters from individual sugars, but this is quite a complex task and in most cases it is unnecessary from a practical point of view.
- Carbonyl compounds (aldehydes and vicinal diketones) are usually associated with a negative impact on beer aroma and taste profile. Their synthesis is almost entirely related to cell growth, as some of the aldehydes are synthesized during wort boiling. The latter cannot be reduced by yeast cells during beer maturation. The reduction of these compounds in the maturation process is extremely important for the final taste and aroma profile of the beer. The rate of the process of reduction of aldehydes and vicinal diketones depends on their current concentration in the green beer and the concentration of viable yeast cells in the fermentation medium [60,62,71,72,73,77].
4. Recent Trends in Modeling in Beer Production
4.1. Dry Hopping of Beer
One of the latest trends in beer production is dry hopping. It is defined as the cold extraction of volatile and non-volatile components of hops in an alcoholic solution [78,79]. As a result, the beer acquires a rich hop taste and aroma, and it is stabilized in terms of microbiology and taste. This approach is mainly used in craft breweries, due to the fact that different types of processes can be realized: static and dynamic extraction, hopping in the fermentation process or in the beer deposit, different fermentation temperatures [78,80]. The modeling of the extraction process of hop components is performed on the basis of known extraction dependencies, taking into account the following parameters: temperature, ethanol concentration, concentration of other wort components, pH, presence of yeast biomass, concentration of some enzymatic groups [81,82,83,84].
4.2. Analytical Approaches for Modeling Cell Growth
The system with differential Equation (17) requires a numerical solution, which usually occurs only after the end of the fermentation process, as it is necessary to accumulate data on the solution of the system [60,62,66,67,68]. This means that it is difficult to make quick decisions if it is necessary to adjust the course of the fermentation process. For this reason, in recent years work on the application of some simpler (analytical methods) for determining the specific rate of cell growth has been done [85]. These approaches include different methods for linearizing the Monod Equation (20), and for this purpose the specific growth rate is given as follows for each local point [85]:
where: XF—final biomass concentration, g/dm3; Xτ+Δτ—current biomass concentration, g/dm3; τF—final fermentation time h; τ + Δτ—current fermentation time, h; τ0—0 h (beginning of fermentation); Δτ—change in time, h—(for correctness of calculations Δτ = const); Xτ0+Δτ—biomass concentration at the beginning of the exponential phase, g/dm3.
Equation (21) assumes a constant decrease in the growth rate, and during the stationary phase μ→0. This does not mean that the cells stop growing, but that the number of newly formed cells will be equal to the number of dying cells and therefore X≈const. Different linearization methods to solve the Monod Equation (20) are used: Lineweaver–Burk (31), Hanes (35), Eadie-Hofstee (33), Warpholomeew-Gurevich (34), Linearization by Substrate Consumption and Product Accumulation (35) [85,86]:
where: S0; Sj; Si—initial concentration of the substrate, g/dm3; substrate concentration corresponding to τj, g/dm3; substrate concentration corresponding to τi, g/dm3, τj − τi—time interval equal to the difference between the final and current process time, h; Pj; Pi—product concentration corresponding to τj, g/dm3; product concentration corresponding to τi, g/dm3, Pm—maximum possible product concentration.
These methods reflect different aspects of the process of assimilation of the substrate and its transformation by the biomass. Our research shows that Equations (25) and (26) are the most suitable equations for use in brewing, as they reflect a substrate-dependent process, i.e., alcoholic fermentation in beer production [87]. At present, these models are used only to determine the specific growth rate of biomass, while the accumulation of metabolites is indirectly determined by fermentation dynamics.
4.3. Development of Functional Beverages Based on Wort
Malt and the wort obtained from it have a number of biological benefits for human health [11,24,88]. In recent years, increasing attention has been paid to the health benefits of beer and efforts to develop new beverage types based on wort are being made. Examples of such beverages are beer obtained with probiotic yeast cells of the species Saccharomyces boulardii [89,90,91,92], fermentation of wort by lactic acid bacteria [93,94], addition of various additives (essential oils from various plants, waste products from different productions, fruits with functional characteristics, herbal raw materials) [89,95,96,97,98]. The purpose of all these developments is to increase the functional value of the drink. With regard to modeling, the approaches already described are applied: one-factor experiments to determine the concentration of additional materials; modeling of mixtures; modeling of microbial growth kinetics (for yeast cells and lactic acid bacteria cells). Usually, the parameters of the beverage’s functionality—concentration of phenolic compounds and antioxidant potential of the wort—are added to the target functions for modeling and optimization. In addition, the organoleptic evaluation of the obtained beverages is a parameter for optimization, as non-traditional raw materials change the known organoleptic of the beverage [99].
5. Conclusions
The present publication discussed different approaches to modeling and optimization of the main processes in beer production. The target functions for optimization and the observed changes related to the process of modeling and optimization of production were highlighted. Some of the data summarized the research of the author’s team in recent years, dedicated to the modeling and optimization of technological processes for beer production. At the end of the work, some new directions in the production of beer and beverages based on wort were discussed and the potential possibilities for modeling the processes were discussed. We hope that this article will provide guidance to scientists working in the field of beer production to develop new assortments to boost the brewing industry. The present review presented a generalized approach to the analysis of mathematical modeling methods used to study the various processes in beer production. The presented models can be applied in the development of different types and styles of beer, adapting them to the respective regimes and specific technological solutions. At the end of the paper, some new trends, both in the field of production and in the field of modeling, were commented. A particular challenge is the production of beverages with functional value, where one of the main target functions is the value of the beverage for human health, which leads modeling and optimization of production to some new challenges that will be the subject of future research.
Author Contributions
Conceptualization, G.K., V.S., R.D.-K.; formal analysis, G.K., V.S.; writing—original draft preparation, G.K., R.D.-K.; writing—review and editing, G.K.; project administration, G.K.; funding acquisition, G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Bulgarian Ministry of Education and Science, under the National Research Programme “Healthy Foods for a Strong Bio-Economy and Quality of Life”, approved by DCM #577/17.08.2018 and by the project “Strengthening the research excellence and innovation capacity of the University of Food Technologies—Plovdiv, through the sustainable development of tailor-made food systems with programmable properties”, part of the European Scientific Networks National Program funded by the Ministry of Education and Science of the Republic of Bulgaria (agreement No. Д01-288/07.10.2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank all our co-authors, students and colleagues who have participated in the research cited in this publication.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Briggs, D.; Boulton, C.; Brookes, P.; Stevens, R. Brewing; Science and Practice, 1st ed.; Woodhead Pub: Cambridge, UK, 2004; p. 881. [Google Scholar]
- Kunze, W. Technology Brewing and Malting, 4th ed.; VLB: Berlin, Germany, 1996; p. 726. [Google Scholar]
- Kunze, W. Technology Brewing and Malting, 5th ed.; VLB: Berlin, Germany, 2004; p. 946. [Google Scholar]
- Puligundla, P.; Smogrovicova, D.; Mok, C.; Obulam, V.S.R. Recent developments in high gravity beer-brewing. Innov. Food Sci. Emerg. Technol. 2020, 64, 102399. [Google Scholar] [CrossRef]
- Karabín, M.; Jelínek, L.; Kotrba, P.; Cejnar, R.; Dostálek, P. Enhancing the performance of brewing yeasts. Biotech. Adv. 2018, 36, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Sanz, V.; Torres, M.D.; Vilariño, J.M.V.; Domínguez, H. What is new on the hop extraction? Trends Food Sci. Technol. 2019, 93, 12–22. [Google Scholar] [CrossRef]
- Trummer, J.; Watson, H.; De Clippeleer, J.; Poreda, A. Brewing with 10% and 20% Malted Lentils—Trials on Laboratory and Pilot Scales. Appl. Sci. 2021, 11, 9817. [Google Scholar] [CrossRef]
- Lehnhardt, F.; Nobis, A.; Skornia, A.; Becker, T.; Gastl, M.A. Comprehensive evaluation of flavor instability of beer (Part 1): Influence of Release of Bound State Aldehydes. Foods 2021, 10, 2432. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, R.; Caballero, I.; Nimubona, D.; Blanco, C.A. Brewing with starchy adjuncts: Its influence on the sensory and nutritional properties of beer. Foods 2021, 10, 1726. [Google Scholar] [CrossRef] [PubMed]
- Ledley, A.J.; Elias, R.J.; Hopfer, H.; Cockburn, D.W. A Modified brewing procedure informed by the enzymatic profiles of gluten-free malts significantly improves fermentable sugar generation in gluten-free brewing. Beverages 2021, 7, 53. [Google Scholar] [CrossRef]
- Shopska, V.; Denkova-Kostova, R.; Dzhivoderova-Zarcheva, M.; Teneva, D.; Denev, P.; Kostov, G. Comparative study on phenolic content and antioxidant activity of different malt types. Antioxidants 2021, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Dabija, A.; Ciocan, M.E.; Chetrariu, A.; Codină, G.G. Maize and sorghum as raw materials for brewing, a Review. Appl. Sci. 2021, 11, 3139. [Google Scholar] [CrossRef]
- Cavallini, N.; Savorani, F.; Bro, R.; Cocchi, M.A. Metabolomic approach to beer characterization. Molecules 2021, 26, 1472. [Google Scholar] [CrossRef]
- Yorke, J.; Cook, D.; Ford, R. Brewing with unmalted cereal adjuncts: Sensory and analytical impacts on beer quality. Beverages 2021, 7, 4. [Google Scholar] [CrossRef]
- Cipollaro, M.; Fabbrizzi, S.; Sottini, V.A.; Fabbri, B.; Menghini, S. Linking sustainability, embeddedness and marketing strategies: A study on the craft beer sector in Italy. Sustainability 2021, 13, 10903. [Google Scholar] [CrossRef]
- Březinová, M. Beer industry in the Czech Republic: Reasons for founding a craft brewery. Sustainability 2021, 13, 9680. [Google Scholar] [CrossRef]
- Iorizzo, M.; Coppola, F.; Letizia, F.; Testa, B.; Sorrentino, E. Role of Yeasts in the Brewing Process: Tradition and Innovation. Processes 2021, 9, 839. [Google Scholar] [CrossRef]
- Salanță, L.C.; Coldea, T.E.; Ignat, M.V.; Pop, C.R.; Tofană, M.; Mudura, E.; Borșa, A.; Pasqualone, A.; Zhao, H. Non-Alcoholic and Craft Beer Production and Challenges. Processes 2020, 8, 1382. [Google Scholar] [CrossRef]
- Cela, N.; Condelli, N.; Caruso, M.C.; Perretti, G.; Di Cairano, M.; Tolve, R.; Galgano, F. Gluten-Free Brewing: Issues and Perspectives. Fermentation 2020, 6, 53. [Google Scholar] [CrossRef]
- Iattici, F.; Catallo, M.; Solieri, L. Designing New Yeasts for Craft Brewing: When Natural Biodiversity Meets Biotechnology. Beverages 2020, 6, 3. [Google Scholar] [CrossRef]
- Guido, L.F. Brewing and Craft Beer. Beverages 2019, 5, 51. [Google Scholar] [CrossRef]
- Mulero-Cerezo, J.; Briz-Redón, Á.; Serrano-Aroca, Á. Saccharomyces Cerevisiae Var. Boulardii: Valuable Probiotic Starter for Craft Beer Production. Appl. Sci. 2019, 9, 3250. [Google Scholar] [CrossRef]
- Kostov, G.; Shopska, V.; Iliev, V. Optimization of Technological and Energy Processes in the Production of Beverages. Part. 1. Brewing, 1st ed.; Academic Publishing House of the University of Food Technology: Plovdiv, Bulgaria, 2019; p. 294. [Google Scholar]
- Shopska, V.; Denkova-Kostova, R.; Ivanova, K.; Kostov, G. Tailor-Made Concept for New Beer Types with High Biological Value. In Beer: From Production to Distribution, 1st ed.; Legault, A., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2020; pp. 1–76. [Google Scholar]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process. and Product Optimization Using Designed Experiments, 4th ed.; John Wiley & Sons: New York, NY, USA, 216; p. 856. In Response Surface Methodology: Process. and Product Optimization Using Designed Experiments, 4th ed.; John Wiley & Sons: New York, NY, USA, 2016; p. 856. [Google Scholar]
- Cornell, J.A. Experiments with Mixtures: Designs, Models, and the Analysis of Mixture Data, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2020; p. 680. [Google Scholar]
- Ivanova, K.; Denkova, R.; Kostov, G.; Petrova, T.; Bakalov, I.; Ruscova, M.; Penov, N. Extrusion of brewers’ spent grains and application in the production of functional food. Characteristics of spent grains and optimization of extrusion. J. Inst. Brew. 2017, 123, 544–552. [Google Scholar] [CrossRef]
- Goode, D.L.; Arendt, E.K. Developments in the Supply of Adjunct Materials for Brewing. In Brewing: New Technologies; Bamforth, W., Ed.; Woodhead Publishing Limited: Sawston, UK; Cambridge, MA, USA, 2006; pp. 30–67. [Google Scholar]
- Fumi, M.D.; Galli, R.; Lambri, M.; Donadini, G.; De Faveri, D.M. Effect of full-scale brewing process on polyphenols in Italian all-malt and maize adjunct lager beers. J. Food Comp. Anal. 2011, 24, 568–573. [Google Scholar] [CrossRef]
- Stewart, G. Chapter 2—Adjuncts. In Brewing Materials and Processes; Bamforth, C.W., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 27–46. [Google Scholar] [CrossRef]
- Krottenthaler, M.; Back, W.; Zarnkow, M. Wort Production. In Handbook of Brewing: Processes, Technology, Markets, 1st ed.; Esslinger, H.M., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 165–205. [Google Scholar]
- Ivanov, K.; Petelkov, I.; Shopska, V.; Denkova, R.; Gochev, V.; Kostov, G. Investigation of mashing regimes for low-alcohol beer production. J. Inst. Brew. 2016, 122, 508–516. [Google Scholar] [CrossRef]
- Montanari, L.; Floridi, S.; Marconi, O.; Tironzelli, M.; Fantozzi, P. Effect of mashing procedures on brewing. Eur. Food Res. Technol. 2005, 221, 175–179. [Google Scholar] [CrossRef]
- Tschoeke, I.C.P.; Silva, J.M.C.L.R.; da Silva, J.P.; Marques, O.M.; Vinhas, G.M.; Santos, A.M.P.; Souza, T.P.C. Kinetic modelling of a brewery mashing: A multidimensional approach. Food Bioprod. Pract. 2019, 116, 130–139. [Google Scholar] [CrossRef]
- Brandam, C.; Meyer, X.M.; Proth, J.; Strehaiano, P.; Pingaud, H. An original kinetic model for the enzymatic hydrolysis of starch during mashing. Biochem. Eng. J. 2003, 13, 43–52. [Google Scholar] [CrossRef]
- Coulter, P.R.; Potter, O.E. A kinetic study of the susceptibility to enzymic hydrolysis of starch in malt. J. Inst. Brew. 1973, 79, 212–218. [Google Scholar] [CrossRef]
- Zanoni, B.; Schiraldi, A.; Simonetta, R. A naive model of starch gelatinization kinetics. J. Food Eng. 1995, 24, 25–33. [Google Scholar] [CrossRef]
- Byong, H.L. Fundamentals of Food Biotechnology, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; pp. 313–430. [Google Scholar]
- Daniels, R. Designing Great Beers: The Ultimate Guide to Brewing Classic Beer Styles, 1st ed.; Brewers Publications: Denver, CO, USA, 1996. [Google Scholar]
- Schultz, S.; Zien, P. Beer, Food, and Flavor: A Guide to Tasting, Pairing, and the Culture of Craft Beer, 1st ed.; Skyhorse: New York, NY, USA, 2012. [Google Scholar]
- Leva, A.; Donida, F.; Maggio, M. Object-oriented modelling of starch mashing for simulation-based control studies. Math. Comp. Mod. Dyn. Sys. 2010, 16, 225–240. [Google Scholar] [CrossRef]
- Viader, R.P.; Yde, M.S.H.; Hartvig, J.W.; Pagenstecher, M.; Carlsen, J.B.; Christensen, T.B.; Andersen, M.L. Optimization of Beer Brewing by Monitoring α-Amylase and β-Amylase Activities during Mashing. Beverages 2021, 7, 13. [Google Scholar] [CrossRef]
- Denk, V.; Felgentraeger, H.G.W.; Flad, W.; Leneol, M.; Michel, R.; Miedaner, H.; Stippler, K.; Hensel, H.; Narziss, L. ; O’Rourke. T. European Brewery Convention—Manual of Good Practice, Wort Boiling and Clarification; Fachverlag Hans Carl: Nurnberg, Germany, 2000; p. 176. [Google Scholar]
- Miedaner, H. Wort boiling today—Old and new aspects. J. Inst. Brew. 1986, 92, 330–335. [Google Scholar] [CrossRef]
- O’Rourke, T. Wort Boiling (part 2). Brew. Guard. 1999, 128, 38–43. [Google Scholar]
- O’Rourke, T. The function of wort boiling. Brew. Int. 2002, 2, 17–19. [Google Scholar]
- Kabzev, Y.; Ignatov, I. Technology of Beer; Academic Press of UFT: Plovdiv, Bulgaria, 2011; p. 338. [Google Scholar]
- Hancock, J.C.J.M.; Andrews, H. Wort boiling. Ferment 1996, 9, 344–351. [Google Scholar]
- Royston, M.G. Wort boiling and cooling. In Modern Brewing Technology; Findlay, W.P.K., Ed.; MacMillan Press: London, UK, 1971; pp. 77–79. [Google Scholar]
- Wilkinson, N.R. Wort Boiling and Clarification. Eur. Brew. Conv. Monograph 1991, 18, 100–105. [Google Scholar]
- Andrews, J.M.H. Proceedings of the 22nd Convention of the Institute Brewing, Australia, and New Zealand Section, Melbourne, Australia, 1–6 March 1992; p. 65.
- Scheuren, H.; Baldus, M.; Methner, F.-J.; Dillenburger, M. Evaporation behaviour of DMS in an aqueous solution at infinite dilution—A review. J. Inst. Brew. 2016, 122, 181–190. [Google Scholar] [CrossRef]
- New Food Magazine. Available online: https://www.newfoodmagazine.com/article/1660/trends-in-brewing-technology-wort-boiling/ (accessed on 10 December 2021).
- Doc Player. Available online: https://docplayer.net/37375319-Wort-boiling-with-the-jetstar-technology-and-energy-management-hand-in-hand-engineering-for-a-better-world.html (accessed on 10 December 2021).
- Huang, Y.; Tippmann, J.; Becker, T. Kinetic modeling of hop acids during wort boiling. Int. J. Biosci. Biochem. Bioinform. 2013, 3, 47–52. [Google Scholar] [CrossRef]
- Intelmann, D.; Haseleu, G.; Dunkel, A.; Lagemann, A.; Stephan, A.; Hofmann, T. Comprehensive sensomics analysis of hop-derived bitter compounds during storage of beer. J. Agric. Food Chem. 2011, 59, 1939–1953. [Google Scholar] [CrossRef] [PubMed]
- Haseleu, G.; Lagemann, A.; Stephan, A.; Intelmann, D.; Dunkel, A.; Hofmann, T. Quantitative sensomics profiling of hop-derived bitter compounds throughout a full-scale beer manufacturing process. J. Agric. Food Chem. 2010, 58, 7930–7939. [Google Scholar] [CrossRef]
- Malowicki, M.G.; Shellhammer, T.H. Isomerization and degradation kinetics of hop (Humulus lupulus) acids in a model wort-boiling system. J. Agric. Food Chem. 2005, 53, 4434–4439. [Google Scholar] [CrossRef]
- Boulton, C. Fermentation of beer. In Brewing–New Technologies; Bamforth, C.W., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 228–253. [Google Scholar]
- Shopska, V.; Denkova, R.; Lyubenova, V.; Kostov, G. Kinetic Characteristics of Alcohol Fermentation in Brewing: State of art and control of the fermentation process. In Fermented Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 529–575. [Google Scholar] [CrossRef]
- Tenge, C. Yeast. In Handbook of Brewing: Processes, Technology, Markets; Eslinger, H.M., Ed.; Wiley-Vch Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; pp. 119–147. [Google Scholar]
- Shopska, V.; Denkova, R.; Kostov, G. Beer production with encapsulated yeast cells. In Beer: Production, Consumption and Health Effects; Salazar, W.H., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2016; pp. 27–100. [Google Scholar]
- Verbelen, P.J.; Delvaux, F.R. Brewing yeast in action: Beer fermentation. In Applied Mycology; Rai, M., Bridge, P.D., Eds.; Cabi: Oxfordshire, UK, 2009; pp. 110–135. [Google Scholar] [CrossRef]
- Munroe, J. Fermentation. In Handbook of Brewing; Priest, D., Stewart, G., Eds.; Tylor and Francis Group: Oxfordshire, UK, 2006; pp. 487–524. [Google Scholar]
- Lodolo, E.J.; Kock, J.L.; Axcell, B.C.; Brooks, M. The yeast Saccharomyces cerevisiae—The main character in beer brewing. FEMS Yeast Res. 2008, 8, 1018–1036. [Google Scholar] [CrossRef]
- Petelkov, I.; Lyubenova, V.; Zlatkova, A.; Shopska, V.; Denkova, R.; Kaneva, M.; Kostov, G. Encapsulation of brewing yeast in alginate/chitosan matrix: Kinetic characteristics of the fermentation process at a constant fermentation temperature. Compt. Rend. Acad. Bulg. Sci. 2016, 69, 1355–1364. [Google Scholar]
- Petelkov, I.; Shopska, V.; Denkova-Kostova, R.; Kostov, G.; Lyubenova, V. Investigation of different regimes of beer fermentation with free and immobilized cells. Per. Pol. Chem. Eng. 2020, 64, 162–171. [Google Scholar] [CrossRef]
- Petelkov, I.; Shopska, V.; Denkova-Kostova, R.; Ivanova, K.; Kostov, G.; Lyubenova, V. Investigation of fermentation regimes for the production of low-alcohol and non-alcohol beers. Per. Pol. Chem. Eng. 2021, 65, 229–237. [Google Scholar] [CrossRef]
- He, Y.; Dong, J.; Yin, H.; Zhao, Y.; Chen, R.; Wan, X.; Chen, P.; Hou, X.; Liu, J.; Chen, L. Wort composition and its impact on the flavour-active higher alcohol and ester formation of beer—A review. J. Inst. Brew. 2014, 120, 157–163. [Google Scholar] [CrossRef]
- Ramirez, W.F.; Maciejowski, J. Optimal Beer Fermentation. J. Inst. Brew. 2007, 113, 325–333. [Google Scholar] [CrossRef]
- Pires, E.; Brányik, T. Biochemistry of Beer Fermentation; Springer International Publishing AG Switzerland: Berlin/Heidelberg, Germany, 2015; pp. 51–80. [Google Scholar]
- Nedović, V.; Gibson, B.; Mantzouridou, T.F.; Bugarski, B.; Djordjević, V.; Kalušević, A.; Paraskevopoulou, A.; Sandell, M.; Šmogrovičová, D.; Yilmaztekin, M. Aroma formation by immobilized yeast cells in fermentation processes. Yeast 2015, 32, 173–216. [Google Scholar] [CrossRef]
- Djordjević, V.; Willaert, R.; Gibson, B.; Nedović, V. Immobilized yeasts and secondary metabolites. In Fungal Metabolites, 1st ed.; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing Switzerland: Berlin/Heidelberg, Germany, 2006; pp. 599–639. [Google Scholar] [CrossRef]
- Saerens, S.M.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef]
- Hiralal, L.; Olaniran, A.O.; Pillay, B. Aroma-active ester profile of ale beer produced under different fermentation and nutritional conditions. J. Biosci. Bioeng. 2014, 117, 57–64. [Google Scholar] [CrossRef]
- Lei, H.; Zhao, H.; Yu, Z.; Zhao, M. Effects of wort gravity and nitrogen level on fermentation performance of brewer’s yeast and the formation of flavor volatiles. Appl. Biochem. Biotechnol. 2012, 166, 1562–1574. [Google Scholar] [CrossRef]
- Krogerus, K.; Gibson, B.R. 125th anniversary review: Diacetyl and its control during brewery fermentation. J. Inst. Brew. 2013, 119, 86–97. [Google Scholar] [CrossRef]
- Lafontaine, S.R.; Shellhammer, T.H. Impact of static dry-hopping rate on the sensory and analytical profiles of beer. J. Inst. Brew. 2018, 124, 434–442. [Google Scholar] [CrossRef]
- Schönberger, C.; Kostelecky, T. 125th anniversary review: The role of hops in brewing. J. Inst. Brew. 2011, 117, 259–267. [Google Scholar] [CrossRef]
- Watson, B. Digging Deeper into Beer Styles, 2017, IRI Group and Brewers Association. Available online: https://www.brewersassociation.org/insights/digging-deeper-beer-styles/ (accessed on 28 December 2021).
- Wolfe, P.H. A Study of Factors Affecting the Extraction of Flavor When Dry-Hopping Beer. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2012. Available online: https://ir.library.oregonstate.edu/concern/graduate_thesis_or_dissertations/rx913t14h (accessed on 28 December 2021).
- Sharp, D.C.; Steensels, J.; Shellhammer, T.H. The effect of hopping regime, cultivar and β-glucosidase activity on monoterpene alcohol concentrations in wort and beer. J. Inst. Brew. 2017, 123, 185–191. [Google Scholar] [CrossRef]
- Takoi, K.; Koie, K.; Itoga, Y.; Katayama, Y.; Shimase, M.; Nakayama, Y.; Watari, J. Biotransformation of hop-derived monoterpene alcohols by lager yeast and their contribution to the flavor of hopped beer. J. Agric. Food Chem. 2010, 58, 5050–5058. [Google Scholar] [CrossRef] [PubMed]
- Oladokun, O.; James, S.; Cowley, T.; Smart, K.; Hort, J.; Cook, D. Dry-hopping: The effects of temperature and hop variety on the bittering profiles and properties of resultant beers. Brew. Sci. 2017, 70, 187–196. [Google Scholar]
- Kostov, G.; Denkova-Kostova, R.; Shopska, V.; Goranov, B. Analytical Approaches to Determine the Specific Biomass Growth Rate. In Brewing. ECMS 2019 Proceedings; Iacono, M., Palmieri, F., Gribaudo, M., Ficco, M., Eds.; European Council for Modeling and Simulation: Wildau, Germany, 2019; pp. 125–131. [Google Scholar] [CrossRef]
- Warpholomeew, S.D. ; Gurevich. K.G. Biokinetic–Practical Course; Fair-Press: Moscow, Russia, 1999; p. 720. (In Russian) [Google Scholar]
- Kostov, G.; Denkova-Kostova, R.; Shopska, V.; Goranov, B.; Ivanova, K. Analytical Approaches for Determining the Effects of Wort Extract on The Specific Growth Rate of The Yeast Population. In ECMS 2020 Proceedings; Steglich, M., Mueller, C., Neumann, G., Walther, M., Eds.; European Council for Modeling and Simulation: Wildau, Germany, 2020; pp. 146–152. [Google Scholar] [CrossRef]
- Carvalho, D.O.; Correia, E.; Lopes, L.; Guido, L.F. Further insights into the role of melanoidins on the antioxidant potential of barley malt. Food Chem. 2014, 160, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Tomova, T.; Petelkov, I.; Shopska, V.; Denkova-Kostova, R.; Kostov, G.; Denkova, Z. Production of probiotic wort-based beverages with grapefruit (Citrus paradisi L.) or tangerine (Citrus reticulata L.) zest essential oil addition. Acta Sci. Pol. Technol. Aliment. 2021, 20, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Pereira de Paula, B.; de Souza Lago, H.; Firmino, L.; Lemos Júnior, W.J.F.; Corrêa, M.F.D.; Guerra, A.F.; Pereira, K.S.; Coelho, M.A.Z. Technological features of Saccharomyces cerevisiae var. boulardii for potential probiotic wheat beer development. LWT 2021, 135, 110233. [Google Scholar] [CrossRef]
- Canonico, L.; Zannini, E.; Ciani, M.; Comitini, F. Assessment of non-conventional yeasts with potential probiotic for protein-fortified craft beer production. LWT 2021, 145, 111361. [Google Scholar] [CrossRef]
- Senkarcinova, B.; Dias, I.A.G.; Nespor, I.; Branyik, T. Probiotic alcohol-free beer made with Saccharomyces cerevisiae var. boulardii. LWT 2019, 100, 362–367. [Google Scholar] [CrossRef]
- Chan, M.; Chua, J.; Toh, M.; Liu, S.-Q. Survival of probiotic strain Lactobacillus paracasei L26 during co-fermentation with S. cerevisiae for the development of a novel beer beverage. Food Microb. 2019, 82, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Dysvik, A.; Leanti, A.K.R.S.; Hovde, L.K.; Myhrer, K.S.; Ostlie, H.M.; De Rouck, G.; Rukke Elling-Olav, B.; Wicklund, T. Co-fermentation Involving Saccharomyces cerevisiae and Lactobacillus Species Tolerant to Brewing-Related Stress Factors for Controlled and Rapid Production of Sour Beer. Front. Microb. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Cirlini, M.; Guido, A.; Liberatore, C.M.; Ganino, T.; Lazzi, C.; Chiancone, B. From Byproduct to Resource: Fermented Apple Pomace as Beer Flavoring. Foods 2019, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, D.R.; Kammerer, J.; Valet, R.; Carle, R. Recovery of polyphenols from the by-products of plant food processing and application as valuable food ingredients. Food Res. Int. 2014, 65, 2–12. [Google Scholar] [CrossRef]
- Paiva, R.A.M.; Mutz, Y.S.; Conte-Junior, C.A. A Review on the Obtaining of Functional Beers by Addition of Non-Cereal Adjuncts Rich in Antioxidant Compounds. Antioxidants 2021, 10, 1332. [Google Scholar] [CrossRef]
- Jahn, A.; Kim, J.; Bashir, K.M.I.; Cho, M.G. Antioxidant Content of Aronia Infused Beer. Fermentation 2020, 6, 71. [Google Scholar] [CrossRef]
- De Francesco, G.; Bravi, E.; Sanarica, E.; Marconi, O.; Cappelletti, F.; Perretti, G. Effect of Addition of Different Phenolic-Rich Extracts on Beer Flavour Stability. Foods 2020, 9, 1638. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).