Papain Enzyme Assisted Extraction of Virgin Coconut Oil as Candidate In-House Reference Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
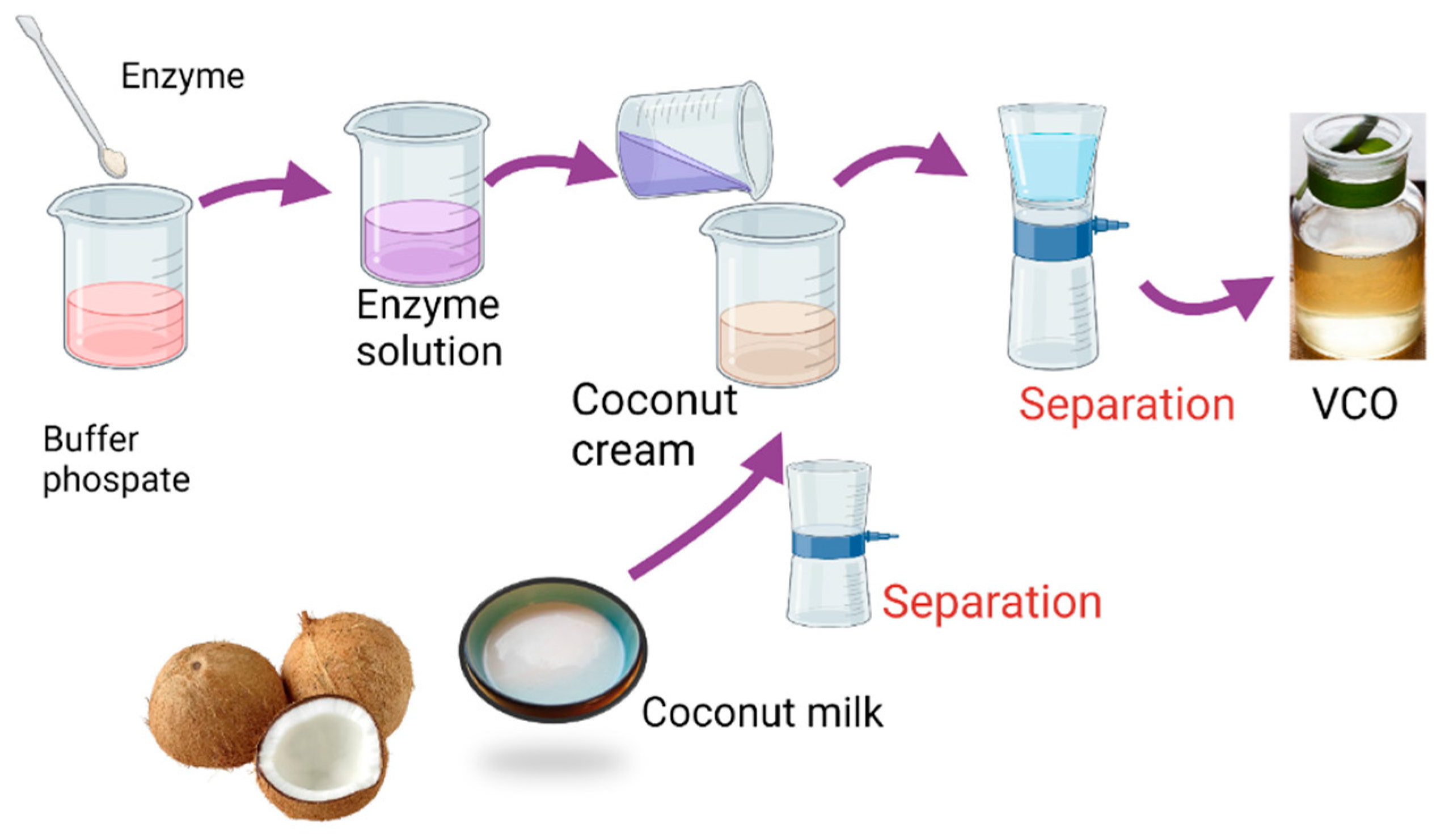
2.2. Extraction of VCO
2.3. Physicochemical Analyses of VCO
2.4. Antibacterial Activity of VCO
2.5. Homogeneity Test
3. Results and Discussion
3.1. VCO Extraction
3.2. Determination of Microbial Contamination
3.3. Homogeneity and Stability Tests
3.4. Antibacterial Activity Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanjaya, C.; Pranata, F.S.; Swasti, Y.R. Quality of Virgin Coconut Oil with Addition of Peppermint Oil. agriTECH 2020, 40, 215. [Google Scholar] [CrossRef]
- Anzaku, A.A. Antimicrobial Activity of Coconut Oil and its Derivative (Lauric Acid) on Some Selected Clinical Isolates. Int. J. Med. Sci. Clin. Invent. 2017, 4, 3173–3177. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.T.A.; Le, T.D.; Phan, H.N.; Tran, L.B. Antibacterial Activity of Free Fatty Acids from Hydrolyzed Virgin Coconut Oil Using Lipase from Candida rugosa. J. Lipids 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Ghani, N.A.A.; Channip, A.A.; Chok Hwee Hwa, P.; Ja’afar, F.; Yasin, H.M.; Usman, A. Physicochemical properties, antioxidant capacities, and metal contents of virgin coconut oil produced by wet and dry processes. Food Sci. Nutr. 2018, 6, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Sundrasegaran, S.; Mah, S.H. Extraction Methods of Virgin Coconut Oil and Palm-pressed Mesocarp Oil and their Phytonutrients. eFood 2020, 1, 381. [Google Scholar] [CrossRef]
- Agarwal, R.K. Extraction Processes of Virgin Coconut Oil. MOJ Food Process. Technol. 2017, 4, 54–56. [Google Scholar] [CrossRef]
- Arslan, A.; Rancke-Madsen, A.; Brask, J. Enzymatic synthesis of estolides from castor oil. Catalysts 2020, 10, 835. [Google Scholar] [CrossRef]
- Díaz-Suárez, P.; Rosales-Quintero, A.; Fernandez-Lafuente, R.; Pola-Sánchez, E.; Hernández-Cruz, M.C.; Ovando-Chacón, S.L.; Rodrigues, R.C.; Tacias-Pascacio, V.G. Aqueous enzymatic extraction of Ricinus communis seeds oil using Viscozyme L. Ind. Crops Prod. 2021, 170, 113811. [Google Scholar] [CrossRef]
- Amri, E.; Mamboya, F. Papain, a plant enzyme of biological importance: A review. Am. J. Biochem. Biotechnol. 2012, 8, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Diyah, N.W.; Purwanto; Susanti, Y.; Dewi, Y.K. Pembuatan Minyak Kelapa secara Enzimatis dengan memanfaatkan Kulit Buah dan Biji Pepaya serta Analisis Sifat Fisikokimianya. Berk. Penelit. Hayati 2010, 15, 181–185. [Google Scholar] [CrossRef]
- Rukman, W.Y.; Safitri, D.; Fadhilah, N.; Wajdi, M. Papain-induced enzyme source to the quality of virgin coconut oil. Int. J. Multidiciplinary Res. Dev. 2018, 5, 57–59. [Google Scholar]
- Raharja, S. dan Dwiyuni, M. Study on Physico-Chemical Characteristics of Virgin Coconut Oil (VCO) Made by Coconut Milk Cream Freezing Method. J. Tek. Ind. Pertan. 2005, 18, 71–78. [Google Scholar]
- Bisht, T.S.; Sharma, S.K.; Sati, R.C.; Rao, V.K.; Yadav, V.K.; DIxit, A.K.; Sharma, A.K.; Chopra, C.S. Improvement of efficiency of oil extraction from wild apricot kernels by using enzymes. J. Food Sci. Technol. 2015, 52, 1543–1551. [Google Scholar] [CrossRef] [Green Version]
- Loung, F.S.; Silalahi, J.; Suryanto, D. Antibacterial activity of enzymatic hydrolyzed of virgin coconut oil and palm kernel oil against staphylococcus aureus, Salmonella thypi and Escherichia coli. Int. J. PharmTech Res. 2014, 6, 628–633. [Google Scholar]
- Effendi, A.M.; Pratjojo, W.; Sumarni, W. Optimalisasi Penggunaan Enzim Bromelin Dari Sari Bonggol Nanas Dalam Pembuatan Minyak Kelapa. Indones. J. Chem. Sci. 2012, 1, 1–6. [Google Scholar]
- Rohyami, Y.; Anjani, R.D.; Purwanti, N.P. The influence of Saccharomyces cerevisiae enzyme ratio on preparation virgin coconut oil for candidate in-house reference materials. AIP Conf. Proc. 2017, 1823, 020086. [Google Scholar] [CrossRef] [Green Version]
- Idrus, N.F.M.; Febrianto, N.A.; Zzaman, W.; Cuang, T.E.; Yang, T.A. Optimization of the aqueous extraction of virgin coconut oil by response surface methodology. Food Sci. Technol. Res. 2013, 19, 729–737. [Google Scholar] [CrossRef]
- Thepakorn, T.; Kanasawud, P.; Halling, P.J. Activity of immobilized papain dehydrated by n-propanol in low-water media. Biotechnol. Lett. 2004, 40, 133–136. [Google Scholar] [CrossRef]
- Tansakul, A.; Chaisawang, P. Thermophysical properties of coconut milk. J. Food Eng. 2006, 73, 276–280. [Google Scholar] [CrossRef]
- Raghavendra, S.N.; Raghavarao, K.S.M.S. Effect of different treatments for the destabilization of coconut milk emulsion. J. Food Eng. 2010, 97, 341–347. [Google Scholar] [CrossRef]
- Asiah, N.; Astuti, R.M.; Cempaka, L.; Setiani, R. Physical and Chemical Characteristic of Virgin Coconut Oil under Mix Culture Fermentation Technique. J. Phys. Conf. Ser. 2019, 1364, 012009. [Google Scholar] [CrossRef]
- Prapun, R.; Cheetangdee, N.; Udomrati, S. Characterization of virgin coconut oil (VCO) recovered by different techniques and fruit maturities. Int. Food Res. J. 2016, 23, 2117–2124. [Google Scholar]
- Mansor, T.S.T.; Che Man, Y.B.; Shuhaimi, M.; Abdul Afiq, M.J.; Ku Nurul, F.K.M. Physicochemical properties of virgin coconut oil extracted from different processing methods. Int. Food Res. J. 2012, 19, 837–845. [Google Scholar]
- Silalahi, J.; Permata, Y.M.; Putra, E.D. Antibacterial activity of hydrolyzed virgin coconut oil. Asian J. Pharm. Clin. Res. 2014, 7, 90–94. [Google Scholar]


| Factor | Value | Water Content (%) | Free Fatty Acid (%) | Peroxide Number (meq/kg) | Iodine Number (g iodine/100 g) |
|---|---|---|---|---|---|
| Enzyme to Oil ratio (g/L) | 0 | 0.99 ± 0.12 | 0.24 ± 0.01 | 0.20 ± 0.01 | 7.36 ± 0.04 |
| 0.2 | 1.01 ± 0.12 | 0.26 ± 0.03 | 0.20 ± 0.01 | 8.13 ± 0.04 | |
| 0.4 | 1.01 ± 0.12 | 0.30 ± 0.03 | 0.20 ± 0.01 | 8.36 ± 0.06 | |
| 0.6 | 1.01 ± 0.12 | 0.30 ± 0.04 | 0.20 ± 0.01 | 8.17 ± 0.01 | |
| 0.8 | 1.01 ± 0.12 | 0.24 ± 0.01 | 0.20 ± 0.01 | 8.45 ± 0.02 | |
| 1.0 | 1.02 ± 0.12 | 0.28 ± 0.01 | 0.20 ± 0.01 | 8.22 ± 0.09 | |
| Volume of water (L/g) | 100 | 1.01 ± 0.22 | 0.23 ± 0.01 | 0.36 ± 0.16 | 7.55 ± 0.01 |
| 200 | 1.01 ± 0.24 | 0.31 ± 0.09 | 0.18 ± 0.01 | 7.73 ± 0.01 | |
| 300 | 1.01 ± 0.21 | 0.31 ± 0.18 | 0.18 ± 0.01 | 7.91 ± 0.05 | |
| 400 | 1.01 ± 0.23 | 0.35 ± 0.06 | 0.18 ± 0.01 | 7.72 ± 0.03 | |
| 500 | 1.01 ± 0.18 | 0.38 ± 0.12 | 0.18 ± 0.01 | 7.49 ± 0.02 | |
| 600 | 1.01 ± 0.20 | 0.42 ± 0.08 | 0.18 ± 0.01 | 7.51 ± 0.01 | |
| Tempeature of water (°C) | 30 | 1.00 ± 0.12 | 0.31 ± 0.02 | 0.18 ± 0.01 | 8.26 ± 1.02 |
| 40 | 1.00 ± 0.13 | 0.27 ± 0.05 | 0.18 ± 0.01 | 8.21 ± 1.66 | |
| 50 | 1.00 ± 0.12 | 0.35 ± 0.06 | 0.18 ± 0.01 | 8.07 ± 1.78 | |
| 60 | 1.00 ± 0.12 | 0.27 ± 0.05 | 0.18 ± 0.01 | 8.33 ± 0.30 | |
| 70 | 0.99 ± 0.13 | 0.19 ± 0.04 | 0.18 ± 0.01 | 8.23 ± 0.97 | |
| Extraction stage | 1 | 0.99 ± 0.13 | 0.35 ± 0.06 | 0.18 ± 0.01 | 8.23 ± 0.01 |
| 2 | 1.00 ± 0.12 | 0.38 ± 0.12 | 0.18 ± 0.01 | 7.65 ± 0.04 | |
| 3 | 0.96 ± 0.14 | 0.27 ± 0.05 | 0.18 ± 0.01 | 7.76 ± 0.03 | |
| 4 | 0.99 ± 0.13 | 0.27 ± 0.0.5 | 0.18 ± 0.01 | 7.81 ± 0.01 | |
| 5 | 0.98 ± 0.12 | 0.35 ± 0.06 | 0.18 ± 0.01 | 8.15 ± 0.05 |
| Methods | Yield (%) | Water Content (%) | Free Fatty Acid (%) | Peroxide Number (meq/kg) | Iodine Number (g iodine/100 g) | Reference |
|---|---|---|---|---|---|---|
| Papain | ||||||
| Papain at optimum conditions | 24.3 | 0.98 | 0.35 | 0.18 | 8.15 | [11] |
| Papain with freezing and thawing | 23 | 0.014 | 0.30 | 0.69 | [12] | |
| Papain | 0.11 ± 0.01 | 0.35 ± 0.01 | 4.26 ± 0.05 | [16] | ||
| 29.8 | 0.18 | 0.22 | [11] | |||
| Peel of papaya | 12.7 ± 1.1 | 0.14 ± 0.00 | 0.17 ± 0.00 | 0.57 ± 0.01 | 8.72 ± 0.12 | [10] |
| Seed of papaya | 12.5 ± 0.5 | 0.14 ± 0.01 | 0.15 ± 0.01 | 0.60 ± 0.00 | 8.23 ± 0.04 | |
| Enzyme | ||||||
| Protease | 0.12 ± 0.01 | 0.53 ± 0.14 | 7.62 ± 0.14 | [22] | ||
| Bromelain | 24.26 ± 0.06 | 0.34 ± 0.01 | 0.29 ± 0.03 | |||
| Fermentation | ||||||
| Natural fermentation | 0.13 ± 0.06 | 0.53 ± 0.15 | 2.8 ± 0.03 | 0.91 ± 0.03 | [4] | |
| Saccharomyces cerevisiae | 12.40 | 0.04 ± 0.01 | 0.31 ± 0.11 | 0.01 ± 0.00 | 2.44 ± 0.20 | [16] |
| 0.06 ± 0.00 | 0.29 ± 0.02 | 4.30 ± 0.07 | [23] | |||
| Bacillus licheniformis | 0.15 ± 0.01 | 0.78 ± 0.08 | 7.26 ± 0.13 | [22] |
| Sample | Dilution | Medium | Incubation | Observation | |||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (Hours) | I | II | Total | |||
| VCO | 10−1 | Plate count agar | 35–37 | 24–48 | 0 | 0 | 0 |
| VCO | 10−2 | Plate count agar | 35–38 | 24–49 | 0 | 0 | 0 |
| Control | 10−1 | Plate count agar | 35–39 | 24–50 | 0 | 0 | 0 |
| Control | 10−2 | Plate count agar | 35–40 | 24–51 | 0 | 0 | 0 |
| Blank | 35–41 | 24–52 | 0 | 0 | 0 | ||
| Medium | Plate count agar | 35–42 | 24–53 | 0 | 0 | 0 | |
| Medium + Diluent solution | Plate count agar-peptone dilution fluid | 35–43 | 24–54 | 0 | 0 | 0 | |
| Total plate count | Plate count agar-lactose broth | 35–44 | 24–55 | 0 | 0 | 0 | |
| Parameter | Mean Square between (MSB) | Mean Square within (MSW) | Ftest |
|---|---|---|---|
| Free fatty acid (%) | 0.0004 | 0.0001 | 3.0146 |
| Peroxide number (meq/kg) | 0.4824 | 0.0614 | 7.8614 |
| Iodine number (mg iodine/100 g) | 0.4417 | 0.0551 | 8.0126 |
| Parameter | Sample 1 | Sample 2 | |
|---|---|---|---|
| Free fatty acid (%) | |||
| Q1 | 0.1532 | 0.1533 | 0.1533 |
| Q3 | 0.2013 | 0.1917 | 0.2003 |
| IQR | 0.0482 | 0.0383 | 0.0470 |
| nIQR (0.7413 × IQR) | 0.0357 | 0.0284 | 0.0349 |
| 0.3 × nIQR | 0.1071 | 0.0852 | 0.1046 |
| 0.0544 | 0.0504 | 0.0509 | |
| 0.0544 < 0.1071 | 0.0504 < 0.0852 | 0.0509 < 0.1046 | |
| Peroxide number (meq/kg) | |||
| Q1 | 1.9956 | 2.7906 | 2.5447 |
| Q3 | 2.7972 | 3.5888 | 2.7958 |
| IQR | 0.8016 | 0.7982 | 0.2511 |
| nIQR (0.7413 × IQR) | 0.5942 | 0.5917 | 0.1862 |
| 0.3 × nIQR | 1.7827 | 1.7750 | 0.5585 |
| −0.0392 | 0.9574 | 0.4591 | |
| 0.0392 < 1.7827 | 0.9574 < 1.7750 | 0.4591 < 0.5585 | |
| Iodine number (mg iodine/100g) | |||
| Q1 | 7.8283 | 7.9354 | 7.9173 |
| Q3 | 8.0926 | 8.0612 | 8.0822 |
| IQR | 0.2643 | 0.1258 | 0.1649 |
| nIQR (0.7413 × IQR) | 0.1959 | 0.0932 | 0.1222 |
| 0.3 × nIQR | 0.5878 | 0.2797 | 0.3667 |
| 1.9507 | 2.0053 | 1.9780 | |
| 1.9507 > 0.5878 | 2.0053 > 0.2797 | 1.9780 > 0.3667 |
| Tested Bacteria | Inhibition Zone for Sample (mm) | |||
|---|---|---|---|---|
| VCO | VCO-2X | VCO-4X | VCO-8X | |
| E. coli | 18.5 | 17 | 11 | 10.5 |
| S. aureus | 10 | 12.5 | 10 | 9 |
| P. acne | 12 | 10.5 | 9 | 9 |
| P. aeruginosa | 10 | 10 | 10 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yulirohyami; Hidayat, H.; Wijaya, A.R.; Fatimah, I. Papain Enzyme Assisted Extraction of Virgin Coconut Oil as Candidate In-House Reference Material. Processes 2022, 10, 315. https://doi.org/10.3390/pr10020315
Yulirohyami, Hidayat H, Wijaya AR, Fatimah I. Papain Enzyme Assisted Extraction of Virgin Coconut Oil as Candidate In-House Reference Material. Processes. 2022; 10(2):315. https://doi.org/10.3390/pr10020315
Chicago/Turabian StyleYulirohyami, Habibi Hidayat, Aprisilia Rizky Wijaya, and Is Fatimah. 2022. "Papain Enzyme Assisted Extraction of Virgin Coconut Oil as Candidate In-House Reference Material" Processes 10, no. 2: 315. https://doi.org/10.3390/pr10020315
APA StyleYulirohyami, Hidayat, H., Wijaya, A. R., & Fatimah, I. (2022). Papain Enzyme Assisted Extraction of Virgin Coconut Oil as Candidate In-House Reference Material. Processes, 10(2), 315. https://doi.org/10.3390/pr10020315








