Recent Advancements in Plant-Derived Nanomaterials Research for Biomedical Applications
Abstract
:1. Introduction to Nanoparticles
2. Different Types of Nanoparticles
2.1. Metallic Nanoparticles
2.1.1. Silver Nanoparticles
2.1.2. Gold Nanoparticles
2.1.3. Copper Nanoparticles
2.1.4. Platinum Nanoparticles (PtNPs)
2.1.5. Metal Oxide Nanoparticles (MO-NPs)
2.1.6. Zinc Oxide Nanoparticles (ZnO-NPs)
2.1.7. Copper Oxide Nanoparticles (CuO-NPs)
2.1.8. Iron Oxide Nanoparticles (Fe3O4-NPs)
2.1.9. Titanium Oxide Nanoparticles (TiO2-NPs)
3. Biomedical Applications of Various Plants-Derived NPs
4. Nanomedicine as Promising Anticancer Agent
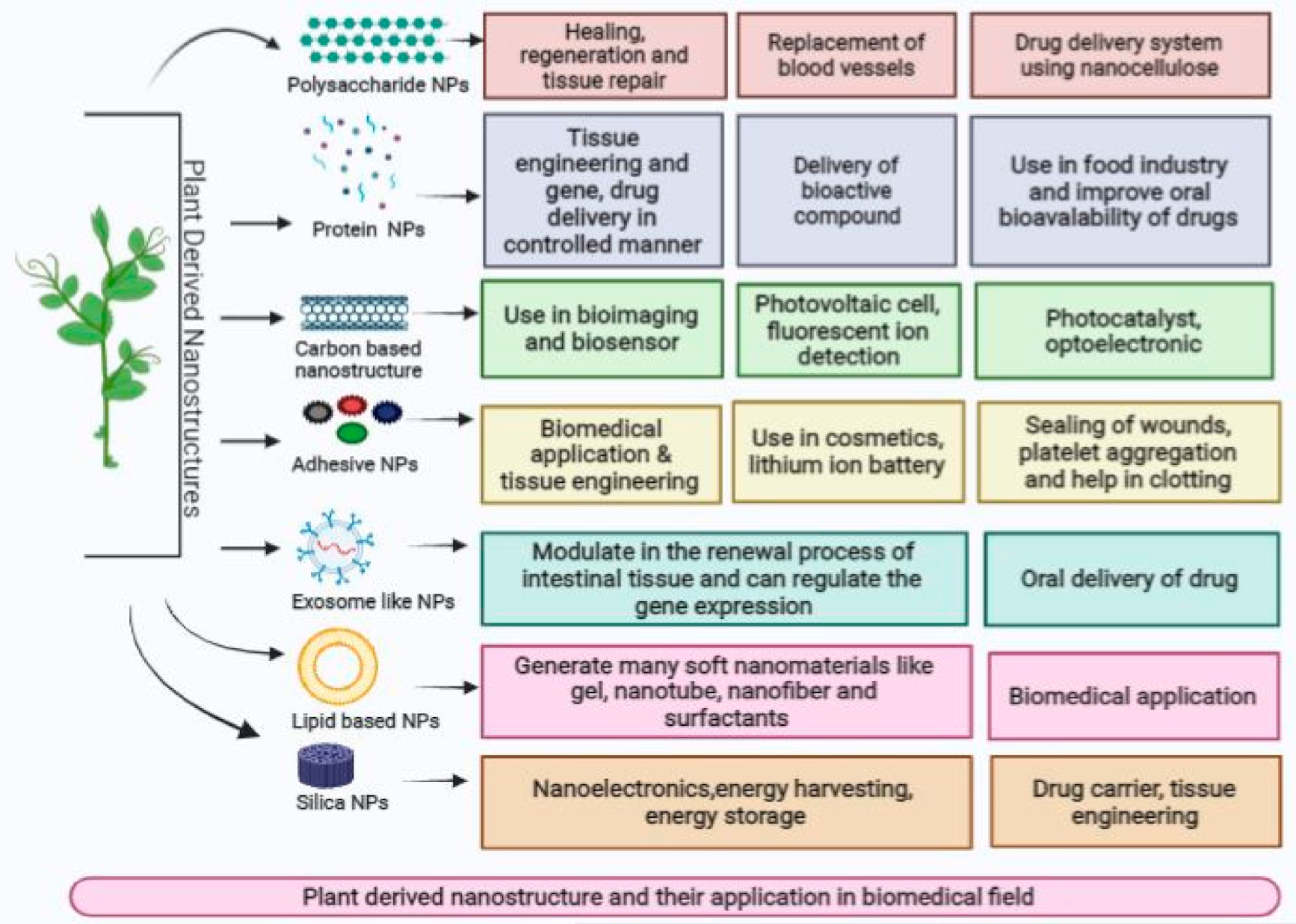
5. Plant-Derived Nanostructures and Their Medicinal Applications
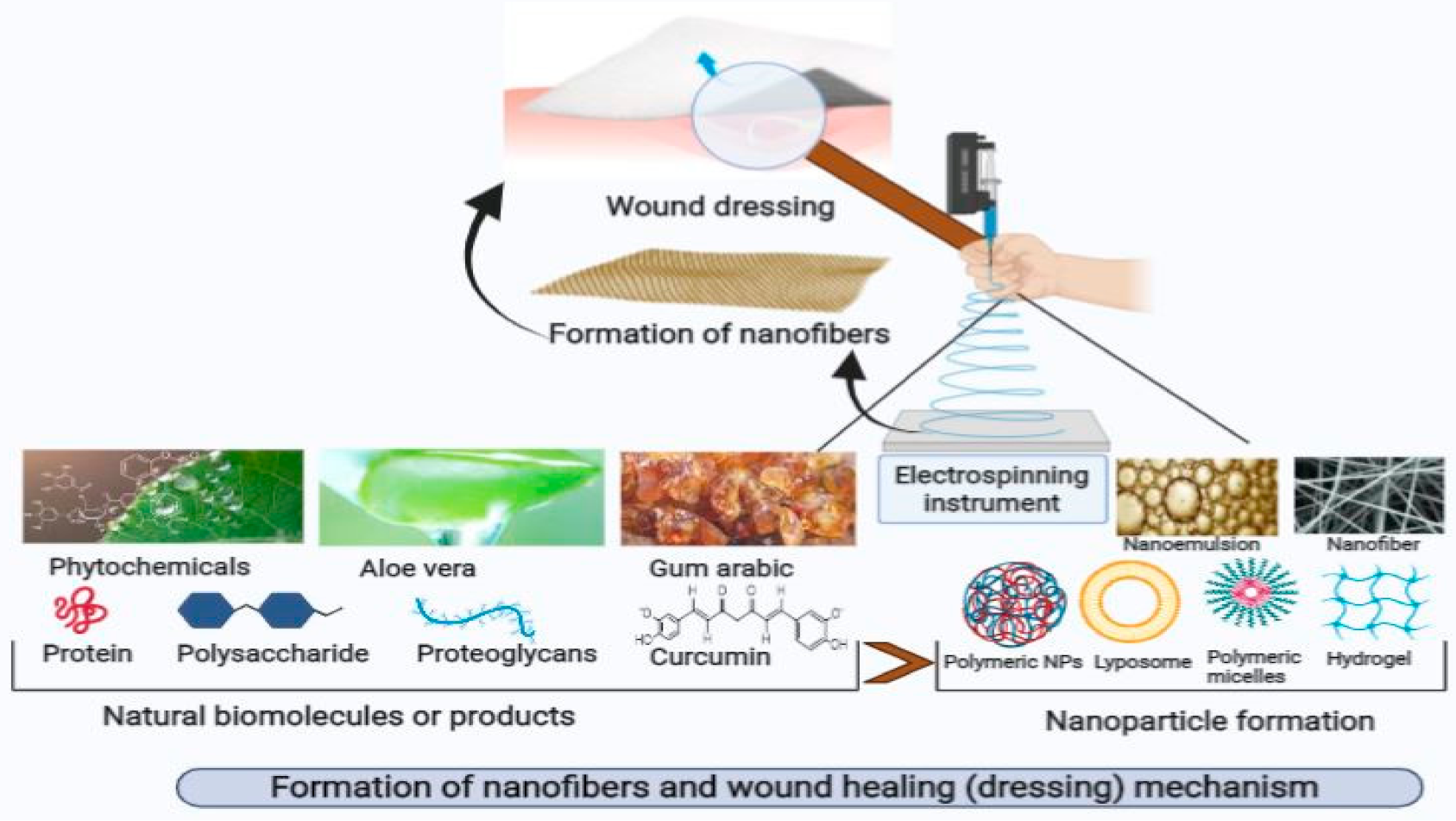
6. Plant-Derived Nanofibers and Their Role in Wound Healing
7. Plant-Derived Nanostructure Biomaterial and Their Role in Bone Regeneration
8. Plant-Based Nanoparticles (PBNPs) as a Promising Nanomedicine against COVID-19
9. Advantages and Disadvantages of Plant-Derived NPs
10. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salam, H.A.; Rajiv, P.; Kamaraj, M.; Jagadeeswaran, P.; Gunalan, S.; Sivaraj, R. Plants: Green route for nanoparticle synthesis. Int. J. Biol. Sci. 2012, 1, 85–90. [Google Scholar]
- Elangovan, K.; Elumalai, D.; Anupriya, S. Phyto mediated biogenic synthesis of silver nanoparticles using leaf extract of Andrographisechioides and its bio-efficacy on anticancer and antibacterial activities. J. Photochem. Photobiol. B Biol. 2015, 151, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green synthesis of metal nanoparticles using plants. GreenChem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 1–24. [Google Scholar] [CrossRef]
- Lengke, M.F.; Sanpawanitchakit, C.; Southam, G. Biosynthesis of Gold Nanoparticles: A Review. In Metal Nanoparticles in Microbiology, 1st ed.; Duran, R.N., Ed.; Springer: New York, NY, USA, 2011; pp. 37–74. [Google Scholar]
- Dahoumane, S.A.; Jeffryes, C.; Mechouet, M.; Agathos, S.N. Biosynthesis of inorganic nanoparticles: A fresh look at the control of shape, size and composition. Bioengineering 2017, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941. [Google Scholar] [CrossRef] [Green Version]
- Holister, P.; Weener, J.W.; Vas, C.R.; Harper, T. Nanoparticles, Technology White Papers nr. 3. Cientifica 2003, 3, 1–11. [Google Scholar]
- Wang, W.; Chen, Q.; Jiang, C.; Yang, D.; Liu, X.; Xu, S. One-step synthesis of biocompatible gold nanoparticles using gallic acid in the presence of poly-(N- vinyl- 2-pyrrolidone). Colloids Surf. A Physicochem. Eng. Aspects 2007, 301, 73–79. [Google Scholar] [CrossRef]
- Schmid, G.; Simon, U. Gold nanoparticles: Assembly and electrical properties in 1–3 dimensions. Chem. Commun. 2005, 6, 697–710. [Google Scholar] [CrossRef]
- Solanki, A.; Kim, J.D.; Lee, K.B. Nanotechnology for regenerative medicine: Nanomaterials for stem cell imaging. Nanomedicine 2008, 3, 567–578. [Google Scholar] [CrossRef]
- Jain, S.K.; Sahni, Y.; Neetu, R.; Vidhi, G. Nanotoxicology: An emerging discipline. Vet. World 2011, 4, 35–40. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.K. Nano-hydroxyapatite composite biomaterials for bone tissue engineering—A review. J. Biomed. Nanotechnol. 2014, 10, 3124–3140. [Google Scholar] [CrossRef]
- Yi, H.; Rehman, F.U.; Zhao, C.; Liu, B.; He, N. Recent advances in nano scaffolds for bone repair. Bone Res. 2016, 4, 1–11. [Google Scholar] [CrossRef]
- Silva, G.A. Introduction to nanotechnology and its applications to medicine. Surg. Neurol. 2004, 61, 216–220. [Google Scholar] [CrossRef]
- Caruthers, S.D.; Wickline, S.A.; Lanza, G.M. Nanotechnological applications in medicine. Curr. Opin. Biotechnol. 2007, 18, 26–30. [Google Scholar] [CrossRef]
- Kubik, T.; Bogunia-Kubik, K.; Sugisaka, M. Nanotechnology on duty in medical applications. Curr. Pharm. Biotechnol. 2005, 6, 17–33. [Google Scholar] [CrossRef]
- Kayser, O.; Lemke, A.; Hernandez-Trejo, N. The impact of nanobiotechnology on the development of new drug delivery systems. Curr. Pharm. Biotechnol. 2005, 6, 3–5. [Google Scholar] [CrossRef]
- Paul, S.; Sasikumar, C.S.; Singh, A.R. Preliminary investigation of synthesizing silver nanoparticles from the different biological source: A modern eco-friendly tool. Int. J. Pharm. Res. 2015, 4, 135–148. [Google Scholar]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, L.; Zhou, X.; Yu, Y.; Li, Z.; Zuo, D.; Wu, Y. Silver nanoparticles induce protective autophagy via Ca2+ /CaMKKβ/AMPK/mTOR pathway in SHSY5Y cells and rat brains. Nanotoxicology 2019, 13, 369–391. [Google Scholar] [CrossRef]
- Radzig, M.A.; Nadtochenko, V.A.; Koksharova, O.A.; Kiwi, J.; Lipasova, V.A.; Khmel, I.A. Antibacterial effects of silver nanoparticles on gram-negative bacteria: Influence on the growth and biofilms formation, mechanisms of action. Colloids Surf. B Biointerfaces 2013, 102, 300–306. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, J.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Hwang, E.T.; Lee, J.H.; Chae, Y.J.; Kim, Y.S.; Kim, B.C.; Sang, B.I.; Gu, M.B. Analysis of the toxic mode of action of silver nanoparticles using stress-specific bioluminescent bacteria. Small 2008, 4, 746–747. [Google Scholar] [CrossRef]
- Sriram, M.I.; Kanth, S.B.; Kalishwaralal, K.; Gurunathan, S. Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. Int. J. Nanomed. 2010, 5, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Sankar, R.; Karthik, A.; Prabu, A.; Karthik, S.; Shivashangari, K.S.; Ravikumar, V. Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf. B Biointerfaces 2013, 108, 80–84. [Google Scholar] [CrossRef]
- Kathiravan, V.; Ravi, S.; Ashokkumar, S. Synthesis of silver nanoparticles from Melia dubia leaf extract and their in vitro anticancer activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 116. [Google Scholar] [CrossRef]
- Lara, H.H.; Ixtepan-Turrent, L.; Treviño, E.N.; Singh, D.K. Use of silver nanoparticles increased inhibition of cell-associated HIV-1 infection by neutralizing antibodies developed against HIV-1 envelope proteins. J. Nanobiotechnol. 2011, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [Green Version]
- Prados, J.; Melguizo, C.; Ortiz, R.; Velez, C.; Alvarez, P.J.; Arias, J.L.; Ruiz, M.A.; Gallardo, V.; Aranega, A. Doxorubicin-loaded nanoparticles: New advances in breast cancer therapy. Anticancer Agents Med. Chem. 2012, 12, 1058–1070. [Google Scholar] [CrossRef]
- Li, W.; Calle, L.M.; Hanford, A.J.; Stambaugh, I.; Callahan, M.R. Investigation of silver biocide as a disinfection technology for spacecraft—an early literature review. In Proceedings of the 48th International Conference on Environmental Systems, Albuquerque, NM, USA, 8–12 July 2018; p. ICES-2018-82. [Google Scholar]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Pal, T. The effect of the interaction between particles on the surface of the nanoparticles. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E. Green synthesis of metallic nanoparticles via biological entities. Materials 2015, 29, 7278–7308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fayaz, M.A.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venkatesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomed. Nanotech. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef]
- Huaizhi, Z.; Yuantao, N. China’s ancient gold drugs. Gold Bull. 2001, 34, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Keller, A.A.; Adeleye, A.S.; Conway, J.R.; Garner, K.L.; Zhao, L.; Cherr, G.N.; Hong, J.; Gardea-Torresdey, J.L.; Godwin, H.A.; Hanna, S.; et al. Comparative environmental fate and toxicity of copper nanomaterials. NanoImpact 2017, 7, 28–40. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Li, X.; Qin, B.; Xing, D.; Guo, Y.; Fan, R. Investigation of the mending effect and mechanism of copper nanoparticles on a tribologically stressed surface. Tribol. Lett. 2004, 17, 961–966. [Google Scholar] [CrossRef]
- Arjunan, N.; Singaravelu, C.M.; Kulanthaivel, J.; Kandasamy, J. A potential photocatalytic, antimicrobial and anticancer activity of chitosan-copper nanocomposite. Int. J. Biol. Macromol. 2017, 104, 1774–1782. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Ahamad, T.; Al-Hajji, A.B.; Ahmed, J.; Chaudhary, A.A.; Alshehri, S.M. Cellulose gum and copper nanoparticles based hydrogel as antimicrobial agents against urinary tract infection (UTI) pathogens. Int. J. Biol. Macromol. 2018, 109, 803–809. [Google Scholar] [CrossRef]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Evaluation of antioxidant and anticancer activity of copper oxide nanoparticles synthesized using medicinally important plant extracts. Biomed. Pharmacother. 2017, 89, 1067–1077. [Google Scholar] [CrossRef]
- Nunes, M.R.; de Souza Maguerroski Castilho, M.; de Lima Veeck, A.P.; da Rosa, C.G.; Noronha, C.M.; Maciel, M.V.O.B.; Barreto, P.M. Antioxidant and antimicrobial methylcellulose films containingLippia alba extract and silver nanoparticles. Carbohydr. Polym. 2018, 192, 37–43. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Z.; Fan, J.; Tan, Y.; Zheng, N. Amine-assisted synthesis of concave polyhedral platinum nanocrystals having {411} high-index facets. J. Am. Chem. Soc. 2011, 133, 4718–4721. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.H.; Kim, J.H. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Takahashi, M.; Shimizu, T.; Shirasawa, T.; Kajita, M.; Kanayama, A.; Miyamoto, Y. Effects of a potent antioxidant, platinum nanoparticle, on the lifespan of Caenorhabditis elegans. Mech. Ageing Dev. 2008, 129, 322–331. [Google Scholar] [CrossRef]
- Saitoh, Y.; Yoshimura, Y.; Nakano, K.; Miwa, N. Platinum nanocolloid-supplemented hydrogen dissolved water inhibits growth of human tongue carcinoma cells preferentially over normal cells. Exp. Oncol. 2009, 31, 156–162. [Google Scholar]
- Tahir, K.; Ahmad, A.; Li, B.; Nazir, S.; Khan, A.U.; Nasir, T.; Khan, Z.U.H.; Naz, R.; Raza, M. Visible light photo catalytic inactivation of bacteria and photo degradation of methylene blue with Ag/TiO2 nanocomposite prepared by a novel method. J. Photochem. Photobiol. B Biol. 2016, 162, 189–198. [Google Scholar] [CrossRef]
- Miri, A.; Sarani, M. Biosynthesis, characterization and cytotoxic activity of CeO2 nanoparticles. Ceram. Int. 2018, 44, 12642–12647. [Google Scholar] [CrossRef]
- Gunalan, S.; Sivaraj, R.; Rajendran, V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. 2012, 22, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnol. 2016, 14, 73. [Google Scholar] [CrossRef] [Green Version]
- Hameed, A.S.H.; Karthikeyan, C.; Ahamed, A.P.; Thajuddin, N.; Alharbi, N.S.; Alharbi, S.A.; Ravi, G. In vitro antibacterial activity of ZnO and Nd doped ZnO nanoparticles against ESBL producing Escherichia coli and Klebsiella pneumoniae. Sci. Rep. 2016, 6, 24312. [Google Scholar] [CrossRef] [Green Version]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bhargava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef]
- Pugazhendhi, T.N.; Edison, J.I.; Karuppusamy, I.; Kathirvel, B. Inorganic nanoparticles: A potential cancer therapy for human welfare. Int. J. Pharm. 2018, 539, 104–111. [Google Scholar] [CrossRef]
- Taylor, J.; Rabe, T.; McGaw, L.; Jäger, A.; Van Staden, J. Towards the scientific validation of traditional medicinal plants. Plant Growth Regul. 2001, 34, 23–37. [Google Scholar] [CrossRef]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioall. Sci. 2010, 2, 282. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.S.; Prasad, D.N.; Singh, S.B.; Kohli, E. Chronic exposure of zinc oxide nanoparticles causes deviant phenotype in Drosophila melanogaster. J. Hazard. Mater. 2017, 327, 180–186. [Google Scholar] [CrossRef]
- Sharma, V.; Anderson, D.; Dhawan, A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis 2012, 17, 852–870. [Google Scholar] [CrossRef]
- Rajakumar, G.; Iruvengadam, M.; Mydhili, G.; Gomathi, T.; Chung, I.M. Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculataleaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess Biosyst. Eng. 2018, 41, 21–30. [Google Scholar] [CrossRef]
- Xiong, H.M. ZnO nanoparticles applied to bioimaging and drug delivery. Adv. Mater. 2013, 25, 5329–5335. [Google Scholar] [CrossRef]
- Zhang, Y.; Nayak, T.R.; Hong, H.; Cai, W. Biomedical applications of zinc oxide nanomaterials. Curr. Mol. Med. 2013, 13, 1633–1645. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.; Weng, Z.; Li, X.; Liu, X.; Wu, S.; Yeung, K.W.K.; Wang, X.; Cui, Z.; Yang, X.; Chu, P.K. Biomedical applications of functionalized ZnO nanomaterials: From biosensors to bioimaging. Adv. Mater. Interfaces 2016, 3, 1500494. [Google Scholar] [CrossRef]
- Hameed, S.; Khalil, A.T.; Ali, M.; Numan, M.; Khamlich, S.; Shinwari, Z.K.; Maaza, M. Greener synthesis of ZnO and Ag–ZnO nanoparticles using Silybum marianum for diverse biomedical applications. Nanomedicine 2019, 14, 655–673. [Google Scholar] [CrossRef]
- Moezzi, A.M.; McDonagh, M.B. Cortie Zinc oxide particles: Synthesis, properties and applications. Chem. Eng. J. 2012, 185, 1–22. [Google Scholar] [CrossRef]
- Hatamie, A.; Khan, A.; Golabi, M.; Turner, A.P.; Beni, V.; Mak, W.C.; Sadollahkhani, Z.; Alnoor, H.; Zargar, B.; Bano, S.; et al. Zinc oxide nanostructure-modified textile and its application to biosensing, photocatalysis, and as antibacterial material. Langmuir 2015, 31, 10913–10921. [Google Scholar] [CrossRef]
- Hu, X.; Cook, S.; Wang, P.; Hwang, H.M. In vitro evaluation of cytotoxicity of engineered metal oxide nanoparticles. Sci. Total Environ. 2009, 407, 3070–3072. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef] [Green Version]
- Santhosh kumar, J.; Kumar, S.V.; Rajeshkumar, S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resour.-Effic. Technol. 2017, 3, 459–465. [Google Scholar]
- Devi, H.S.; Singh, T.D. Synthesis of copper oxide nanoparticles by a novel method and its application in the degradation of methyl orange. Adv. Electr. Electron. Eng. 2014, 4, 83–88. [Google Scholar]
- Bhattacharjee, A.; Ahmaruzzaman, M. CuO nanostructures: Facile synthesis and applications for enhanced photodegradation of organic compounds and reduction of pnitrophenol from aqueous phase. RSC Adv. 2016, 6, 41348–41363. [Google Scholar] [CrossRef]
- Rakhshani, E. Preparation, characteristics and photovoltaic properties of cuprous oxide—A review. Solid-State Electron. 1986, 29, 7–17. [Google Scholar] [CrossRef]
- Nabila, M.I.; Kannabiran, K. Biosynthesis, characterization and antibacterial activity of copper oxide nanoparticles (CuO NPs) from actinomycetes. Biocatal. Agric. Biotechnol. 2018, 15, 56–62. [Google Scholar] [CrossRef]
- Mirza, A.U.; Khan, M.S.; Nami, S.A.; Kareem, A.; Rehman, S.; Bhat, S.A.; Nishat, N. Copper oxide nanomaterials Derived from Zanthoxylum armatum DC. and Berberis lyciumRoyle plant species: Characterization, assessment of free radical scavenging and antibacterial activity. Chem. Biodivers. 2019, 16, e1900145. [Google Scholar] [CrossRef]
- Lee, S.W.; Woo, K.J.; Kim, C.S. Crystallographic and magnetic properties of iron oxide nanoparticles for applications in biomedicine. J. Magn. 2004, 9, 83–85. [Google Scholar] [CrossRef] [Green Version]
- Muthukumar, H.; Chandrasekaran, N.I.; Mohammed, S.N.; Pichiah, S.; Manickam, M. Iron oxide nano-material: Physicochemical traits and in vitro antibacterial propensity against multidrug resistant bacteria. J. Ind. Eng. Chem. 2017, 45, 121–130. [Google Scholar] [CrossRef]
- Chauhan, S.; Upadhyay, L.S.B. Biosynthesis of iron oxide nanoparticles using plant derivatives of Lawsoniainermis (Henna) and its surface modification for biomedical application. Nanotechnol. Environ. Eng. 2019, 4, 8. [Google Scholar] [CrossRef]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer therapy with iron oxide nanoparticles: Agents of thermal and immune therapies. Adv. Drug Deliv. Rev. 2020, 163, 65–83. [Google Scholar] [CrossRef]
- Ahmad, W.; Kumar Jaiswal, K.; Amjad, M. Euphorbia herita leaf extract as a reducing agent in a facile green synthesis of iron oxide nanoparticles and antimicrobial activity evaluation. Inorg. Nano-Met. Chem. 2021, 51, 1147–1154. [Google Scholar] [CrossRef]
- Yin, Z.F.; Wu, L.; Yang, H.G.; Su, Y.H. Recent progress in biomedical applications of titanium dioxide. Phys. Chem. Chem. Phys. 2013, 15, 4844–4858. [Google Scholar] [CrossRef]
- Wu, K.C.-W.; Yamauchi, Y.; Hong, C.-Y.; Yang, Y.-H.; Liang, Y.-H.; Funatsu, T.; Tsunoda, M. Biocompatible, surface functionalized mesoporous titania nanoparticles for intracellular imaging and anticancer drug delivery. Chem. Commun. 2011, 47, 5232–5234. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Roopan, S.M.; Kirthi, A.V.; Venkatesan, J.; Kim, S.-K.; Iyappan, M.; Siva, C. Biological Approach to Synthesize TiO2 Nanoparticles Using Aeromonas hydrophila and Its Antibacterial Activity. Spectrochim. Acta Part A 2013, 107, 82–89. [Google Scholar] [CrossRef]
- MuhdJulkapli, N.; Bagheri, S.; Bee Abd Hamid, S. Recent Advances in Heterogeneous Photocatalytic Decolorization of Synthetic Dyes. Sci. World J. 2014, 2014, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Visai, L.; De Nardo, L.; Punta, C.; Melone, L.; Cigada, A.; Imbriani, M.; Arciola, C.R. Titanium Oxide Antibacterial Surfaces in Biomedical Devices. Int. J. Artif. Organs 2011, 34, 929–946. [Google Scholar] [CrossRef]
- Ren, W.; Zeng, L.; Shen, Z.; Xiang, L.; Gong, A.; Zhang, J.; Mao, C.; Li, A.; Paunesku, T.; Woloschak, G.E.; et al. Enhanced doxorubicin transport to multidrug resistant breast cancer cells via TiO2 nanocarriers. RSC Adv. 2013, 3, 20855–20861. [Google Scholar] [CrossRef]
- Velayutham, K.; Rahuman, A.A.; Rajakumar, G.; Santhoshkumar, T.; Marimuthu, S.; Jayaseelan, C.; Bagavan, A.; Kirthi, A.V.; Kamaraj, C.; Zahir, A.A. Evaluation of Catharanthus Roseus Leaf Extract-mediated Biosynthesis of Titanium Dioxide Nanoparticles Against Hippobosca Maculata and Bovicola Ovis. Parasitol. Res. 2012, 111, 2329–2337. [Google Scholar] [CrossRef]
- Marimuthu, S.; Rahuman, A.A.; Jayaseelan, C.; Kirthi, A.V.; Santhoshkumar, T.; Velayutham, K.; Bagavan, A.; Kamaraj, C.; Elango, G.; Iyappan, M. Acaricidal Activity of Synthesized Titanium Dioxide Nanoparticles Using Calotropis Gigantea Against Rhipicephalus microplus and Haemaphysalisbispinosa. Asian Pacific J. Trop. Med. 2013, 6, 682–688. [Google Scholar] [CrossRef] [Green Version]
- Rajakumar, G.; Rahuman, A.A.; Priyamvada, B.; Khanna, V.G.; Kumar, D.K.; Sujin, P. EcliptaProstrata Leaf Aqueous Extract Mediated Synthesis of Titanium Dioxide Nanoparticles. Mater. Lett. 2012, 68, 115–117. [Google Scholar] [CrossRef]
- Durairaj, B.; Xavier, T.; Muthu, S. Fungal Generated Titanium Dioxide Nanoparticles: A Potent Mosquito (Aedesaegypti) Larvicidal Agent. Sch. Acad. J. Biosci. 2014, 2, 651–658. [Google Scholar]
- Srinivasan, M.; Venkatesan, M.; Arumugam, V.; Natesan, G.; Saravanan, N.; Murugesan, S.; Pugazhendhi, A. Green synthesis and characterization of titanium dioxide nanoparticles (TiO2 NPs) using Sesbania grandiflora and evaluation of toxicity in zebrafish embryos. Process Biochem. 2019, 80, 197–202. [Google Scholar] [CrossRef]
- El-Shabouri, M.H. Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int. J. Pharm. 2002, 249, 101–108. [Google Scholar] [CrossRef]
- Hu, L.; Tang, X.; Cui, F. Solid lipid nanoparticles (SLNs) to improve oral bioavailability of poorly soluble drugs. J. Pharm. Pharmacol. 2004, 56, 1527–1535. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [Green Version]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interf. Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Hano, C.; Abbasi, B.H. Plant-Based Green Synthesis of Nanoparticles: Production, Characterization and Applications. Biomolecules 2022, 12, 31. [Google Scholar] [CrossRef]
- Shivashankar, M.; Sisodia, G. Biosynthesis of silver nanoparticles obtained from plant extracts of Moringa oleifera. Int. J. Life Sci. Biotechnol. Pharm. Res. 2012, 1, 182–185. [Google Scholar]
- Paul, S.; Basak, P.; Majumder, R.; Mukherjee, A.; Ghosh, J.; Patra, S.; Jana, N.K. Biochemical estimation of Moringaoleifera leaf extract for synthesis of silver nanoparticle mediated drug delivery system. J. Plant Biochem. Biotechnol. 2020, 29, 86–93. [Google Scholar] [CrossRef]
- Sharma, A.K.; Swami, A.K.; Jangir, D.; Saran, M.; Upadhyay, T.K.; Prajapat, R.K.; Mathur, M. An eco-friendly green synthesis of tungsten nanoparticles from Moringa oleifera Lam. and their pharmacological studies. GMJ 2020, 31, 719–725. [Google Scholar]
- Sivaranjani, V.; Philominathan, P.J.W.M. Synthesize of Titanium dioxide nanoparticles using Moringa oleifera leaves and evaluation of wound healing activity. Wound Med. 2016, 12, 1–5. [Google Scholar] [CrossRef]
- Sasidharan, S.; Pottail, L. Antimicrobial activity of metal and non-metallic nanoparticles from Cyperus rotundus root extract on infectious disease causing pathogens. J. Plant Biochem. Biotechnol. 2020, 29, 134–143. [Google Scholar] [CrossRef]
- Yasmin, A.; Ramesh, K.; Rajeshkumar, S. Optimization and stabilization of gold nanoparticles by using herbal plant extract with microwave heating. Nano Converg. 2014, 1, 12. [Google Scholar] [CrossRef] [Green Version]
- Adavallan, K.; Krishnakumar, N. Mulberry leaf extract mediated synthesis of gold nanoparticles and its anti-bacterial activity against human pathogens. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 025018. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmed, A.; Akkamwar, B.; Sastry, M.; Rai, A.; Singh, A. Biological synthesis of triangular gold nanoprism. Nature 2004, 3, 482. [Google Scholar] [CrossRef]
- Ponarulselvam, S.; Panneerselvam, C.; Murugan, K.; Aarthi, N.; Kalimuthu, K.; Thangamani, S. Synthesis of silver nanoparticles using leaves of Catharanthus roseus Linn. G. Don and their antiplasmodial activities. Asian Pac. J. Trop. Biomed. 2012, 2, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Suresh, U.; Murugan, K.; Benelli, G.; Nicoletti, M.; Barnard, D.R.; Panneerselvam, C.; Chandramohan, B. Tackling the growing threat of dengue: Phyllanthus niruri-mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedesaegypti (Diptera: Culicidae). Parasitol. Res. 2015, 114, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Rane, R.V.; Meenakshi, K.; Shah, M.; Georg, I.A. Biological synthesis of silver nanoparticles using Abelmoschusmoschatus. Indian J. Biotechnol. 2014, 13, 342–346. [Google Scholar]
- Wen, X.; Wang, Q.; Dai, T.; Shao, J.; Wu, X.; Jiang, Z.; Jiang, C. Identification of possible reductants in the aqueous leaf extract of mangrove plant Rhizophora apiculata for the fabrication and cytotoxicity of silver nanoparticles against human osteosarcoma MG-63 cells. Mater. Sci. Eng. C 2020, 116, 111252. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.; Kumar, D.; Agrawal, V. Green synthesis of silver nanoparticles using Indian Belladonna extract and their potential antioxidant, anti-inflammatory, anticancer and larvicidal activities. Plant Cell Rep. 2020, 39, 921–939. [Google Scholar] [CrossRef]
- Tippayawat, P.; Phromviyo, N.; Boueroy, P.; Chompoosor, A. Green synthesis of silver nanoparticles in aloe vera plant extract prepared by a hydrothermal method and their synergistic antibacterial activity. Peer J. 2016, 4, e2589. [Google Scholar] [CrossRef]
- Vélez, E.; Campillo, G.; Morales, G.; Hincapié, C.; Osorio, J.; Arnache, O. Silver nanoparticles obtained by aqueous or ethanolic aloe vera extracts: An assessment of the antibacterial activity and mercury removal capability. J. Nanomater. 2018, 2018, 7215210. [Google Scholar] [CrossRef] [Green Version]
- Ramteke, C.; Chakrabarti, T.; Sarangi, B.K.; Pandey, R.A. Synthesis of silver nanoparticles from the aqueous extract of leaves of Ocimum sanctum for enhanced antibacterial activity. J. Chem. 2013, 2013, 278925. [Google Scholar] [CrossRef] [Green Version]
- Nancy, B.A.; Elumalai, K. Synthesis of Silver Nanoparticles Using Pelargonium graveolens Essential Oil and Anti-Fungal Activity. Int. J. Pharm. Biol. Sci.-IJPBSTM 2019, 9, 176–185. [Google Scholar]
- Bere, A.W.; Mulati, O.; Kimotho, J.; Ng’ong’a, F. Carica papaya Leaf Extract Silver Synthesized Nanoparticles Inhibit Dengue Type 2 Viral Replication In Vitro. Pharmaceuticals 2021, 14, 718. [Google Scholar] [CrossRef]
- Gunti, L.; Dass, R.S.; Kalagatur, N.K. Phytofabrication of selenium nanoparticles from Emblica officinalis fruit extract and exploring its biopotential applications: Antioxidant, antimicrobial, and biocompatibility. Front. Microbiol. 2019, 10, 931. [Google Scholar] [CrossRef] [Green Version]
- Goyal, P.; Bhardwaj, A.; Mehta, B.K.; Mehta, D. Research article green synthesis of zirconium oxide nanoparticles (ZrO2NPs) using Helianthus annuus seed and their antimicrobial effects. J. Indian Chem. Soc. 2021, 98, 100089. [Google Scholar] [CrossRef]
- Muhammad, W.; Khan, M.A.; Nazir, M.; Siddiquah, A.; Mushtaq, S.; Hashmi, S.S.; Abbasi, B.H. Papaver somniferum L. mediated novel bioinspired lead oxide (PbO) and iron oxide (Fe2O3) nanoparticles: In-vitro biological applications, biocompatibility and their potential towards HepG2 cell line. Mater. Sci. Eng. C 2019, 103, 109740. [Google Scholar] [CrossRef]
- Mani, M.; Pavithra, S.; Mohanraj, K.; Kumaresan, S.; Alotaibi, S.S.; Eraqi, M.M.; Kaviyarasu, K. Studies on the spectrometric analysis of metallic silver nanoparticles (Ag NPs) using Basella alba leaf for the antibacterial activities. Environ. Res. 2021, 199, 111274. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, J.; He, H.; Wang, Y.; Zhao, Y.; Lu, Q.; Peng, Y. Green synthesis of silver nanoparticles with black rice (Oryza sativa L.) extract endowing carboxymethyl chitosan modified cotton with high anti-microbial and durable properties. Cellulose 2021, 28, 1827–1842. [Google Scholar] [CrossRef]
- Chauhan, N.; Tyagi, A.K.; Kumar, P.; Malik, A. Antibacterial potential of Jatropha curcas synthesized silver nanoparticles against food borne pathogens. Front. Microbiol. 2016, 7, 1748. [Google Scholar] [CrossRef] [Green Version]
- Vimalraj, S.; Ashokkumar, T.; Saravanan, S. Biogenic gold nanoparticles synthesis mediated by Mangifera indica seed aqueous extracts exhibits antibacterial, anticancer and anti-angiogenic properties. Biomed. Pharmacother. 2018, 440–448. [Google Scholar] [CrossRef]
- Qamar, H.; Rehman, S.; Chauhan, D.K.; Tiwari, A.K.; Upmanyu, V. Green synthesis, characterization and antimicrobial activity of copper oxide nanomaterial derived from Momordica charantia. Int. J. Nanomed. 2020, 15, 2541. [Google Scholar] [CrossRef] [Green Version]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: Pathways for translational development and commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Bhat, R.; Sharanabasava, V.G.; Deshpande, R.; Shetti, U.; Sanjeev, G.; Venkataraman, A. Photo-bio-synthesis of irregular shaped functionalized gold nanoparticles using edible mushroom Pleurotusflorida and its anticancer evaluation. J. Photochem. 2013, 125, 63–69. [Google Scholar]
- Guo, Y.; Sun, L.; Chen, X.P.; Zhang, D.S. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural. Regen. Res. 2013, 8, 2003–2014. [Google Scholar]
- Arakha, M.; Roy, J.; Nayak, P.S.; Mallick, B.; Jha, S. Zinc oxide nanoparticle energy band gap reduction triggers the oxidative stress resulting into autophagy-mediated apoptotic cell death. Free. Radic. Biol. Med. 2017, 110, 42–53. [Google Scholar] [CrossRef]
- Asharani, P.V.; Hande, M.P.; Valiyaveettil, S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 2009, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Raja, G.; Kim, S.; Yoon, D.; Yoon, C.; Kim, S. 1H NMR based metabolomics studies of the toxicity of titanium dioxide nanoparticles in zebrafish (Danio rerio). Bull. Korean Chem. Soc. 2018, 39, 33–39. [Google Scholar] [CrossRef]
- Saeed, M.; Iqbal, M.Z.; Ren, W.Z.; Xia, Y.Z.; Liu, C.; Khan, W.S.; Wu, A.G. Controllable synthesis of Fe3O4 nanoflowers: Enhanced imaging guided cancer therapy and comparison of photothermal efficiency with black-TiO2. J. Mater. Chem. B. 2018, 6, 3800–3810. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Ren, W.Z.; Saeed, M.; Chen, T.X.; Ma, X.H.; Yu, X.; Zhang, J.C.; Zhang, L.L.; Li, A.G.; Wu, A.G. A facile fabrication route for binary transition metal oxide-based Janus nanoparticles for cancer theranostic applications. Nano Res. 2018, 11, 5735–5750. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, Z.; Qiu, N.; Shen, Y. Rational design of cancer nanomedicine: Nanoproperty integration and synchronization. Adv. Mater. 2017, 29, 1606628. [Google Scholar] [CrossRef]
- Hoffman, A.J.; Yee, H.; Mills, G.; Hoffmann, M.R. Photoinitiated polymerization of methyl methacrylate using Q-sized zinc oxide colloids. J. Phys. Chem. 1992, 96, 5540–5546. [Google Scholar] [CrossRef]
- Schmid, G. Large clusters and colloids. Metals in the embryonic state. Chem. Rev. 1992, 92, 1709–1727. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Rah, D.K.; Han, D.W.; Baek, H.S.; Hyon, S.H.; Park, B.Y.; Park, J.C. Protection of rabbit kidney from ischemia/reperfusion injury by green tea polyphenol pretreatment. Arch. Pharm. Res. 2007, 30, 1447–1454. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Karimi, S.; Iravani, S.; Varma, R.S. Plant-derived nanostructures: Types and applications. Green Chem. 2016, 18, 20–52. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Karimi, S.; Tahir, P.M.; Karimi, A.; Dufresne, A.; Abdulkhani, A. Kenaf bast cellulosic fibers hierarchy: A comprehensive approach from micro to nano. Carbohydr. Polym. 2014, 101, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Berglund, L. Cellulose-based nanocomposites. In Natural Fibers, Biopolymers and Biocomposites; Mohanty, A.K., Misra, M., Drzal, L.T., Eds.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Jha, A.K.; Prasad, K.; Prasad, K.; Kulkarni, A.R. Plant system: Nature’s nanofactory. Colloids Surf. B Biointerfaces 2009, 73, 219–223. [Google Scholar] [CrossRef]
- Kesharwani, J.; Yoon, K.Y.; Hwang, J.; Rai, M. Phytofabrication of silver nanoparticles by leaf extract of Datura metel: Hypothetical mechanism involved in synthesis. J. Bionanosci. 2009, 3, 39–44. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, Q.; Reddy, N.; Yang, Y. Hollow nanoparticles from zein for potential medical applications. J. Mater. Chem. 2011, 21, 18227–18235. [Google Scholar] [CrossRef]
- Muramatsu, H.; Kim, Y.A.; Yang, K.S.; Cruz-Silva, R.; Toda, I.; Yamada, T.; Saitoh, H. Rice husk-derived graphene with nano-sized domains and clean edges. Small 2014, 10, 2766–2770. [Google Scholar] [CrossRef]
- Chen, X.W.; Timpe, O.; Hamid, S.B.; Schlögl, R.; Su, D.S. Direct synthesis of carbon nanofibers on modified biomass-derived activated carbon. Carbon 2009, 47, 340–343. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, J.; Kwong, F.L.; Ng, D.H.L.; Tjong, S.C. Synthesis of multiwalled carbon nanotubes from bamboo charcoal and the roles of minerals on their growth. Biomass Bioenergy 2012, 36, 12–19. [Google Scholar] [CrossRef]
- Xia, L.; Lenaghan, S.C.; Wills, A.B.; Chen, Y.; Zhang, M. Evaluation of the nanofibrillar structure of Dioscorea opposite extract for cell attachment. Colloids Surf. B Biointerfaces 2011, 88, 425–431. [Google Scholar] [CrossRef]
- Wang, B.; Zhuang, X.; Deng, Z.B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef] [Green Version]
- Voirin, C.; Caillol, S.; Sadavarte, N.V.; Tawade, B.V.; Boutevin, B.; Wadgaonkar, P.P. Functionalization of cardanol: Towards biobased polymers and additives. Polym. Chem. 2014, 5, 3142–3162. [Google Scholar] [CrossRef]
- Balachandran, V.S.; Jadhav, S.R.; Vemula, P.K.; John, G. Recent advances in cardanol chemistry in a nutshell: From a nut to nanomaterials. Chem. Soc. Rev. 2013, 42, 427–438. [Google Scholar] [CrossRef]
- Liu, N.; Huo, K.; McDowell, M.T.; Zhao, J.; Cui, Y. Rice husks as a sustainable source of nanostructured silicon for high performance Li-ion battery anodes. Sci. Rep. 2013, 3, 1919. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, J.; Jang, M.; Kwak, M.H.; Go, J.; Kho, E.K.; Song, S.H.; Sung, J.E.; Lee, J.; Hwang, D.Y. Accelerated healing of cutaneous wounds using phytochemically stabilized gold nanoparticle deposited hydrocolloid membranes. Biomater. Sci. 2015, 3, 509–519. [Google Scholar] [CrossRef]
- Naraginti, S.; Kumari, P.L.; Das, R.K.; Sivakumar, A.; Patil, S.H.; Andhalkar, V.V. Amelioration of excision wounds by topical application of green synthesized, formulated silver and gold nanoparticles in albino Wistar rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 293–300. [Google Scholar] [CrossRef]
- Krychowiak, M.; Grinholc, M.; Banasiuk, R.; Krauze-Baranowska, M.; Głód, D.; Kawiak, A.; Królicka, A. Combination of silver nanoparticles and Droserabinata extract as a possible alternative for antibiotic treatment of burn wound infections caused by resistant Staphylococcus aureus. PLoS ONE 2014, 9, e115727. [Google Scholar]
- Ramazan, E. Advances in fabric structures for wound care. In Advanced Textiles for WoundCare; Rajendran, S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 509–540. [Google Scholar]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6027439/ (accessed on 4 January 2022). [CrossRef] [Green Version]
- Tayi, A.S.; Pashuck, E.T.; Newcomb, C.J.; McClendon, M.T.; Stupp, S.I. Electrospinning bioactive supramolecular polymers from water. Biomacromolecules 2014, 15, 1323–1327. [Google Scholar] [CrossRef]
- Dias Antunes, M.; da Silva Dannenberg, G.; Fiorentini, A.M.; Pinto, V.Z.; Lim, L.T.; daRosaZavareze, E.; Dias, A.R.G. Antimicrobial electrospun ultrafine fibers from zein containing eucalyptus essential oil/cyclodextrin inclusion complex. Int. J. Biol. Macromol. 2017, 104, 874–882. [Google Scholar] [CrossRef]
- Moreno, M.A.; Orqueda, M.E.; Go’mez-Mascaraque, L.G.; Isla, M.I.; Lo´pez-Rubio, A. Crosslinked electrospun zein-based food packaging coatings containing bioactive chilto fruit extracts. Food Hydrocoll. 2019, 95, 496–505. [Google Scholar] [CrossRef]
- Miguel, S.P.; Sequeira, R.S.; Moreira, A.F.; Cabral, C.S.; Mendonça, A.G.; Ferreira, P.; Correia, I.J. An overview of electrospun membranes loaded with bioactive molecules for improving the wound healing process. Eur. J. Pharm. Biopharm. 2019, 139, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Suwantong, O.; Opanasopit, P.; Ruktanonchai, U.; Supaphol, P. Electrospun cellulose acetate fiber mats containing curcumin and release characteristic of the herbal substance. Polymer 2007, 48, 7546–7557. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Haftcheshmeh, S.M.; Esmaeili, S.A.; Johnston, T.P.; Abdollahi, E.; Sahebkar, A. Curcumin: A natural modulator of immune cells in systemic lupus erythematosus. Autoimmun. Rev. 2017, 17, 125–135. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Nicolin, V.; De Tommasi, N.; Nori, S.L.; Costantinides, F.; Berton, F.; Di Lenarda, R. Modulatory effects of plant polyphenols on bone remodeling: A prospective view from the bench to bedside. Front. Endocrinol. 2019, 10, 494. [Google Scholar] [CrossRef]
- Park, Y.H.; Han, D.W.; Suh, H.; Ryu, G.H.; Hyon, S.H.; Cho, B.K.; Park, J.C. Protective effects of green tea polyphenol against reactive oxygen species-induced oxidative stress in cultured rat calvarial osteoblast. Cell Biol. Toxicol. 2003, 19, 325–337. [Google Scholar] [CrossRef]
- Vu, M.N.; Kelly, H.G.; Kent, S.J.; Wheatley, A.K. Current and future nanoparticle vaccines for COVID-19. EBioMedicine 2021, 74, 103699. [Google Scholar] [CrossRef]
- Jagessar, R.C. Plant Extracts Based Nanoparticles, Potential Nanomedicine in Fight against COVID-19. J. Nanosci. Res. Rep. 2020, 2, 1–4. [Google Scholar]
- Medicago and GSK Announce Positive Phase 3 Efficacy and Safety Results for Adjuvanted Plant-Based COVID-19 Vaccine Candidate. Available online: https://www.gsk.com/en-gb/media/press-releases/medicago-and-gsk-announce-positive-phase-3-efficacy-and-safety-results/ (accessed on 4 January 2022).
- Prabha, S.; Durgalakshmi, D.; Rajendran, S.; Lichtfouse, E. Plant-derived silica nanoparticles and composites for biosensors, bioimaging, drug delivery and supercapacitors: A review. Environ. Chem. Lett. 2020, 19, 1667–1691. [Google Scholar] [CrossRef]
- Parveen, K.; Banse, V.; Ledwani, L. Green synthesis of nanoparticles: Their advantages and disadvantages. In Proceedings of the 2nd International Conference on Emerging Technologies: Micro to Nano 2015, Rajasthan, India, 24–25 October 2015; Volume 1724, p. 020048. [Google Scholar]



| S. No. | Source Plant | Type of NPs Prepared | Color of NPs | Phase of NPs | Biomedical Application | Reference |
|---|---|---|---|---|---|---|
| 1. | Moringa oleifera | Poly Vinyl Alcohol-Silver Nanoparticles (PVA-AgNPs) | Green black | Liquid | Cervical cancer cell line (HeLa) proliferation was halted by newly formed drug-loaded PVA-AgNp and developed an antineoplastic (drug that stopped cellular proliferation, i.e., by inhibiting DNA synthesis) compound. | [94,95] |
| Moringa oleifera Lam. | Tungsten Nanoparticles (W-NPs) | Dark brown | Liquid | It shows antibacterial, antifungal activity against Bacillus subtilis and Fusarium exosporium, respectively. It shows remarkable cytotoxicity against MCF-7 (breast cancer cell line) and 3T3 (fibroblast cell line). NPs were potent at 200µg/mL and 0 µg/mL concentrations, respectively. | [96] | |
| Moringa oleifera | Titanium dioxide nanoparticles (TiNPs) | Brown color | Liquid | In an animal model (excision wound model), TiNPs of Moringa oleifera showedenhancedcontactions in wound compared to any standard animal excision model in the experiment. | [97] | |
| 2. | Cyperus rotundus(CR) | Gold Nanoparticles (Au-NPs) | Grayish pink | Liquid | CRnp (Cyperus rotundus gold nanoparticles) shows antibacterial activity against various Gram-positive (G+) (Staphylococcus aureus, Bacillus subtilis) and Gram-negative (G−) (Salmonella paratyphi, Escherichia coli) bacterial species. | [98] |
| 3. | Hibiscus rosa-Sinensis | Gold Nanoparticles (Au-NPs) | Ruby red color | Liquid | It is nontoxic, highly stable and therapeutic, and diagnostic purposes can be used for biomedical and sensor applications. | [99] |
| 4. | Morus alba (white mulberry) | Gold Nanoparticles (Au-NPs) | Purple, pink color | Liquid | It shows inhibition against human pathogens, i.e., Vibrio cholera (G−) and Staphylococcus aureus (G+) bacteria. | [100] |
| 5. | Catharanthus roseus Linn. G. | Silver Nanoparticles (Ag-NPs) | Dark yellowish-brown | Liquid | It shows anti-plasmodial activity against P. falciparum. | [101,102] |
| 6. | Phyllanthus niruri | Silver Nanoparticles (Ag-NPs) | Brown yellow | Liquid | These NPs have mosquitocidal properties against the dengue vector Aedes aegypti species. | [103] |
| 7. | Abelmoschus moschatus | Silver Nanoparticles (Ag-NPs) | Brownish-yellow | Liquid | Shows antimicrobial activity against P. aeruginosa, S. aureus, and B. subtilis. | [104] |
| 8. | Rhizophora apiculata | Silver Nanoparticles (Ag-NPs) | Yellowish-brown | Liquid | Synthesized NPs were tested against osteosarcoma cells (MG-63) in vitro and showed promising results against bone cancer cell lines. | [105] |
| 9. | Atropa acuminata (Indian Belladonna) | Silver Nanoparticles (Ag-NPs) | Yellowish-brown | Liquid | (i) Synthesized Ag-NPs effectively controlled autoantigen production that occurs during the inflammation process. (ii) Biosynthesized AgNPs impart potent cytotoxicity against the HeLa (cervical cancer) cell line. | [106] |
| Atropa acuminata | Silver Nanoparticles (Ag-NPs) | Red wine colour | Liquid | Ag-NPs of belladone shows the potential halting of BSA protein denauturation. Mainly atropine and apoatropine were major antiinflammatory agents/compounds present in it. | [2] | |
| 10. | Passiflora caerulea L. (Passifloraceae) | Zinc oxide Nanoparticles (ZnONPs) | Yellow color | Liquid | It shows antibacterial activity against infectious disease urinary tract infection (UTI) pathogens such asthe B. subtilis, Klebsiella pneumonia, E. coli, Serratia, and Streptococcus species. | [67] |
| 11. | Aloe vera | Silver Nanoparticles (Ag-NPs) | Gray color | Solid ppt. | The bactericidal effect was observed against pathogenic S. epidermidis (G+) and P. aeruginosa (G−). | [107] |
| It also shows antibacterial activity against Kocuriavarians(G+). | [108] | |||||
| 12. | Ocimum sanctum (holy basil or tulsi) | Silver Nanoparticles (Ag-NPs) | Yellow-brown color | Liquid | The Ag-NPs show the zone of inhibition against the flourishing of E. coli and Staphylococcusaureus bacterial species. | [109] |
| 13. | Pelargonium graveolens | Silver Nanoparticles (Ag-NPs) | Dark brownish color | Liquid | It exhibits antifungal activity in Candida tropicalis and Candida kefir fungal species. | [110] |
| Pelargonium graveolens | Silver Nanoparticles (Ag-NPs) | Dark brown color | Liquid | These Ag-NPs exhibited antibacterial against Candida albicans, Candida kefyr Candida tropicalis, Aspergillus niger,and Aspergillus flavus and had fungicidal activity. Theyalso show good wound healing activity and can be used in the field of medicine. | [111] | |
| 14. | Emblica Officinalis (Amla) | Phyto-fabricated Selenium Nanoparticles (PF-SeNPs) | Brick-red color | Liquid | It exhibits potent antibacterial activity towards G+ bacteria (S. aureus MTCC 96, E. faecalis MTCC 439, and L. monocytogenes MTCC 657) compared to G -bacteria (E. coli MTCC 4). | [112] |
| 15. | Helianthus annuus (Sunflower) seed | Zirconium oxide nanoparticles (ZrO2NPs) | Black powder | Liquid | It shows the inhibition of G bacteria (E. coli, P. aeruginosa, and K. pneumonia) in comparison to G+ S. aureus bacteria. | [113] |
| 16. | Papaver somniferum L. | Lead oxide (PbO) and iron oxide (Fe2O3) nanoparticles | Violet color | Solid pellets | (i) These Biogenic NPs showed potency against most of the pathogenic strains such as G+ (Bacillus subtilis and Staphylococcus epidermidis) and G− (Klebsiella pneumonia and Pseudomonas aeruginosa) bacterial species, while S. epidermidis was found prone to Fe2O3 NPs. (ii) It also exhibits an antifungal tendency against fungal pathogens such as Aspergillus flavus, Fusarium solani, Mucormycosis, Aspergillus niger, and Aspergillus fumigates. The PbO NPs show that the highest inhibition was found against F. solani. While Fe2O3 NPs also show it, the highest antifungal characteristics werefound towards F. solani. (iii) Cell culture study: Cytotoxicity screening performedagainst HepG2 (Human liver cancer) tumorigenic cell line. PbO NPs showed elevated cytotoxicity in contrast to Fe2O3 NPs. | [114] |
| 17. | Basella alba L. | Metallic Silver Nanoparticles (Ag-NPs) | Yellowish-brown color | Liquid | It shows antibacterial properties towards pathogens G+ (S. aureus, P. aeruginosa) and G− (Enterococcus feces, E. coli). S. aureus and E. coli bacterial species show a broad region of inhibition. | [115] |
| 18. | Oryza sativa (Black rice) | Silver Nanoparticles (Ag-NPs) | Brownish color | Liquid | Ag-NPs-loaded samples against E. coli and S. aureus clearly show inhibition zones in a bacterial culture plate. | [116] |
| 19. | Jatropha curcas(JC) | Silver Nanoparticles (Ag-NPs) | Brownish–gray color | Liquid | JC-AgNps show action against Listeria monocytogenes (Food born pathogen), a bacterial pathogen. | [117] |
| 20. | Carica papaya | Silver Nanoparticles (Ag-NPs) | Dark brown | Liquid | It highly inhibited DENV-2 replication in vitro conditions with a viral inhibition percentage >90. | [112] |
| 21. | Mangifera indica | Gold Nanoparticles (Au-NPs) | Deep purple red/ruby red color. | Liquid | It was found that Au-NPs were effective at low concentrations when used against G− (E. coli) and G + (S. aureus) bacterial species. | [118] |
| 22. | P. caerulea L. (Passifloraceae) | Zinc oxide Nanoparticle (ZnO-NPs) | Yellow color | Liquid | It shows antibacterial activity against urinary tract infection (UTI) pathogens such as Streptococcus sp., Serratia sp., E. coli, Klebsiella pneumonia, and B. subtilis. | [67] |
| 23. | Momordica charantia | Copper Oxide Nanoparticles (CuO-NPs) | Brown color | Liquid | CuO-NPs were effective against Multi-Drug Resistance (MDR) bacterial strains such as G+ bacteria Bacillus cereus, Streptococcus mutans, Streptococcus viridans, etc., and G− bacteria such as Proteus Vulgaris, Escherichia coli, and Pseudomonas aeruginosa. | [119] |
| 24. | Berberis lycium ROYLE(SU) | Copper Oxide Nanoparticle (CuO-NPs) | Brown color | Liquid | Its NPs are sensitive against the G+ and G−bacterial sp. The highest zone of inhibition in the case of G+ bacteria is towards Streptococcus Mutants,while in the case of G− bacteria, it is towards Escherichia coli. | [72] |
| 25. | Zanthoxylum armatum DC. | Copper Oxide Nanoparticle (CuO-NPs) | Black color | Liquid | It shows antibacterial activity and maximum inhibition against Streptococcus mutans (G+) bacterium, while in case of G− bacterium it shows maximum inhibition against Pseudomonas aeruginosa. | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivedi, R.; Upadhyay, T.K.; Mujahid, M.H.; Khan, F.; Pandey, P.; Sharangi, A.B.; Muzammil, K.; Nasir, N.; Hassan, A.; Alabdallah, N.M.; et al. Recent Advancements in Plant-Derived Nanomaterials Research for Biomedical Applications. Processes 2022, 10, 338. https://doi.org/10.3390/pr10020338
Trivedi R, Upadhyay TK, Mujahid MH, Khan F, Pandey P, Sharangi AB, Muzammil K, Nasir N, Hassan A, Alabdallah NM, et al. Recent Advancements in Plant-Derived Nanomaterials Research for Biomedical Applications. Processes. 2022; 10(2):338. https://doi.org/10.3390/pr10020338
Chicago/Turabian StyleTrivedi, Rashmi, Tarun Kumar Upadhyay, Mohd Hasan Mujahid, Fahad Khan, Pratibha Pandey, Amit Baran Sharangi, Khursheed Muzammil, Nazim Nasir, Atiq Hassan, Nadiyah M. Alabdallah, and et al. 2022. "Recent Advancements in Plant-Derived Nanomaterials Research for Biomedical Applications" Processes 10, no. 2: 338. https://doi.org/10.3390/pr10020338
APA StyleTrivedi, R., Upadhyay, T. K., Mujahid, M. H., Khan, F., Pandey, P., Sharangi, A. B., Muzammil, K., Nasir, N., Hassan, A., Alabdallah, N. M., Anwar, S., Siddiqui, S., & Saeed, M. (2022). Recent Advancements in Plant-Derived Nanomaterials Research for Biomedical Applications. Processes, 10(2), 338. https://doi.org/10.3390/pr10020338










