Recent Progress Using Solid-State Materials for Hydrogen Storage: A Short Review
Abstract
:1. Introduction
2. Hydrogen Storage Techniques
3. Hydrogen Storage Materials for Physisorption Methods
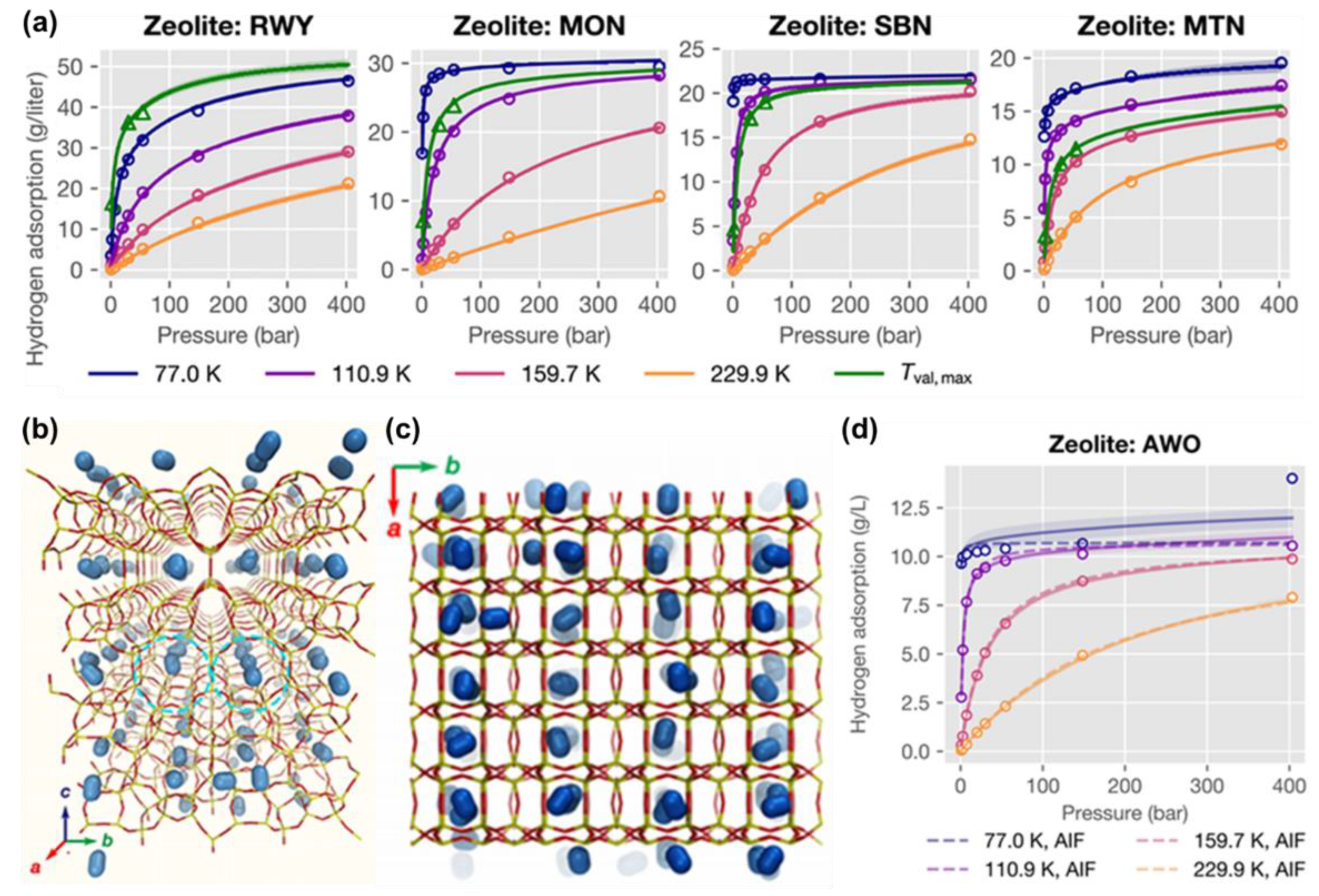
3.1. Non-Carbonaceous Materials for Hydrogen Storage
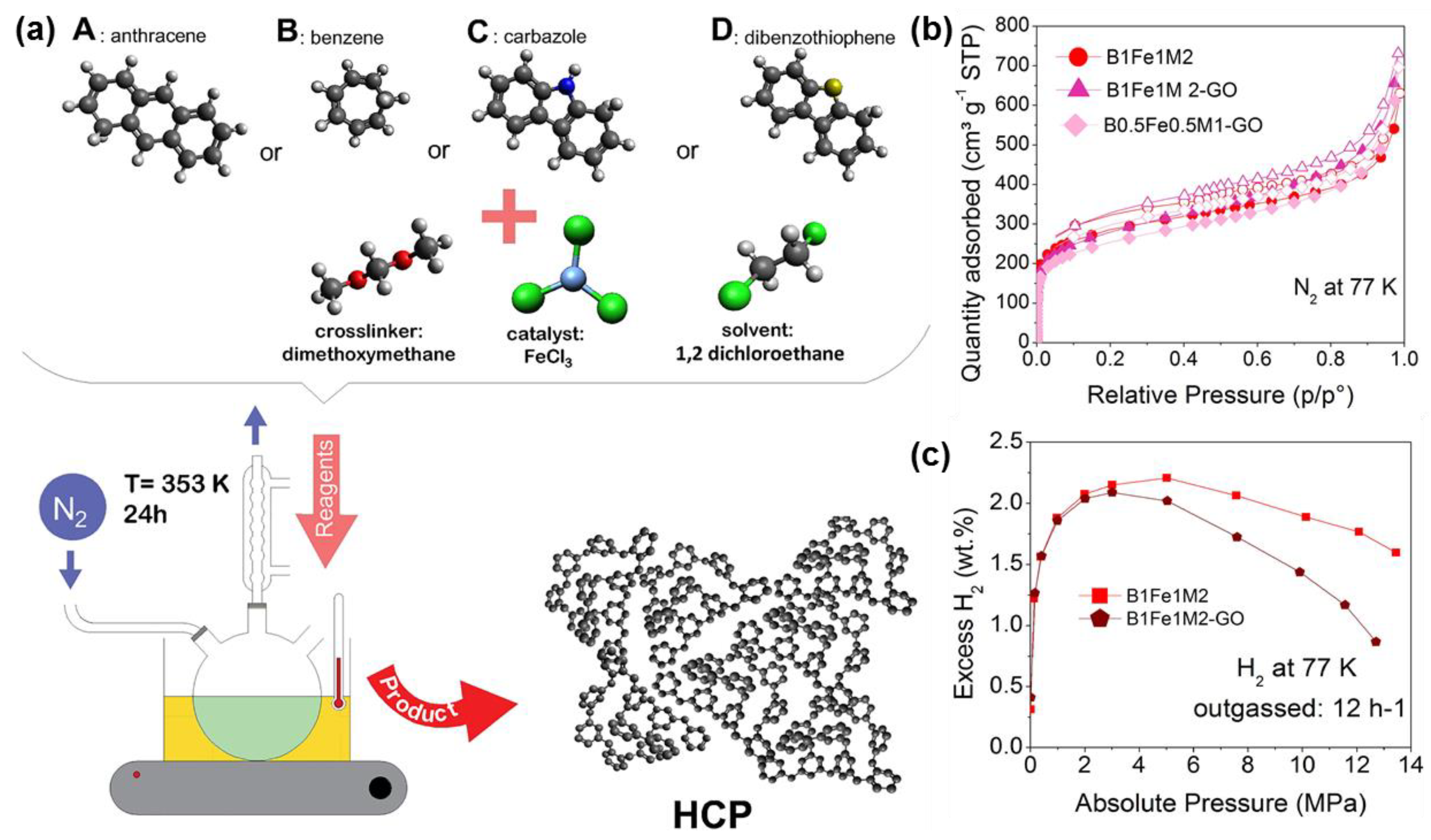
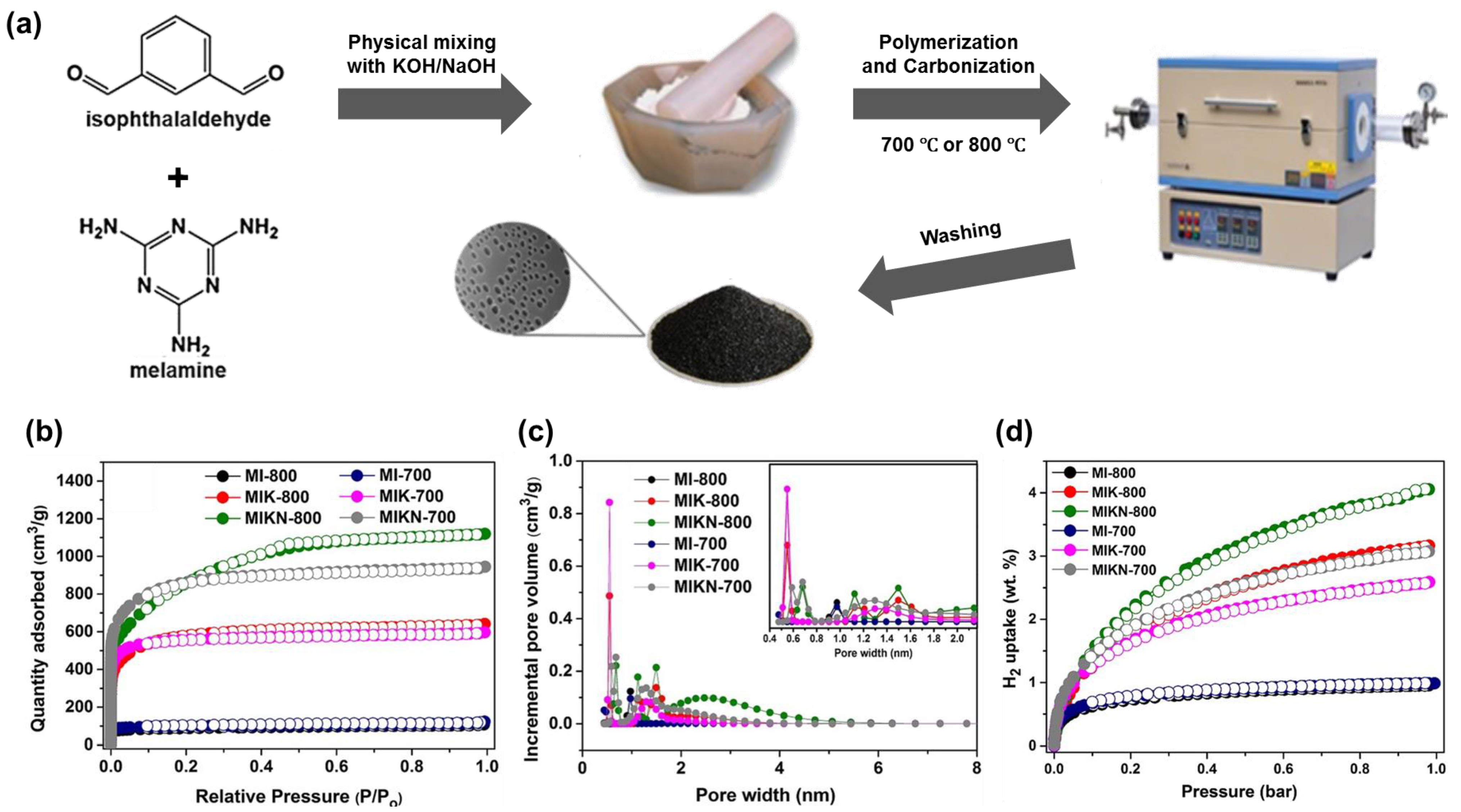
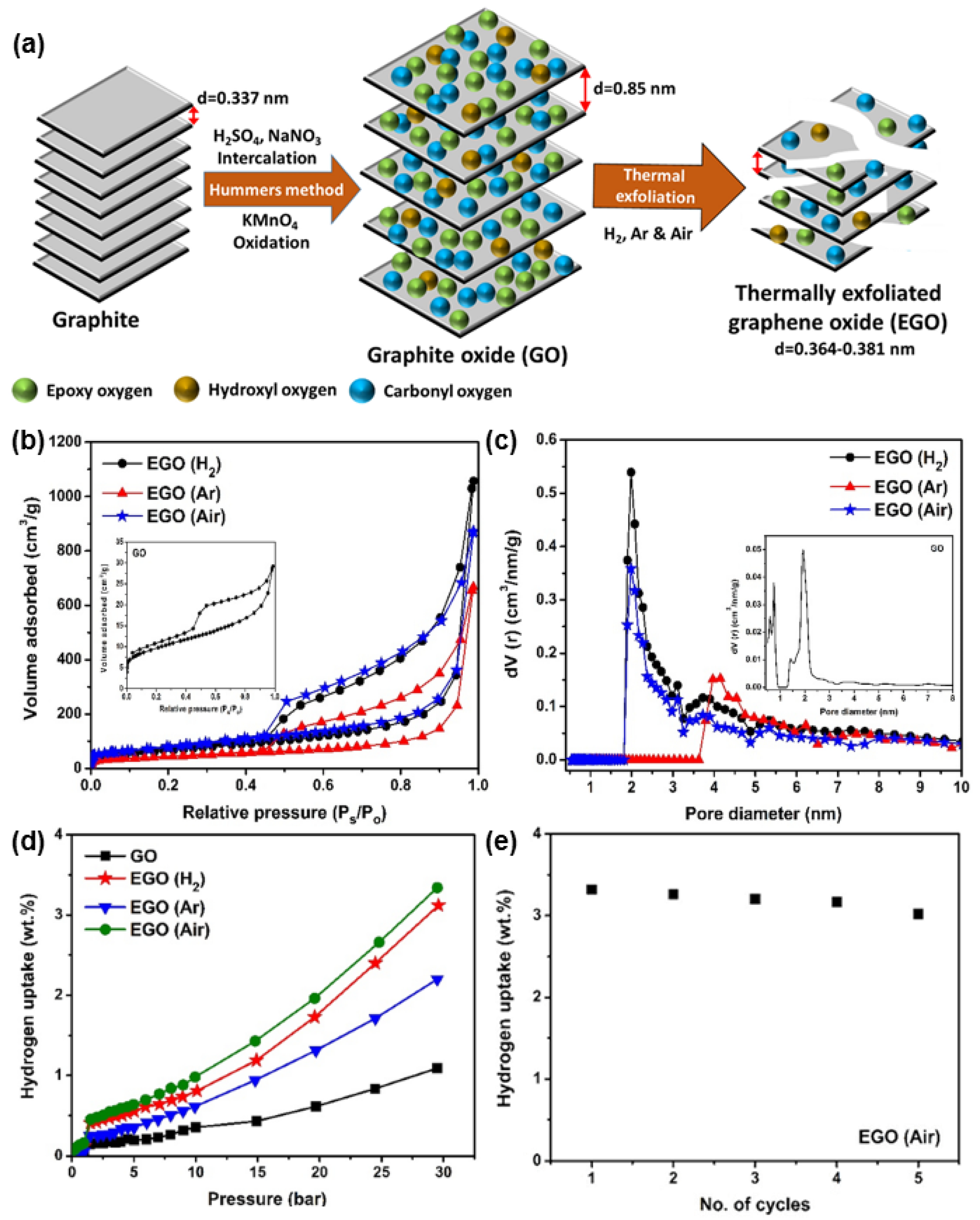
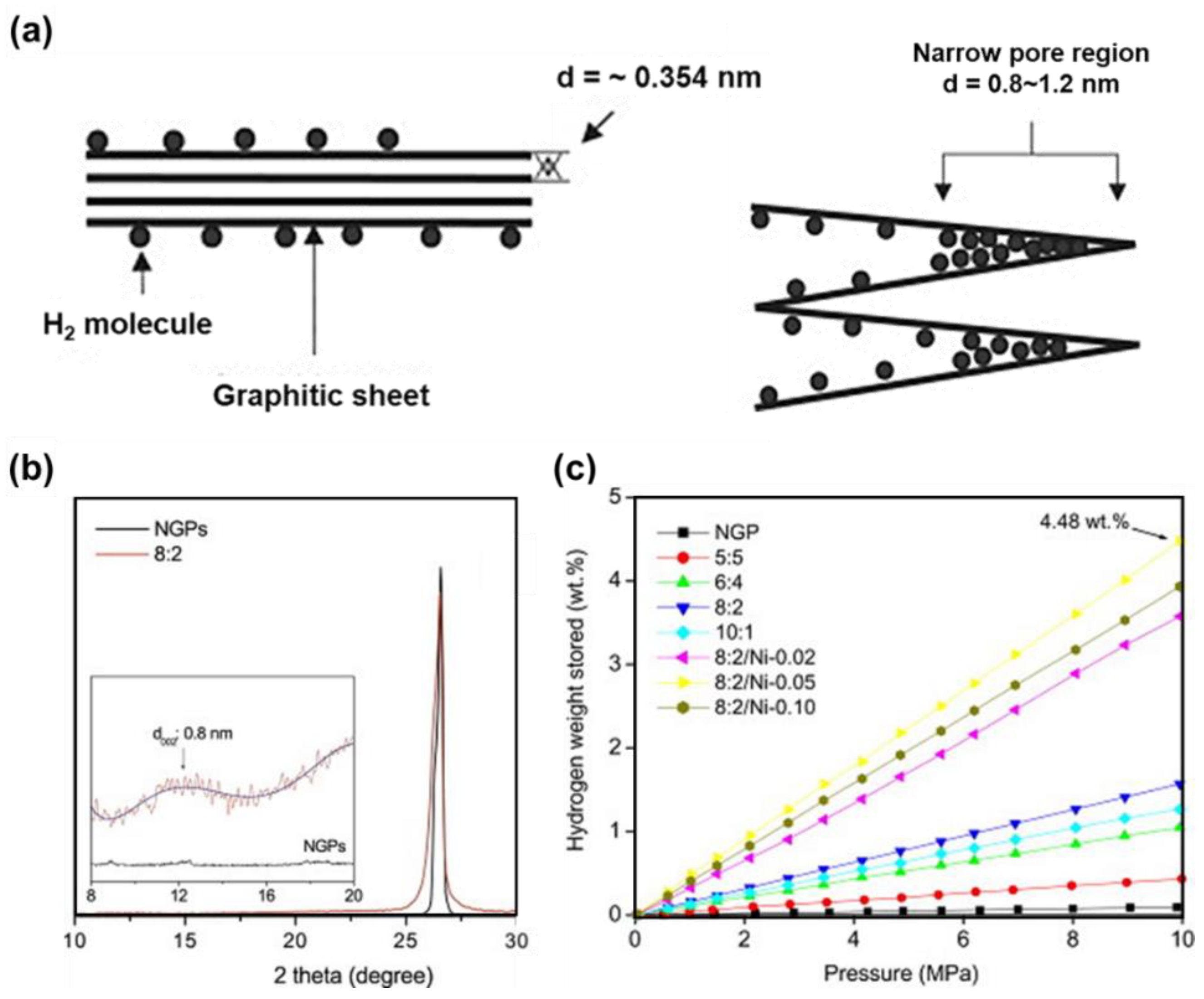
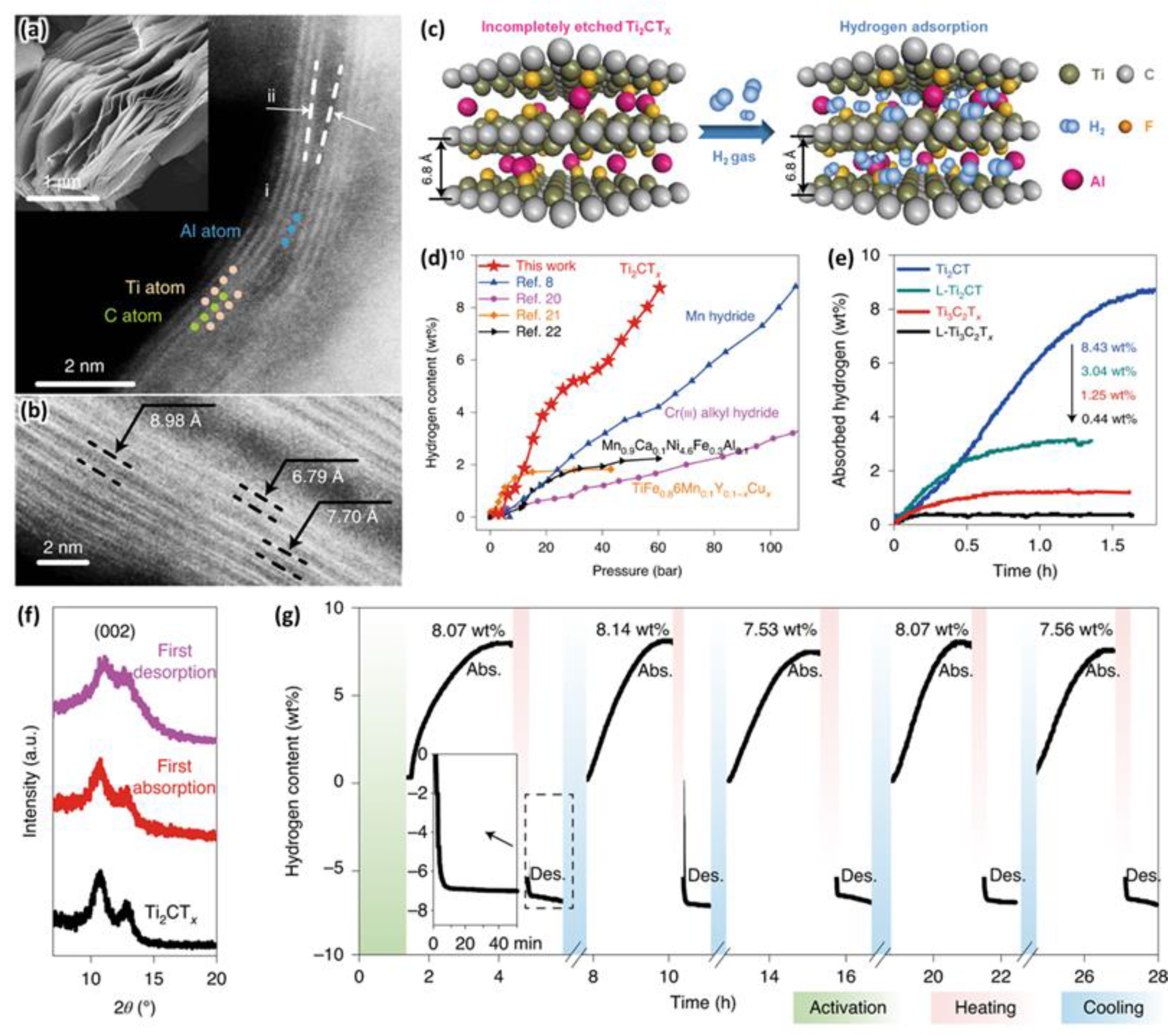
3.2. Carbonaceous Materials for Hydrogen Storage
4. The Adsorption Models for Hydrogen Storage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schlapbach, L.; Zuttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Schoedel, A.; Ji, Z.; Yaghi, O.M. The role of metal-organic frameworks in a carbon-neutral energy cycle. Nat. Energy 2016, 1, 16034. [Google Scholar] [CrossRef]
- Schneemann, A.; White, J.L.; Kang, S.; Jeong, S.; Wan, L.W.F.; Cho, E.S.; Heo, T.W.; Prendergast, D.; Urban, J.J.; Wood, B.C.; et al. Nanostructured Metal Hydrides for Hydrogen Storage. Chem. Rev. 2018, 118, 10775–10839. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Allendorf, M.D.; Hulvey, Z.; Gennett, T.; Ahmed, A.; Autrey, T.; Camp, J.; Cho, E.S.; Furukawa, H.; Haranczyk, M.; Head-Gordon, M.; et al. An assessment of strategies for the development of solid-state adsorbents for vehicular hydrogen storage. Energy Environ. Sci. 2018, 11, 2784–2812. [Google Scholar] [CrossRef] [Green Version]
- Ueckerdt, F.; Bauer, C.; Dirnaichner, A.; Everall, J.; Sacchi, R.; Luderer, G. Potential and risks of hydrogen-based e-fuels in climate change mitigation. Nat. Clim. Chang. 2021, 11, 384–393. [Google Scholar] [CrossRef]
- Doe, U. Target explanation document: Onboard hydrogen storage for light-duty fuel cell vehicles. US Drive 2017, 1, 1–29. [Google Scholar]
- Morris, L.; Hales, J.J.; Trudeau, M.L.; Georgiev, P.; Embs, J.P.; Eckert, J.; Kaltsoyannis, N.; Antonelli, D.M. A manganese hydride molecular sieve for practical hydrogen storage under ambient conditions. Energy Environ. Sci. 2019, 12, 1580–1591. [Google Scholar] [CrossRef] [Green Version]
- Czarna-Juszkiewicz, D.; Cader, J.; Wdowin, M. From coal ashes to solid sorbents for hydrogen storage. J. Clean. Prod. 2020, 270, 122355. [Google Scholar] [CrossRef]
- Shiraz, H.G.; Tavakoli, O. Investigation of graphene-based systems for hydrogen storage. Renew. Sustain. Energy Rev. 2017, 74, 104–109. [Google Scholar] [CrossRef]
- Hassan, I.A.; Ramadan, H.S.; Saleh, M.A.; Hissel, D. Hydrogen storage technologies for stationary and mobile applications: Review, analysis and perspectives. Renew. Sustain. Energy Rev. 2021, 149, 111311. [Google Scholar] [CrossRef]
- Xiao, R.F.; Tian, G.; Hou, Y.; Chen, S.T.; Cheng, C.; Chen, L. Effects of cooling-recovery venting on the performance of cryo-compressed hydrogen storage for automotive applications. Appl. Energy 2020, 269, 115143. [Google Scholar] [CrossRef]
- Ramirez-Vidal, P.; Canevesi, R.L.S.; Sdanghi, G.; Schaefer, S.; Maranzana, G.; Celzard, A.; Fierro, V. A Step Forward in Understanding the Hydrogen Adsorption and Compression on Activated Carbons. ACS Appl. Mater. Interfaces 2021, 13, 12562–12574. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L. Progress and problems in hydrogen storage methods. Renew. Sustain. Energy Rev. 2005, 9, 395–408. [Google Scholar] [CrossRef]
- Thornton, A.W.; Simon, C.M.; Kim, J.; Kwon, O.; Deeg, K.S.; Konstas, K.; Pas, S.J.; Hill, M.R.; Winkler, D.A.; Haranczyk, M.; et al. Materials Genome in Action: Identifying the Performance Limits of Physical Hydrogen Storage. Chem. Mater. 2017, 29, 2844–2854. [Google Scholar] [CrossRef]
- Li, M.X.; Bai, Y.F.; Zhang, C.Z.; Song, Y.X.; Jiang, S.F.; Grouset, D.; Zhang, M.J. Review on the research of hydrogen storage system fast refueling in fuel cell vehicle. Int. J. Hydrogen Energy 2019, 44, 10677–10693. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Lv, H.; Zhou, W.; Zhang, C. Review of the Hydrogen Permeability of the Liner Material of Type IV On-Board Hydrogen Storage Tank. World Electr. Veh. J. 2021, 12, 130. [Google Scholar] [CrossRef]
- Benitez, A.; Wulf, C.; de Palmenaer, A.; Lengersdorf, M.; Roding, T.; Grube, T.; Robinius, M.; Stolten, D.; Kuckshinrichs, W. Ecological assessment of fuel cell electric vehicles with special focus on type IV carbon fiber hydrogen tank. J. Clean. Prod. 2021, 278, 123277. [Google Scholar] [CrossRef]
- Liu, H.R.; Wang, C.J.; Chen, B.; Zhang, Z. A further study of pyrolysis of carbon fibre-epoxy composite from hydrogen tank: Search optimization for kinetic parameters via a Shuffled Complex Evolution. J. Hazard. Mater. 2019, 374, 20–25. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.J.; Huang, G.; Liu, H.R.; Yang, S.L.; Zhang, A.F. Thermal degradation behaviors and reaction mechanism of carbon fibre-epoxy composite from hydrogen tank by TG-FTIR. J. Hazard. Mater. 2018, 357, 73–80. [Google Scholar] [CrossRef]
- Kim, W.J.; Heo, Y.J.; Lee, J.H.; Rhee, K.Y.; Park, S.J. Effect of Atmospheric-Pressure Plasma Treatments on Fracture Toughness of Carbon Fibers-Reinforced Composites. Molecules 2021, 26, 3698. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.Q.; Roh, H.S.; Ahluwalia, R.K. Performance assessment of 700-bar compressed hydrogen storage for light duty fuel cell vehicles. Int. J. Hydrogen Energy 2017, 42, 25121–25129. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sdanghi, G.; Maranzana, G.; Celzard, A.; Fierro, V. Review of the current technologies and performances of hydrogen compression for stationary and automotive applications. Renew. Sustain. Energy Rev. 2019, 102, 150–170. [Google Scholar] [CrossRef]
- Reuss, M.; Grube, T.; Robinius, M.; Preuster, P.; Wasserscheid, P.; Stolten, D. Seasonal storage and alternative carriers: A flexible hydrogen supply chain model. Appl. Energy 2017, 200, 290–302. [Google Scholar] [CrossRef]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.-W. Hydrogen storage in metal–organic frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, H.; Wang, C.G.; Ye, E.Y.; Xu, J.W.; Loh, X.J.; Li, Z.B. Current research progress and perspectives on liquid hydrogen rich molecules in sustainable hydrogen storage. Energy Storage Mater. 2021, 35, 695–722. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Gong, M.Q.; Zhou, Y.; Dong, X.Q.; Shen, J. Thermodynamics analysis of hydrogen storage based on compressed gaseous hydrogen, liquid hydrogen and cryo-compressed hydrogen. Int. J. Hydrogen Energy 2019, 44, 16833–16840. [Google Scholar] [CrossRef]
- Wei, G.M.; Zhang, J.F. Numerical Study of the Filling Process of a Liquid Hydrogen Storage Tank under Different Sloshing Conditions. Processes 2020, 8, 1020. [Google Scholar] [CrossRef]
- Bai, X.S.; Yang, W.W.; Tang, X.Y.; Yang, F.S.; Jiao, Y.H.; Yang, Y. Optimization of tree-shaped fin structures towards enhanced absorption performance of metal hydride hydrogen storage device: A numerical study. Energy 2021, 220, 119738. [Google Scholar] [CrossRef]
- Gao, S.C.; Liu, H.Z.; Xu, L.; Li, S.Q.; Wang, X.H.; Yan, M. Hydrogen storage properties of nano-CoB/CNTs catalyzed MgH2. J. Alloy. Compd. 2018, 735, 635–642. [Google Scholar] [CrossRef]
- Lotoskyy, M.; Denys, R.; Yartys, V.A.; Eriksen, J.; Goh, J.; Nyamsi, S.N.; Sita, C.; Cummings, F. An outstanding effect of graphite in nano-MgH2-TiH2 on hydrogen storage performance. J. Mater. Chem. A 2018, 6, 10740–10754. [Google Scholar] [CrossRef]
- Cho, E.S.; Ruminski, A.M.; Liu, Y.S.; Shea, P.T.; Kang, S.Y.; Zaia, E.W.; Park, J.Y.; Chuang, Y.D.; Yuk, J.M.; Zhou, X.W.; et al. Hierarchically Controlled Inside-Out Doping of Mg Nanocomposites for Moderate Temperature Hydrogen Storage. Adv. Funct. Mater. 2017, 27, 1704316. [Google Scholar] [CrossRef]
- Zhang, J.G.; Zhu, Y.F.; Lin, H.J.; Liu, Y.N.; Zhang, Y.; Li, S.Y.; Ma, Z.L.; Li, L.Q. Metal Hydride Nanoparticles with Ultrahigh Structural Stability and Hydrogen Storage Activity Derived from Microencapsulated Nanoconfinement. Adv. Mater. 2017, 29, 1700760. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Luo, Q.; Gu, Q.F. Insights into the composition exploration of novel hydrogen storage alloys: Evaluation of the Mg-Ni-Nd-H phase diagram. J. Mater. Chem. A 2017, 5, 3848–3864. [Google Scholar] [CrossRef]
- Liu, Y.S.; Wang, S.; Li, Z.L.; Gao, M.X.; Liu, Y.F.; Sun, W.P.; Pan, H.G. Enhanced Hydrogen Storage Performance of MgH2 by the Catalysis of a Novel Intersected Y2O3/NiO Hybrid. Processes 2021, 9, 892. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Shabani, B. Review of metal hydride hydrogen storage thermal management for use in the fuel cell systems. Int. J. Hydrogen Energy 2021, 46, 31699–31726. [Google Scholar] [CrossRef]
- Hang, Z.M.; Hu, Z.C.; Xiao, X.Z.; Jiang, R.C.; Zhang, M. Enhancing Hydrogen Storage Kinetics and Cycling Properties of NaMgH3 by 2D Transition Metal Carbide MXene Ti3C2. Processes 2021, 9, 1690. [Google Scholar] [CrossRef]
- Lin, X.; Xie, W.; Zhu, Q.; Yang, H.G.; Li, Q. Rational optimization of metal hydride tank with LaNi4.25Al0.75 as hydrogen storage medium. Chem. Eng. J. 2021, 421, 127844. [Google Scholar] [CrossRef]
- Rizo-Acosta, P.; Cuevas, F.; Latroche, M. Hydrides of early transition metals as catalysts and grain growth inhibitors for enhanced reversible hydrogen storage in nanostructured magnesium. J. Mater. Chem. A 2019, 7, 23064–23075. [Google Scholar] [CrossRef]
- Wang, H.; Wu, G.; Cao, H.; Pistidda, C.; Chaudhary, A.L.; Garroni, S.; Dornheim, M.; Chen, P. Near ambient condition hydrogen storage in a synergized tricomponent hydride system. Adv. Energy Mater. 2017, 7, 1602456. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, R.B.; Wang, J.; Wang, B.; Liang, B.; Yildirim, T.; Zhang, J.; Zhou, W.; Chen, B.L. Optimization of the Pore Structures of MOFs for Record High Hydrogen Volumetric Working Capacity. Adv. Mater. 2020, 32, 1907995. [Google Scholar] [CrossRef] [PubMed]
- Eberle, U.; Felderhoff, M.; Schuth, F. Chemical and Physical Solutions for Hydrogen Storage. Angew. Chem. Int. Edit 2009, 48, 6608–6630. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Aulakh, D.; Purewal, J.; Siegel, D.J.; Veenstra, M.; Matzger, A.J. Optimizing Hydrogen Storage in MOFs through Engineering of Crystal Morphology and Control of Crystal Size. J. Am. Chem. Soc. 2021, 143, 10727–10734. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.J.; Yeon, S.H.; Park, S.J. Defining contribution of micropore size to hydrogen physisorption behaviors: A new approach based on DFT pore volumes. Carbon 2019, 143, 288–293. [Google Scholar] [CrossRef]
- Zuo, Z.Q.; Sun, P.J.; Jiang, W.B.; Qin, X.J.; Li, P.; Huang, Y.H. Thermal stratification suppression in reduced or zero boil-off hydrogen tank by self-spinning spray bar. Int. J. Hydrogen Energy 2019, 44, 20158–20172. [Google Scholar] [CrossRef]
- Sun, Y.Z.S.; DeJaco, R.F.; Li, Z.; Tang, D.; Glante, S.; Sholl, D.S.; Colina, C.M.; Snurr, R.Q.; Thommes, M.; Hartmann, M.; et al. Fingerprinting diverse nanoporous materials for optimal hydrogen storage conditions using meta-learning. Sci. Adv. 2021, 7, eabg3983. [Google Scholar] [CrossRef]
- Weng, Q.H.; Zeng, L.L.; Chen, Z.W.; Han, Y.X.; Jiang, K.; Bando, Y.; Golberg, D. Hydrogen Storage in Carbon and Oxygen Co-Doped Porous Boron Nitrides. Adv. Funct. Mater. 2021, 31, 2007381. [Google Scholar] [CrossRef]
- Balderas-Xicohtencatl, R.; Schmieder, P.; Denysenko, D.; Volkmer, D.; Hirscher, M. High Volumetric Hydrogen Storage Capacity using Interpenetrated Metal-Organic Frameworks. Energy Technol.-Ger. 2018, 6, 510–512. [Google Scholar] [CrossRef] [Green Version]
- Rozzi, E.; Minuto, F.D.; Lanzini, A. Dynamic modeling and thermal management of a Power-to-Power system with hydrogen storage in microporous adsorbent materials. J. Energy Storage 2021, 41, 102953. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.H.; Yuan, L.H.; Zhang, M.L.; Zhang, C.R. Scandium Decoration of Boron Doped Porous Graphene for High-Capacity Hydrogen Storage. Molecules 2019, 24, 2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.Q.; Kobayashi, H.; Taylor, J.M.; Ikeda, R.; Kubota, Y.; Kato, K.; Takata, M.; Yamamoto, T.; Toh, S.; Matsumura, S.; et al. Hydrogen storage in Pd nanocrystals covered with a metal-organic framework. Nat. Mater. 2014, 13, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Reardon, H.; Hanlon, J.M.; Hughes, R.W.; Godula-Jopek, A.; Mandal, T.K.; Gregory, D.H. Emerging concepts in solid-state hydrogen storage: The role of nanomaterials design. Energy Environ. Sci. 2012, 5, 5951–5979. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. Preparation and characterization of ordered porous carbons for increasing hydrogen storage behaviors. J. Solid State Chem. 2011, 184, 2655–2660. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. Influence of oxygen-functional groups on carbon replicas for hydrogen adsorption. Phys Status Solidi A 2012, 209, 694–697. [Google Scholar] [CrossRef]
- Liang, X.Y.; Ng, S.P.; Ding, N.; Wu, C.M.L. Strain-induced switch for hydrogen storage in cobalt-decorated nitrogen-doped graphene. Appl. Surf. Sci. 2019, 473, 174–181. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, T.J.D. Electronic Structure Calculations of Hydrogen Storage in Lithium Decorated Metal-Graphyne Framework. ACS Appl. Mater. Interfaces 2017, 9, 28659–28666. [Google Scholar] [CrossRef]
- Makowski, P.; Thomas, A.; Kuhn, P.; Goettmann, F. Organic materials for hydrogen storage applications: From physisorption on organic solids to chemisorption in organic molecules. Energy Environ. Sci. 2009, 2, 480–490. [Google Scholar] [CrossRef]
- Guo, C.X.; Wang, Y.; Li, C.M. Hierarchical Graphene-Based Material for Over 4.0 Wt % Physisorption Hydrogen Storage Capacity. ACS Sustain. Chem. Eng. 2013, 1, 14–18. [Google Scholar] [CrossRef]
- Dong, J.X.; Wang, X.Y.; Xu, H.; Zhao, Q.; Li, J.P. Hydrogen storage in several microporous zeolites. Int. J. Hydrogen Energy 2007, 32, 4998–5004. [Google Scholar] [CrossRef]
- Chae, H.K.; Siberio-Perez, D.Y.; Kim, J.; Go, Y.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Langmi, H.W.; Book, D.; Walton, A.; Johnson, S.R.; Al-Mamouri, M.M.; Speight, J.D.; Edwards, P.P.; Harris, I.R.; Anderson, P.A. Hydrogen storage in ion-exchanged zeolites. J. Alloy. Compd. 2005, 404, 637–642. [Google Scholar] [CrossRef]
- Forster, P.M.; Eckert, J.; Chang, J.S.; Park, S.E.; Ferey, G.; Cheetham, A.K. Hydrogen adsorption in nanoporous Nickel(II) phosphates. J. Am. Chem. Soc. 2003, 125, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Arean, C.O.; Palomino, G.T.; Carayol, M.R.L. Variable temperature FT-IR studies on hydrogen adsorption on the zeolite (Mg,Na)-Y. Appl. Surf. Sci. 2007, 253, 5701–5704. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. Synthesis of zeolite-casted microporous carbons and their hydrogen storage capacity. J. Colloid Interface Sci. 2012, 384, 116–120. [Google Scholar] [CrossRef]
- Kapelewski, M.T.; Runcevski, T.; Tarver, J.D.; Jiang, H.Z.H.; Hurst, K.E.; Parilla, P.A.; Ayala, A.; Gennett, T.; FitzGerald, S.A.; Brown, C.M.; et al. Record High Hydrogen Storage Capacity in the Metal-Organic Framework Ni-2(m-dobdc) at Near-Ambient Temperatures. Chem. Mater. 2018, 30, 8179–8189. [Google Scholar] [CrossRef]
- Rostami, S.; Pour, A.N.; Salimi, A.; Abolghasempour, A. Hydrogen adsorption in metal- organic frameworks (MOFs): Effects of adsorbent architecture. Int. J. Hydrogen Energy 2018, 43, 7072–7080. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. Effect of platinum doping of activated carbon on hydrogen storage behaviors of metal-organic frameworks-5. Int. J. Hydrogen Energy 2011, 36, 8381–8387. [Google Scholar] [CrossRef]
- Daglar, H.; Gulbalkan, H.C.; Avci, G.; Aksu, G.O.; Altundal, O.F.; Altintas, C.; Erucar, I.; Keskin, S. Effect of Metal-Organic Framework (MOF) Database Selection on the Assessment of Gas Storage and Separation Potentials of MOFs. Angew. Chem. Int. Edit 2021, 60, 7828–7837. [Google Scholar] [CrossRef]
- Camp, J.; Stavila, V.; Allendorf, M.D.; Prendergast, D.; Haranczyk, M. Critical Factors in Computational Characterization of Hydrogen Storage in Metal-Organic Frameworks. J. Phys. Chem. C 2018, 122, 18957–18967. [Google Scholar] [CrossRef]
- Jaramillo, D.E.; Jiang, H.Z.H.; Evans, H.A.; Chakraborty, R.; Furukawa, H.; Brown, C.M.; Head-Gordon, M.; Long, J.R. Ambient-Temperature Hydrogen Storage via Vanadium(II)-Dihydrogen Complexation in a Metal-Organic Framework. J. Am. Chem. Soc. 2021, 143, 6248–6256. [Google Scholar] [CrossRef] [PubMed]
- Witman, M.; Ling, S.L.; Gladysiak, A.; Stylianou, K.C.; Smit, B.; Slater, B.; Haranczyk, M. Rational Design of a Low-Cost, High-Performance Metal-Organic Framework for Hydrogen Storage and Carbon Capture. J. Phys. Chem. C 2017, 121, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Seth, S.; Purewal, J.; Wong-Foy, A.G.; Veenstra, M.; Matzger, A.J.; Siegel, D.J. Exceptional hydrogen storage achieved by screening nearly half a million metal-organic frameworks. Nat. Commun. 2019, 10, 1568. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.A.; Shulda, S.; Parilla, P.A.; Le Magueres, P.; Richardson, R.K.; Morris, W.; Brown, C.M.; McGuirk, C.M. Structural resolution and mechanistic insight into hydrogen adsorption in flexible ZIF-7. Chem. Sci. 2021, 12, 15620–15631. [Google Scholar] [CrossRef] [PubMed]
- Panchariya, D.K.; Rai, R.K.; Kumar, E.A.; Singh, S.K. Core-Shell Zeolitic Imidazolate Frameworks for Enhanced Hydrogen Storage. ACS Omega 2018, 3, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ji, D.; Zhou, H.; Yan, X.F.; Yuan, A.H. Nickel-Platinum Nanoparticles Supported on Zeolitic Imidazolate Framework/Graphene Oxide as High-Performance Adsorbents for Ambient-Temperature Hydrogen Storage. J. Nanosci. Nanotechnol. 2017, 17, 1400–1406. [Google Scholar] [CrossRef]
- Ethiraj, J.; Palla, S.; Reinsch, H. Insights into high pressure gas adsorption properties of ZIF-67: Experimental and theoretical studies. Microporous Mesoporous Mater. 2020, 294, 109867. [Google Scholar] [CrossRef]
- Zhan, G.W.; Zeng, H.C. Hydrogen spillover through Matryoshka-type (ZIFs@)(n-1)ZIFs nanocubes. Nat. Commun. 2018, 9, 3778. [Google Scholar] [CrossRef] [Green Version]
- Ke, Z.P.; Cheng, Y.Y.; Yang, S.Y.; Li, F.; Ding, L.F. Modification of COF-108 via impregnation/functionalization and Li-doping for hydrogen storage at ambient temperature. Int. J. Hydrogen Energy 2017, 42, 11461–11468. [Google Scholar] [CrossRef]
- Tong, M.M.; Zhu, W.C.; Li, J.; Long, Z.Y.; Zhao, S.; Chen, G.J.; Lan, Y.S. An easy way to identify high performing covalent organic frameworks for hydrogen storage. Chem. Commun. 2020, 56, 6376–6379. [Google Scholar] [CrossRef]
- Braunecker, W.A.; Shulda, S.; Martinez, M.B.; Hurst, K.E.; Koubek, J.T.; Zaccarine, S.; Mow, R.E.; Pylypenko, S.; Sellinger, A.; Gennett, T.; et al. Thermal Activation of a Copper-Loaded Covalent Organic Framework for Near-Ambient Temperature Hydrogen Storage and Delivery. ACS Mater. Lett. 2020, 2, 227–232. [Google Scholar] [CrossRef]
- Ramirez-Vidal, P.; Suarez-Garcia, F.; Canevesi, R.L.S.; Castro-Muniz, A.; Gadonneix, P.; Paredes, J.I.; Celzard, A.; Fierro, V. Irreversible deformation of hyper-crosslinked polymers after hydrogen adsorption. J. Colloid Interface Sci. 2022, 605, 513–527. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Zhu, Y.W.; Liu, Q.Y.; Li, X.W. Preparation of porous carbon materials from biomass pyrolysis vapors for hydrogen storage. Appl. Energy 2022, 306, 118131. [Google Scholar] [CrossRef]
- Naheed, L.; Koppel, M.; Paalo, M.; Alsabawi, K.; Lamb, K.E.; Gray, E.M.; Janes, A.; Webb, C.J. Hydrogen adsorption properties of carbide-derived carbons at ambient temperature and high pressure. Int. J. Hydrogen Energy 2021, 46, 15761–15772. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.J. Recent advances in preparations and applications of carbon aerogels: A review. Carbon 2020, 163, 1–18. [Google Scholar] [CrossRef]
- Choi, Y.K.; Park, S.J. Hydrogen storage capacity of highly porous carbons synthesized from biomass-derived aerogels. Carbon Lett. 2015, 16, 127–131. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.K.; Park, S.J. Preparation and characterization of sucrose-based microporous carbons for increasing hydrogen storage. J. Ind. Eng. Chem. 2015, 28, 32–36. [Google Scholar] [CrossRef]
- Im, J.S.; Kwon, O.; Kim, Y.H.; Park, S.J.; Lee, Y.S. The effect of embedded vanadium catalyst on activated electrospun CFs for hydrogen storage. Microporous Mesoporous Mater. 2008, 115, 514–521. [Google Scholar] [CrossRef]
- Jung, M.J.; Kim, J.W.; Im, J.S.; Park, S.J.; Lee, Y.S. Nitrogen and hydrogen adsorption of activated carbon fibers modified by fluorination. J. Ind. Eng. Chem. 2009, 15, 410–414. [Google Scholar] [CrossRef]
- Ustinov, E.A.; Gavrilov, V.Y.; Mel’gunov, S.; Sokolov, V.V.; Berveno, V.P.; Berveno, A.V. Characterization of activated carbons with low-temperature hydrogen adsorption. Carbon 2017, 121, 563–573. [Google Scholar] [CrossRef]
- Gaboardi, M.; Amade, N.S.; Aramini, M.; Milanese, C.; Magnani, G.; Sanna, S.; Ricco, M.; Pontiroli, D. Extending the hydrogen storage limit in fullerene. Carbon 2017, 120, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Alhameedi, K.; Hussain, T.; Bae, H.; Jayatilaka, D.; Lee, H.; Karton, A. Reversible hydrogen storage properties of defect-engineered C4N nanosheets under ambient conditions. Carbon 2019, 152, 344–353. [Google Scholar] [CrossRef]
- Kim, B.J.; Bae, K.M.; An, K.H.; Park, S.J. Effects of Mechanical Ball Milling with Active Gases on Hydrogen Adsorption Behaviors of Graphite Flakes. J. Nanosci. Nanotechnol. 2012, 12, 5713–5718. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Park, B.H.; Park, S.J.; Kim, C.K. Modeling studies on the uptake of hydrogen molecules by graphene. J. Mol. Modeling 2015, 21, 240. [Google Scholar] [CrossRef]
- Lee, H.I.; Kim, W.J.; Heo, Y.J.; Son, Y.R.; Park, S.J. Control of interlayer spacing of expanded graphite for improved hydrogen storage capacity. Carbon Lett. 2018, 27, 117–120. [Google Scholar] [CrossRef]
- Kaskun, S.; Akinay, Y.; Kayfeci, M. Improved hydrogen adsorption of ZnO doped multi-walled carbon nanotubes. Int. J. Hydrogen Energy 2020, 45, 34949–34955. [Google Scholar] [CrossRef]
- Wang, Y.X.; Hu, X.D.; Guo, T.; Tian, W.G.; Hao, J.; Guo, Q.J. The competitive adsorption mechanism of CO2, H2O and O2 on a solid amine adsorbent. Chem. Eng. J. 2021, 416, 129007. [Google Scholar] [CrossRef]
- Lee, J.H.; Rhee, K.Y.; Park, S.J. Effects of cryomilling on the structures and hydrogen storage characteristics of multi-walled carbon nanotubes. Int. J. Hydrogen Energy 2010, 35, 7850–7857. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. Effect of temperature on activated carbon nanotubes for hydrogen storage behaviors. Int. J. Hydrogen Energy 2010, 35, 6757–6762. [Google Scholar] [CrossRef]
- Lee, S.Y.; Rhee, K.Y.; Nahm, S.H.; Park, S.J. Effect of p-type multi-walled carbon nanotubes for improving hydrogen storage behaviors. J. Solid State Chem. 2014, 210, 256–260. [Google Scholar] [CrossRef]
- Han, Y.J.; Park, S.J. Influence of nickel nanoparticles on hydrogen storage behaviors of MWCNTs. Appl. Surf. Sci. 2017, 415, 85–89. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. Effect of chemical treatments on hydrogen storage behaviors of multi-walled carbon nanotubes. Mater. Chem. Phys. 2010, 124, 1011–1014. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. Hydrogen Adsorption of Acid-treated Multi-walled Carbon Nanotubes at Low Temperature. Bull. Korean Chem. Soc. 2010, 31, 1596–1600. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Park, S.J. Influence of the pore size in multi-walled carbon nanotubes on the hydrogen storage behaviors. J. Solid State Chem. 2012, 194, 307–312. [Google Scholar] [CrossRef]
- Dillon, A.C.; Jones, K.M.; Bekkedahl, T.A.; Kiang, C.H.; Bethune, D.S.; Heben, M.J. Storage of hydrogen in single-walled carbon nanotubes. Nature 1997, 386, 377–379. [Google Scholar] [CrossRef]
- Pupysheva, O.V.; Farajian, A.A.; Yakobson, B.I. Fullerene nanocage capacity for hydrogen storage. Nano Lett. 2008, 8, 767–774. [Google Scholar] [CrossRef]
- Blankenship, T.S.; Blankenship, T.S.; Balahmar, N.; Mokaya, R. Oxygen-rich microporous carbons with exceptional hydrogen storage capacity. Nat. Commun. 2017, 8, 1545. [Google Scholar] [CrossRef] [Green Version]
- Sdanghi, G.; Nicolas, V.; Mozet, K.; Schaefer, S.; Maranzana, G.; Celzard, A.; Fierro, V. A 70 MPa hydrogen thermally driven compressor based on cyclic adsorption-desorption on activated carbon. Carbon 2020, 161, 466–478. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, S.Y.; Park, S.J. Efficient micropore sizes for carbon dioxide physisorption of pine cone-based carbonaceous materials at different temperatures. J. CO2 Util. 2021, 54, 101770. [Google Scholar] [CrossRef]
- Han, Y.J.; Park, S.J. Effect of nickel on hydrogen storage behaviors of carbon aerogel hybrid. Carbon Lett. 2015, 16, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.J.; Park, S.J. Hydrogen Storage Behaviors of Porous Carbons Derived from Poly(vinylidene fluoride). J. Nanosci. Nanotechnol. 2017, 17, 8075–8080. [Google Scholar] [CrossRef]
- Kim, B.J.; An, K.H.; Park, S.J. Preparation and Characterization of Highly Porous Carbons for Hydrogen Storage. J. Nanosci. Nanotechnol. 2011, 11, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Park, S.J. Influence of surface treatments on micropore structure and hydrogen adsorption behavior of nanoporous carbons. J. Colloid Interface Sci. 2007, 311, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, Y.P.; Sun, Y. A comparative study of hydrogen adsorption on superactivated carbon versus carbon nanotubes. Int. J. Hydrogen Energy 2004, 29, 475–479. [Google Scholar] [CrossRef]
- Im, J.S.; Park, S.J.; Kim, T.J.; Kim, Y.H.; Lee, Y.S. The study of controlling pore size on electrospun carbon nanofibers for hydrogen adsorption. J. Colloid Interface Sci. 2008, 318, 42–49. [Google Scholar] [CrossRef]
- Im, J.S.; Park, S.J.; Lee, Y.S. Superior prospect of chemically activated electrospun carbon fibers for hydrogen storage. Mater. Res. Bull. 2009, 44, 1871–1878. [Google Scholar] [CrossRef]
- Im, J.S.; Park, S.J.; Lee, Y.S. The metal-carbon-fluorine system for improving hydrogen storage by using metal and fluorine with different levels of electronegativity. Int. J. Hydrogen Energy 2009, 34, 1423–1428. [Google Scholar] [CrossRef]
- Lee, H.M.; Heo, Y.J.; An, K.H.; Jung, S.C.; Chung, D.C.; Park, S.J.; Kim, B.J. A study on optimal pore range for high pressure hydrogen storage behaviors by porous hard carbon materials prepared from a polymeric precursor. Int. J. Hydrogen Energy 2018, 43, 5894–5902. [Google Scholar] [CrossRef]
- Park, J.H.; Park, S.J. Expansion of effective pore size on hydrogen physisorption of porous carbons at low temperatures with high pressures. Carbon 2020, 158, 364–371. [Google Scholar] [CrossRef]
- Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250–1280. [Google Scholar] [CrossRef] [Green Version]
- Bastos-Neto, M.; Patzschke, C.; Lange, M.; Mollmer, J.; Moller, A.; Fichtner, S.; Schrage, C.; Lassig, D.; Lincke, J.; Staudt, R.; et al. Assessment of hydrogen storage by physisorption in porous materials. Energy Environ. Sci. 2012, 5, 8294–8303. [Google Scholar] [CrossRef]
- Heo, Y.J.; Park, S.J. Synthesis of activated carbon derived from rice husks for improving hydrogen storage capacity. J. Ind. Eng. Chem. 2015, 31, 330–334. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.J. Potassium Oxalate as an Alternative Activating Reagent of Corn Starch-Derived Porous Carbons for Methane Storage. J. Nanosci. Nanotechnol. 2020, 20, 7124–7129. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Park, S.J. Microporous carbons derived from melamine and isophthalaldehyde: One-pot condensation and activation in a molten salt medium for efficient gas adsorption. Sci. Rep. 2018, 8, 6092. [Google Scholar] [CrossRef] [Green Version]
- Koh, J.; Choi, E.; Sakaki, K.; Kim, D.; Han, S.M.; Kim, S.; Cho, E.S. Uncovering the encapsulation effect of reduced graphene oxide sheets on the hydrogen storage properties of palladium nanocubes. Nanoscale 2021, 13, 16942–16951. [Google Scholar] [CrossRef]
- Dhar, P.; Gaur, S.S.; Kumar, A.; Katiyar, V. Cellulose Nanocrystal Templated Graphene Nanoscrolls for High Performance Supercapacitors and Hydrogen Storage: An Experimental and Molecular Simulation Study. Sci. Rep. 2018, 8, 3886. [Google Scholar] [CrossRef] [Green Version]
- Ruse, E.; Buzaglo, M.; Pri-Bar, I.; Shunak, L.; Nadiv, R.; Pevzner, S.; Siton-Mendelson, O.; Skripnyuk, V.M.; Rabkin, E.; Regev, O. Hydrogen storage kinetics: The graphene nanoplatelet size effect. Carbon 2018, 130, 369–376. [Google Scholar] [CrossRef]
- Mun, S.J.; Park, S.J. Graphitic Carbon Nitride Materials for Photocatalytic Hydrogen Production via Water Splitting: A Short Review. Catalysts 2019, 9, 805. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Lee, K.; Sun, Y.Y.; Lucking, M.; Chen, Z.F.; Zhao, J.J.; Zhang, S.B.B. Graphene Oxide as an Ideal Substrate for Hydrogen Storage. ACS Nano 2009, 3, 2995–3000. [Google Scholar] [CrossRef]
- Singh, S.B.; De, M. Effects of gaseous environments on physicochemical properties of thermally exfoliated graphene oxides for hydrogen storage: A comparative study. J. Porous Mater. 2021, 28, 875–888. [Google Scholar] [CrossRef]
- Kim, B.J.; Park, S.J. Optimization of the pore structure of nickel/graphite hybrid materials for hydrogen storage. Int. J. Hydrogen Energy 2011, 36, 648–653. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. Hydrogen Storage Behaviors of Ni-Doped Graphene Oxide/MIL-101 Hybrid Composites. J. Nanosci. Nanotechnol. 2013, 13, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Singh, S.; Hashmi, S.A.R.; Kim, K.H. MXenes: Emerging 2D materials for hydrogen storage. Nano Energy 2021, 85, 105989. [Google Scholar] [CrossRef]
- Jiang, R.C.; Xiao, X.Z.; Zheng, J.G.; Chen, M.; Chen, L.X. Remarkable hydrogen absorption/desorption behaviors and mechanism of sodium alanates in-situ doped with Ti-based 2D MXene. Mater. Chem. Phys. 2020, 242, 122529. [Google Scholar] [CrossRef]
- Kannan, K.; Sadasivuni, K.K.; Abdullah, A.M.; Kumar, B. Current Trends in MXene-Based Nanomaterials for Energy Storage and Conversion System: A Mini Review. Catalysts 2020, 10, 495. [Google Scholar] [CrossRef]
- Murali, G.; Rawal, J.; Modigunta, J.K.R.; Park, Y.H.; Lee, J.H.; Lee, S.Y.; Park, S.J.; In, I. A review on MXenes: New-generation 2D materials for supercapacitors. Sustain. Energy Fuels 2021, 5, 5672–5693. [Google Scholar] [CrossRef]
- Hu, Q.K.; Sun, D.D.; Wu, Q.H.; Wang, H.Y.; Wang, L.B.; Liu, B.Z.; Zhou, A.G.; He, J.L. MXene: A New Family of Promising Hydrogen Storage Medium. J. Phys. Chem. A 2013, 117, 14253–14260. [Google Scholar] [CrossRef]
- Yadav, A.; Dashora, A.; Patel, N.; Miotello, A.; Press, M.; Kothari, D.C. Study of 2D MXene Cr2C material for hydrogen storage using density functional theory. Appl. Surf. Sci. 2016, 389, 88–95. [Google Scholar] [CrossRef]
- Sun, S.J.; Liao, C.; Hafez, A.M.; Zhu, H.L.; Wu, S.P. Two-dimensional MXenes for energy storage. Chem. Eng. J. 2018, 338, 27–45. [Google Scholar] [CrossRef]
- Liu, S.Y.; Liu, J.Y.; Liu, X.F.; Shang, J.X.; Xu, L.; Yu, R.H.; Shui, J.L. Hydrogen storage in incompletely etched multilayer Ti2CTx at room temperature. Nat. Nanotechnol. 2021, 16, 331–336. [Google Scholar] [CrossRef]
- Shi, R.; Yan, H.X.; Zhang, J.G.; Gao, H.G.; Zhu, Y.F.; Liu, Y.N.; Hu, X.H.; Zhang, Y.; Li, L.Q. Vacancy-Mediated Hydrogen Spillover Improving Hydrogen Storage Properties and Air Stability of Metal Hydrides. Small 2021, 17, 2100852. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.C.; Ou, Y.S.; Li, G.L.; Chang, J.K.; Wang, C.Y. Room-Temperature Hydrogen Adsorption via Spillover in Pt Nanoparticle-Decorated UiO-66 Nanoparticles: Implications for Hydrogen Storage. ACS Appl. Nano Mater. 2021, 4, 11269–11280. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, Y.S.; Park, S.J. A study on the hydrogen storage capacity of Ni-plated porous carbon nanofibers. Int. J. Hydrogen Energy 2008, 33, 4112–4115. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, Y.S.; Park, S.J. Novel porous carbons synthesized from polymeric precursors for hydrogen storage. Int. J. Hydrogen Energy 2008, 33, 2254–2259. [Google Scholar] [CrossRef]
- Karim, W.; Spreafico, C.; Kleibert, A.; Gobrecht, J.; VandeVondele, J.; Ekinci, Y.; van Bokhoven, J.A. Catalyst support effects on hydrogen spillover. Nature 2017, 541, 68–71. [Google Scholar] [CrossRef]
- Plerdsranoy, P.; Thaweelap, N.; Poo-arporn, Y.; Khajondetchairit, P.; Suthirakun, S.; Fongkaew, I.; Chanlek, N.; Utke, O.; Pangon, A.; Utke, R. Hydrogen adsorption of O/N-rich hierarchical carbon scaffold decorated with Ni nanoparticles: Experimental and computational studies. Int. J. Hydrogen Energy 2021, 46, 5427–5440. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, Y.S.E.; Park, S.J. Preparation of platinum-decorated porous graphite nanofibers, and their hydrogen storage behaviors. J. Colloid Interface Sci. 2008, 318, 530–533. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. Influence of CO2 activation on hydrogen storage behaviors of platinum-loaded activated carbon nanotubes. J. Solid State Chem. 2010, 183, 2951–2956. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, S.Y. A study on hydrogen-storage behaviors of nickel-loaded mesoporous MCM-41. J. Colloid Interface Sci. 2010, 346, 194–198. [Google Scholar] [CrossRef]
- Pyle, D.S.; Gray, E.M.; Webb, C.J. Hydrogen storage in carbon nanostructures via spillover. Int. J. Hydrogen Energy 2016, 41, 19098–19113. [Google Scholar] [CrossRef]
- Wang, L.F.; Yang, R.T. New sorbents for hydrogen storage by hydrogen spillover—A review. Energy Environ. Sci. 2008, 1, 268–279. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, S.Y. Hydrogen storage behaviors of platinum-supported multi-walled carbon nanotubes. Int. J. Hydrogen Energy 2010, 35, 13048–13054. [Google Scholar] [CrossRef]
- Prins, R. Hydrogen Spillover. Facts and Fiction. Chem. Rev. 2012, 112, 2714–2738. [Google Scholar] [CrossRef] [PubMed]
- Lachawiec, A.J.; Qi, G.S.; Yang, R.T. Hydrogen storage in nanostructured carbons by spillover: Bridge-building enhancement. Langmuir 2005, 21, 11418–11424. [Google Scholar] [CrossRef]
- Li, Y.W.; Yang, R.T. Hydrogen storage in metal-organic frameworks by bridged hydrogen spillover. J. Am. Chem. Soc. 2006, 128, 8136–8137. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, B.J.; Lee, Y.S.; Cho, M.J. Influence of copper electroplating on high pressure hydrogen-storage behaviors of activated carbon fibers. Int. J. Hydrogen Energy 2008, 33, 1706–1710. [Google Scholar] [CrossRef]
- Hoang, T.K.A.; Antonelli, D.M. Exploiting the Kubas Interaction in the Design of Hydrogen Storage Materials. Adv. Mater. 2009, 21, 1787–1800. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, J.H.; Kim, Y.H.; Rhee, K.Y.; Park, S.J. Roles of London Dispersive and Polar Components of Nano-Metal-Coated Activated Carbons for Improving Carbon Dioxide Uptake. Coatings 2021, 11, 691. [Google Scholar] [CrossRef]
- Im, J.S.; Park, S.J.; Kim, T.; Lee, Y.S. Hydrogen storage evaluation based on investigations of the catalytic properties of metal/metal oxides in electrospun carbon fibers. Int. J. Hydrogen Energy 2009, 34, 3382–3388. [Google Scholar] [CrossRef]






| Units | 2020 | 2025 | Ultimate | |
|---|---|---|---|---|
| Storage capacity | ||||
| System gravimetric capacity | ||||
| Usable, specific energy from H2 (net useful energy/max system mass) | kWh/kg (kg H2/kg system) | 1.5 (0.045) | 1.8 (0.055) | 2.2 (0.065) |
| System volumetric capacity | ||||
| Usable energy density from H2 (net useful energy/max system volume) | kWh/L (kg H2/L system) | 1.0 (0.030) | 1.3 (0.040) | 1.7 (0.050) |
| Storage system cost | ||||
| Storage system cost | USD/kWh net (USD/kg H2) | 10 (333) | 9 (300) | 8 (266) |
| Fuel cost | USD/gge at pump | 4 | 4 | 4 |
| Durability/operability | ||||
| Operating ambient temperature | °C | −40/60 (Sun) | −40/60 (Sun) | −40/60 (Sun) |
| Min/max delivery temperature | °C | −40/85 | −40/85 | −40/85 |
| Operational cycle life (1/4 tank to full) | cycles | 1500 | 1500 | 1500 |
| Min/max delivery pressure from storage system | bar (abs) | 5/12 | 5/12 | 5/12 |
| Onboard efficiency | % | 90 | 90 | 90 |
| “Well” to power plant efficiency | % | 60 | 60 | 60 |
| Charging–discharging rates | ||||
| System fill time | min | 3–5 | 3–5 | 3–5 |
| Minimum/average full flow rate | (g/s)/kW | 0.02/0.004 | 0.02/0.004 | 0.02/0.004 |
| Start time to full flow (−20 °C/20 °C) | s | 15/5 | 15/5 | 15/5 |
| Transient response at operating temperature | s | 0.75 | 0.75 | 0.75 |
| Dormancy | ||||
| Dormancy time target | days | 7 | 10 | 14 |
| Boil-off loss target | % | 10 | 10 | 10 |
| Environmental health and safety | ||||
| Permeation and leakage | - | Meet or exceed SAE J2579 for system safety | ||
| Toxicity | - | Meet or exceed applicable standards | ||
| Safety | - | Conduct and evaluate failure analysis | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-Y.; Lee, J.-H.; Kim, Y.-H.; Kim, J.-W.; Lee, K.-J.; Park, S.-J. Recent Progress Using Solid-State Materials for Hydrogen Storage: A Short Review. Processes 2022, 10, 304. https://doi.org/10.3390/pr10020304
Lee S-Y, Lee J-H, Kim Y-H, Kim J-W, Lee K-J, Park S-J. Recent Progress Using Solid-State Materials for Hydrogen Storage: A Short Review. Processes. 2022; 10(2):304. https://doi.org/10.3390/pr10020304
Chicago/Turabian StyleLee, Seul-Yi, Jong-Hoon Lee, Yeong-Hun Kim, Jong-Woo Kim, Kyu-Jae Lee, and Soo-Jin Park. 2022. "Recent Progress Using Solid-State Materials for Hydrogen Storage: A Short Review" Processes 10, no. 2: 304. https://doi.org/10.3390/pr10020304
APA StyleLee, S.-Y., Lee, J.-H., Kim, Y.-H., Kim, J.-W., Lee, K.-J., & Park, S.-J. (2022). Recent Progress Using Solid-State Materials for Hydrogen Storage: A Short Review. Processes, 10(2), 304. https://doi.org/10.3390/pr10020304







