Brine and Post-Frying Oil Management in the Fish Processing Industry—A Concept Based on Oleaginous Yeast Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strain
2.2. Fish Processing Wastes
2.3. Culture Conditions
2.4. Simple Culture Measurements
2.5. Cellular oil Extraction and Fatty Acids Profile Determination
2.6. Statistical Analysis
3. Results
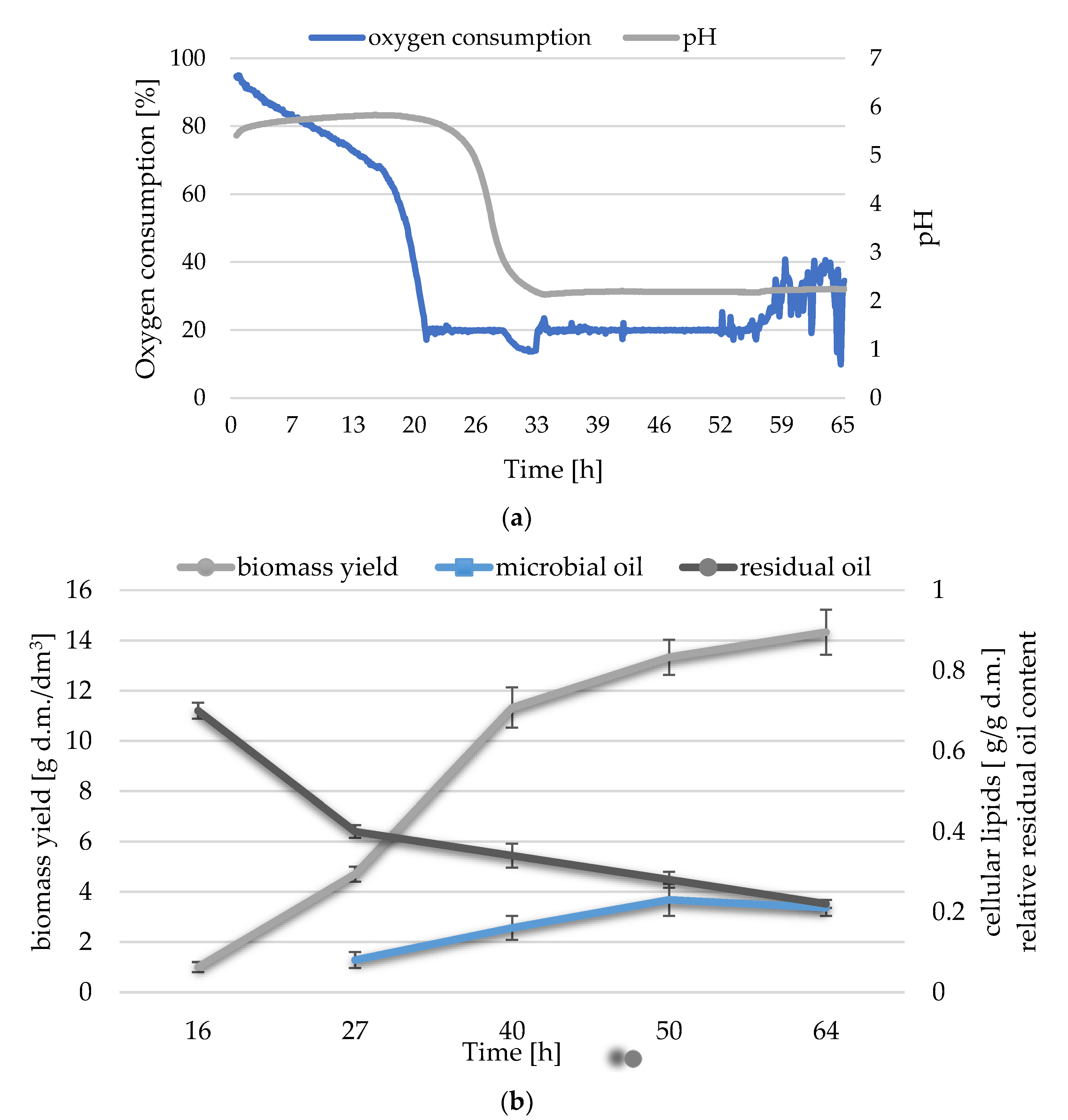
3.1. Waste Post-Frying Rapeseed Oil Management in Y. lipolytica Culture
3.2. Brine Management in Y. lipolytica Culture
3.3. Characteristics of Microbial Oil—Fatty Acids Profile
4. Discussion
4.1. Bioreactor Batch Cultures of Y. lipolytica in Media with Post-Frying Oil
4.2. Waste Brine Utilization in Yeast Cultures
4.3. Single Cell Oil Composition of Yeast Cultured in Waste Medium
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pauly, D.; Zeller, D. Comments on FAOs State of World Fisheries and Aquaculture (SOFIA 2016). Mar. Policy 2017, 77, 176–181. [Google Scholar] [CrossRef]
- The State of World Fisheries and Aquaculture (SOFIA 2020), Food and Agriculture Organization of the United Nations 2020. Available online: https://www.fao.org/publications/sofia/en (accessed on 27 December 2021).
- Bücker, F.; Marder, M.; Peiter, M.R.; Lehn, D.N.; Esquerdo, V.M.; Pinto, L.A.; Konrad, O. Fish waste: An efficient alternative to biogas and methane production in an anaerobic mono-digestion system. Renew. Energy 2020, 147, 708–805. [Google Scholar] [CrossRef]
- Kara, K.; Ouanji, F.; Lotfi, E.M.; Mahi, M.E.; Kacimi, M.; Ziyad, M. Biodiesel production from waste fish oil with high free fatty acid content from Moroccan fish-processing industries. Egypt. J. Pet. 2018, 27, 249–255. [Google Scholar] [CrossRef]
- Mo, W.Y.; Man, Y.B.; Wong, M.H. Use of food waste, fish waste and food processing waste for China’s aquaculture industry: Needs and challenge. Sci. Total Environ. 2018, 613–614, 635–643. [Google Scholar] [CrossRef]
- Lopes, C.; Antelo, L.T.; Franco-Uría, A.; Alonso, A.A.; Pérez-Martín, R. Valorisation of fish by-products against waste management treatments—Comparison of environmental impacts. Waste Manag. 2015, 46, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Kruijssen, F.; Tedesco, I.; Ward, A.; Pincus, L.; Love, D.; Thorne-Lyman, A.L. Loss and waste in fish value chains: A review of the evidence from low and middle-income countries. Glob. Food Secur. 2020, 26, 100434. [Google Scholar] [CrossRef]
- Rebah, F.B.; Miled, N. Fish processing wastes for microbial enzyme production: A review. 3 Biotech 2013, 3, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Coppola, D.; Lauritano, C.; Palma Esposito, F.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef]
- Wierzchowska, K.; Zieniuk, B.; Fabiszewska, A. Use of Non-Conventional Yeast Yarrowia lipolytica in Treatment or Upgradation of Hydrophobic Industry Wastes. Waste Biomass Valorization 2021, 13, 757–779. [Google Scholar] [CrossRef]
- Wierzchowska, K.; Zieniuk, B.; Nowak, D.; Fabiszewska, A. Phosphorus and Nitrogen Limitation as a Part of the Strategy to Stimulate Microbial Lipid Biosynthesis. Appl. Sci. 2021, 11, 11819. [Google Scholar] [CrossRef]
- Snopek, P.; Nowak, D.; Zieniuk, B.; Fabiszewska, A. Aeration and Stirring in Yarrowia lipolytica Lipase Biosynthesis during Batch Cultures with Waste Fish Oil as a Carbon Source. Fermentation 2021, 7, 88. [Google Scholar] [CrossRef]
- Fabiszewska, A.U.; Zieniuk, B.; Kozłowska, M.; Mazurczak-Zieniuk, P.M.; Wołoszynowska, M.; Misiukiewicz-Stępień, P.; Nowak, D. Studies on Upgradation of Waste Fish Oil to Lipid-Rich Yeast Biomass in Yarrowia lipolytica Batch Cultures. Foods 2021, 10, 436. [Google Scholar] [CrossRef]
- Fabiszewska, A.; Misiukiewicz-Stępień, P.; Paplińska-Goryca, M.; Zieniuk, B.; Białecka-Florjańczyk, E. An Insight into Storage Lipid Synthesis by Yarrowia lipolytica Yeast Relating to Lipid and Sugar Substrates Metabolism. Biomolecules 2019, 9, 685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabiszewska, A.; Pielińska, A.; Mazurczak, P.; Zieniuk, B.; Wołoszynowska, M. Impact of the selected factors on extraction yield and composition of fatty acids of microbial oil produced by the yeast cells of Yarrowia lipolytica. Żywność. Nauka. Technol. Jakość. 2017, 24, 58–68. (In Polish) [Google Scholar]
- Rakicka, M.; Lazar, Z.; Dulermo, T.; Fickers, P.; Nicaud, J.M. Lipid production by the oleaginous yeast Yarrowia lipolytica using industrial by-products under different culture conditions. Biotechnol. Biofuels 2015, 8, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabra, W.; Bommareddy, R.R.; Maheshwari, G.; Papanikolaou, S.; Zeng, A.P. Substrates and oxygen dependent citric acid production by Yarrowia lipolytica: Insights through transcriptome and fluxome analyses. Microb. Cell Fact. 2017, 16, 78. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Chevalot, I.; Galiotou-Panayotou, M.; Komaitis, M.; Marc, I.; Aggelis, G. Industrial derivative of tallow: A promising renewable substrate for microbial lipid, single-cell protein and lipase production by Yarrowia lipolytica. Electron. J. Biotechnol. 2007, 10, 425–435. [Google Scholar] [CrossRef] [Green Version]
- Otto, C.; Holz, M.; Barth, G. Production of Organic Acids by Yarrowia lipolytica. In Yarrowia lipolytica. Microbiol. Monographs; Barth, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 25, pp. 137–149. [Google Scholar]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part II: Technology and potential applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part I: Biochemistry of single-cell oil production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar] [CrossRef]
- Tomaszewska, L.; Rakicka, M.; Rymowicz, W.; Rywińska, A. A comparative study on glycerol metabolism to erythritol and citric acid in Yarrowia lipolytica yeast cells. FEMS Yeast Res. 2014, 14, 966–976. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Kwak, R.; Kwon, H.; Pham, V.S.; Kim, M.; Al-Anzi, B.; Lim, G.; Han, J. Purification of high salinity brine by multi-stage ion concentration polarization desalination. Sci. Rep. 2016, 6, 31850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; van Dijck, P.W.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, A.; Drzymała, K.; Rzechonek, D.; Mituła, P.; Mirończuk, A. Lipid production from waste materials in seawater-based medium by the yeast Yarrowia lipolytica. Front. Microbiol. 2019, 10, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamimed, S.; Barkaoui, T.; Trabelsi, I.; Landoulsi, A.; Chatti, A. High-performance biological treatment of tuna wash processing wastewater using Yarrowia lipolytica. Environ. Sci. Pollut. Res. 2021, 28, 1545–1554. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Chevalot, I.; Komaitis, M.; Aggelis, G.; Marc, I. Kinetic profile of the cellular lipid composition in an oleaginous Yarrowia lipolytica capable of producing a cocoa-butter substitute from industrial fats. Antonie Leeuwenhoek 2001, 80, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Aggelis, G. Selective uptake of fatty acids by the yeast Yarrowia lipolytica. Eur. J. Lipid Sci. Technol. 2003, 105, 651–655. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Muniglia, L.; Chevalot, I.; Aggelis, G.; Marc, I. Accumulation of a cocoa-butter-like lipid by Yarrowia lipolytica cultivated on agro-industrial residues. Curr. Microbiol. 2003, 46, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Saygün, A.; Şahin-Yeşilçubuk, N.; Aran, N. Effects of different oil sources and residues on biomass and metabolite production by Yarrowia lipolytica YB 423-12. J. Am. Oil Chem. Soc. 2014, 91, 1521–1530. [Google Scholar] [CrossRef]

| Culture Symbol | Mineral Medium | (NH4)2SO4 g/dm3 | Waste Oil Pre-Treatment | Brine, %v/v | Aeration Regulation |
|---|---|---|---|---|---|
| A | pfWO-N-0.25 | 2.5 | no | 0 | yes |
| B | pfWO-N-0.50 | 5.0 | no | 0 | no |
| C | H-pfWO-N-0.25 | 2.5 | yes | 0 | no |
| D | pfWO-N-0.25 | 2.5 | no | 20.0 | yes |
| Culture Medium | Citric Acid Concentration during Lag Phase 1 [g/dm3] | Citric Acid Concentration during Log Phase 1 [g/dm3] | Cellular Lipids Content in Log Phase 1 [g d.m./g] |
|---|---|---|---|
| A-pfWO-N-0.25-Aer-reg | 0.06 ± 0.01 b * | 0.08 ± 0.02 a | 0.43 ± 0.04 a |
| B-pfWO-N-0.50-no-Aer-reg | 0.01 ± 0.00 a | 0.14 ± 0.02 a | 0.31 ± 0.02 b |
| C-H-pfWO-N-0.25-no-Aer-reg | 0.08 ± 0.02 b | 0.78 ± 0.05 b | 0.04 ± 0.01 c |
| Brine Share [%] | Cell Dry Mass [g d.m./dm3] | Yeast Cells Number [log CFU/cm3] |
|---|---|---|
| 0 | 16.2 ± 0.2 a | 9. 14 ± 0.01 |
| 10.0 | 15.7 ± 0.4 a | 9.10 ± 0.01 |
| 20.0 | 14.7 ± 0.3 a | 9.05 ± 0.01 |
| 40.0 | 9.7 ± 1.0 b | 8.16 ± 0.05 |
| 60.0 | 5.3 ± 0.1 c | 7.82 ± 0.09 |
| 80.0 | 2.2 ± 0.5 d | 7.06 ± 0.15 |
| 100.0 1 | 1.0 ± 0.3 d | 4.88 ± 0.41 |
| Fatty Acids | Post-Frying Waste Oil—Initial Substrate [%] | Microbial Oil [%] | Residual Oil in Culture Medium [%] |
|---|---|---|---|
| C14:0 | 0.15 ± 0.01 | 0.44 ± 0.12 | ND * |
| C16:0 | 3.08 ± 0.16 | 4.84 ± 0.43 | 3.18 ± 0.22 |
| C18:0 | 12.89 ± 2.19 | 9.39 ± 0.68 | 15.20 ± 0.44 |
| C18:1 | 60.12 ± 1.46 | 62.42 ± 1.32 | 70.32 ± 1.69 |
| C18:2 | 11.90 ± 1.26 | 13.22 ± 2.04 | 9.39 ± 1.76 |
| C18:3 | 4.26 ± 0.84 | 4.23 ± 0.39 | 1.90 ± 0.15 |
| C20:0 | 6.91 ± 1.31 | 2.96 ± 1.01 | ND |
| C22:0 | 0.68 ± 0.08 | 2.13 ± 0.50 | ND |
| C22:1 | ND | 0.37 ± 0.06 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabiszewska, A.; Wierzchowska, K.; Nowak, D.; Wołoszynowska, M.; Zieniuk, B. Brine and Post-Frying Oil Management in the Fish Processing Industry—A Concept Based on Oleaginous Yeast Culture. Processes 2022, 10, 294. https://doi.org/10.3390/pr10020294
Fabiszewska A, Wierzchowska K, Nowak D, Wołoszynowska M, Zieniuk B. Brine and Post-Frying Oil Management in the Fish Processing Industry—A Concept Based on Oleaginous Yeast Culture. Processes. 2022; 10(2):294. https://doi.org/10.3390/pr10020294
Chicago/Turabian StyleFabiszewska, Agata, Katarzyna Wierzchowska, Dorota Nowak, Małgorzata Wołoszynowska, and Bartłomiej Zieniuk. 2022. "Brine and Post-Frying Oil Management in the Fish Processing Industry—A Concept Based on Oleaginous Yeast Culture" Processes 10, no. 2: 294. https://doi.org/10.3390/pr10020294
APA StyleFabiszewska, A., Wierzchowska, K., Nowak, D., Wołoszynowska, M., & Zieniuk, B. (2022). Brine and Post-Frying Oil Management in the Fish Processing Industry—A Concept Based on Oleaginous Yeast Culture. Processes, 10(2), 294. https://doi.org/10.3390/pr10020294








