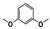
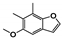
Abstract
To investigate the effects of different aging times, three An–tea samples were selected for comparison with a non-aged sample as the control (CK, one-year-old) and labeled as high-aged (HAS, 12 years old), medium-aged (MAS, 7 years old), and low-aged samples (LAS, 2 years old). Changes in the volatile components of the different An–tea samples were investigated using headspace–solid-phase microextraction (HS-SPME) combined with gas chromatography–mass spectrometry (GC-MS). The volatile components of An–tea consisted of eight types of compounds. Aldehydes and hydrocarbons were dominant in LAS, while esters, ketones, alcohols, nitrogen-containing compounds, and ethers were the most abundant compound in MAS. Esters were dominant in HAS, while phenols were only present in LAS and CK. As aging time increased, the number of identical compounds gradually decreased, while the relative contents of the alcohols also decreased. Except for CK, the contents of ketones and aldehydes gradually decreased as the aging time increased. The content of aldehydes greatly increased during the initial aging period. Ketones and esters had markedly increased in HAS, while their abundance in LAS was close to that in CK. The comprehensive quality score of the volatile components obtained by principal component analysis was highest for MAS, followed by HAS and LAS, with CK having the lowest score.
1. Introduction
An–tea is a type of post-fermented and pressed tea mainly produced in 15 southwestern townships of Qimen County, such as Luxi and Rongkou in southern Anhui, China. An–tea has a history of about 300 years and is part of the provincial intangible cultural heritage [1,2,3,4]. Owing to its “mellow and sweet taste, rich and long aroma”, and medicinal value, An–tea has been included in the Chinese national geographical indication product list [5,6]. Relatively ripe tea leaves before and after “grain rain” are picked to make the tea, then the coarse (green) and acerbic characteristics are removed by a unique stock fermentation and aging process. Aging can be conducted indoors [2], which not only solves the problem of the wastage of fresh leaves in summer and autumn, but also avoids the need for cold storage. However, in contrast to the production of Keemun and other famous teas with their strong development momentum in the region, the disorder and confusion regarding An–tea production and management has meant that its production and sales volume have remained at a low level. Relevant research and product development have also lagged behind, with the basic understanding of the medicinal value of An–tea still remaining at the level of ancient writings and legend.
Information on the composition of the volatile aromas produced during the aging process of post-fermented tea is essential for understanding tea quality [7,8,9]. Studies have shown that, with increasing aging time, the contents of the functional and aromatic components in post-fermented teas, such as Pu’er tea [9,10,11,12], Liupao tea [13], and Green brick tea [14], can change significantly. Compared with a post-fermented tea, such as Pu’er, there have been few studies on the compositional changes in the volatile substances of An–tea during the aging process, so the identity of the main aromatic components of aged An–tea remain unclear. In this study, the main volatile components, such as ketones, aldehydes, alcohols, esters, phenols, hydrocarbons, and nitrogenous and other compounds in aged An–tea, will be quantified and identified by headspace–solid-phase microextraction (HS-SPME) combined with gas chromatography–mass spectrometry (GC-MS). The objectives of this study were: (1) to determine the chemical composition of An–tea after different aging times; (2) to determine the key compounds responsible for its aroma; and (3) to comprehensively evaluate the relationship between the composition of the volatile components and the aging time, which causes the main quality changes in An–tea.
2. Materials and Methods
2.1. Sample Collection and Preparation
Tea samples aged for 12, 7, and 2 years were selected from the largest An–tea processing plant in Luxi Township, Qimen County, China, which is the core protected area for An–tea production, and were labeled as high-aging (HAS), medium-aging (MAS), and low-aging (LAS) samples, respectively. One-year-old unfermented tea recently placed in a storage room was used as the control sample (CK). Three replicates were prepared for each sample. The basic sample information is shown in Table 1.

Table 1.
Information on An–tea samples.
2.2. Primary Instrument
GC-MS was performed using an Agilent 7890-5975 instrument (Agilent Technologies Inc., Santa Clara, CA, USA) with a fused silica capillary column (30 m × 250 mm × 0.25 µm; DB-5MS, Agilent Technologies Inc.) and a carboxen (CAR)/divinylbenzene (DVB)/polydimethylsiloxane (PDMS) SPME fiber (50/30 µm; 57298-U, Supelco, Bellefonte, PA, USA).
2.3. SPME Extraction Method
The tea powder sample (4.0 g) was placed in a 250 mL extraction bottle. Boiling water (50 mL) was then added and the bottle sealed. A CAR/DVB/PDMS SPME fiber (50/30 µm) was inserted into the extraction head at 60 °C for 60 min, followed by desorption for 3 min.
2.4. GC/MS Analysis
GC was performed using an injector temperature of 250 °C with high–purity helium as the carrier gas. The column phase heating program was as follows: 40 °C held for 2 min, increased to 85 °C at 5 °C/min and held for 2 min, increased to 110 °C at 2 °C/min, increased to 139 °C at 7 °C/min, and then increased to 230 °C at 5 °C/min and held for 8 min.
Mass spectrometry was conducted using electron ionization (EI) with an ion source temperature of 230 °C, ionization energy of 70 eV, and mass scan range of 35–500 amu.
2.5. Identification of Volatile Components
The National Institute of Standards and Technology (NIST)08 standard spectrum library was used for retrieval matching and manual parsing. The chemical composition and structure of the volatile components were identified according to a standard of mass spectrometry matching of >90% and the relative contents were determined by the peak area normalization method [15,16,17].
2.6. Data Processing
SPSS 19.0 software (IBM, Armonk, NY, USA) was used for principal component analysis. The graphics were processed using Origin 8.0 (OriginLab Corp., Northampton, MA, USA) and Chemsketch v11 (ACD/Labs, Toronto, ON, Canada) software.
3. Results
3.1. Composition of Volatile Substances in An–tea of Different Aaging Times
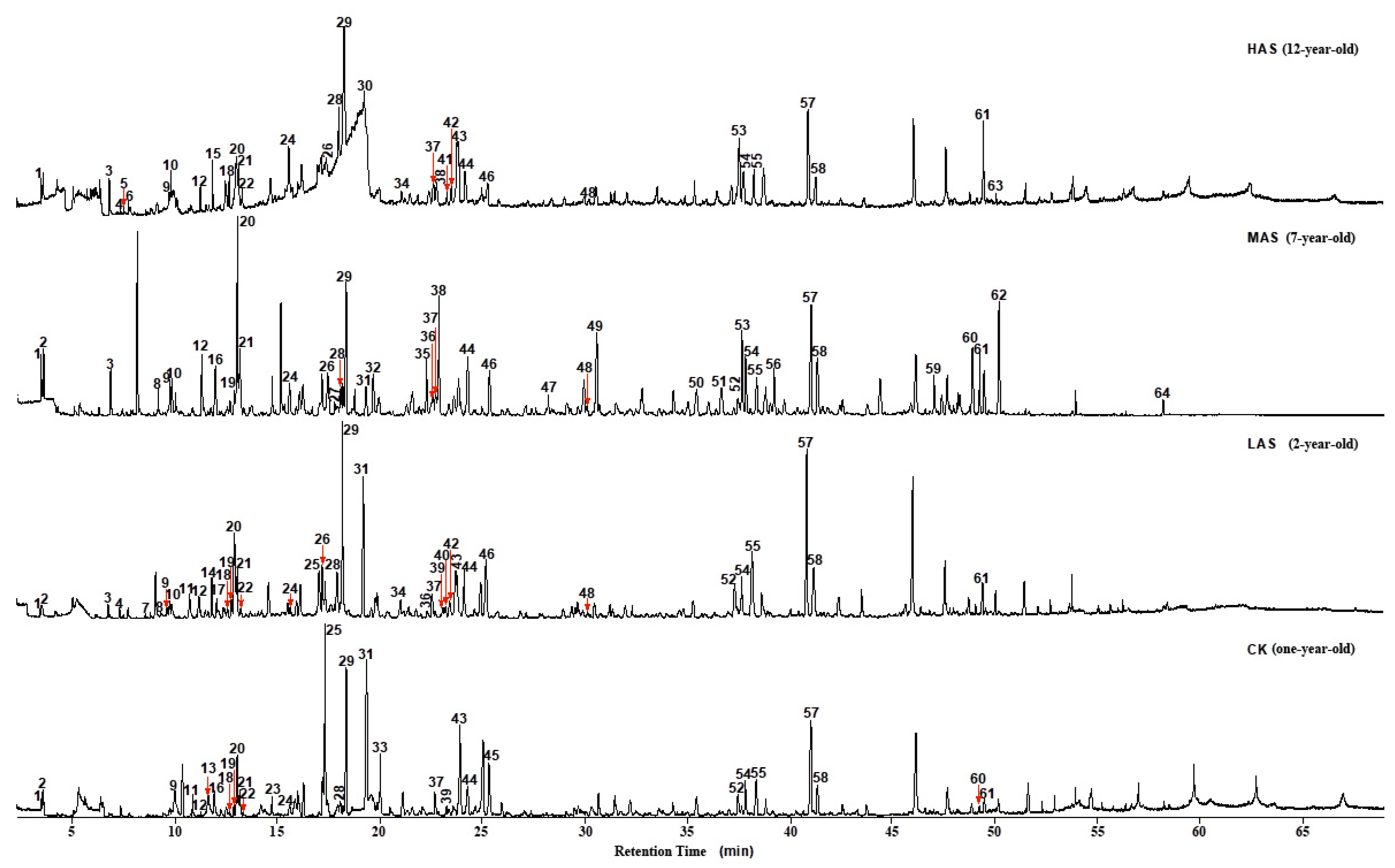
By integrating the GC-MS total ion chromatograms of the four An–tea samples, 64 peaks of volatile substances were identified (Figure 1). The HAS, MAS, LAS, and CK samples exhibited 34, 41, 39, and 31 peaks, respectively. Seven types of compounds, esters, ketones, aldehydes, alcohols, phenols, hydrocarbons, and nitrogenous compounds, as well as small amounts of furans and ethers, were identified in the four An–tea samples—with eight types of compounds identified among the volatile components (Table 2). The identification results showed that the number of volatile compounds in An–tea increased with aging time: the LAS and CK samples contained 24 of the same volatile compounds, while MAS and HAS had only 21 and 18 compounds in common with CK, respectively. Meanwhile, LAS and MAS had 26 compounds in common, while MAS and HAS shared 22 compounds. The volatile compounds in LAS An–tea were similar to those in CK. With increasing aging time, the similarity with new tea decreased, with MAS and HAS having fewer compounds in common with CK. Meanwhile, the aged samples were highly similar. To some extent, the differences in the number of volatile compounds reflected the intensity of the transformations caused by biochemical reactions during aging. This indicated that more transformations occurred in An–tea during the later stages of aging than the early stages.
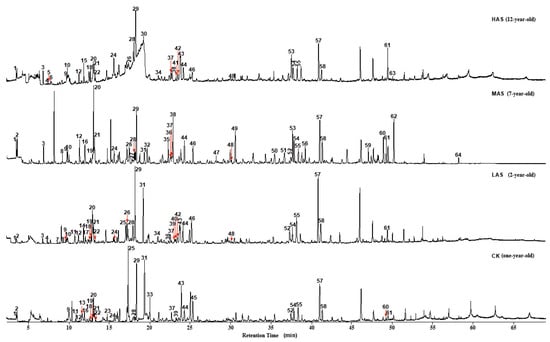
Figure 1.
Total ion chromatogram of four An–tea samples.

Table 2.
Compounds identified in the An–tea samples.
3.2. Relative Compositions of Volatile Compounds in Differently Aged An–tea Samples
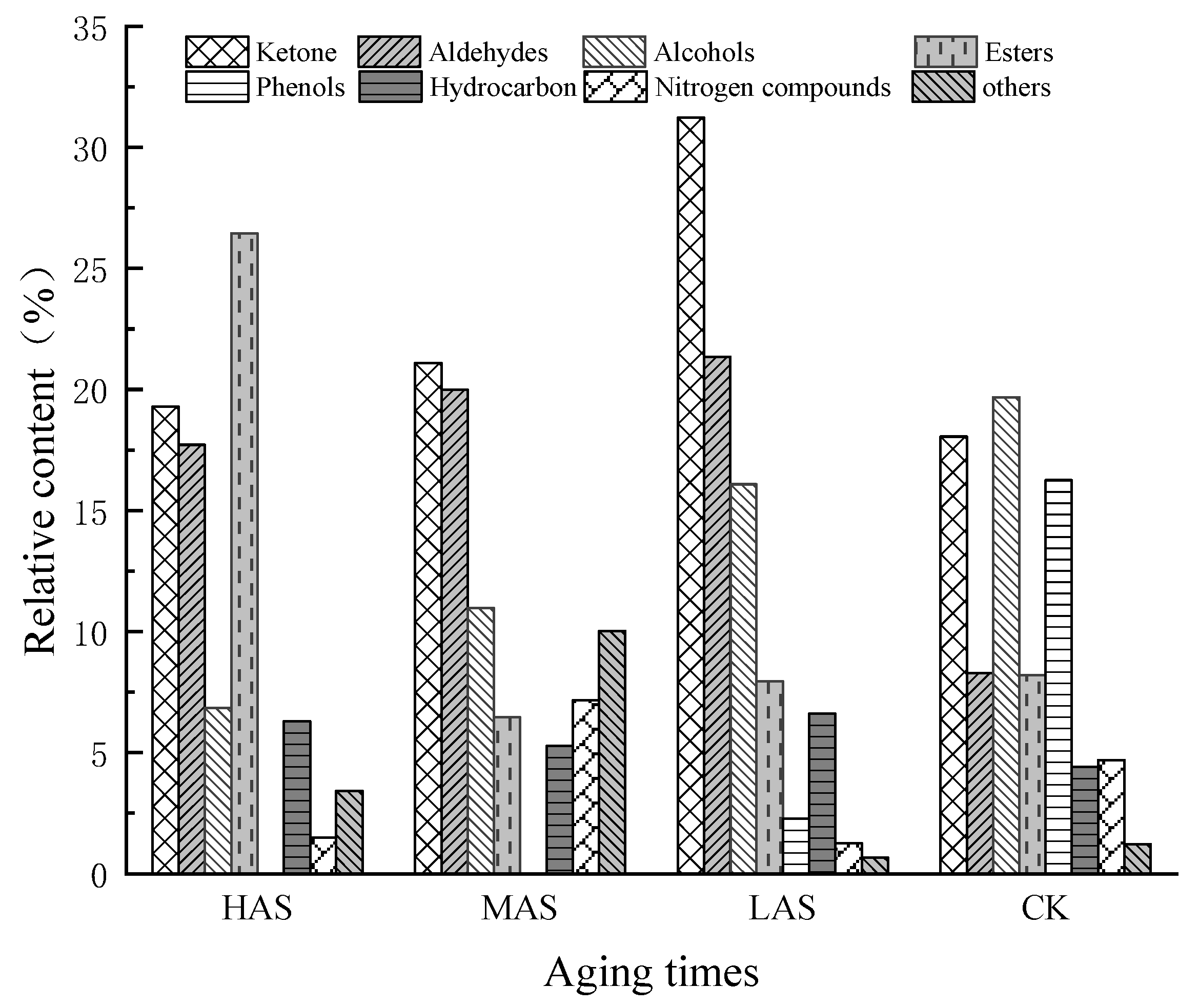
The relative contents of ketones, aldehydes, and alcohols in the three samples with different aging times were generally higher than those of esters, hydrocarbons, nitrogen-containing compounds, and other classes, except for the higher relative contents of esters in HAS and other classes in MAS (Figure 2).
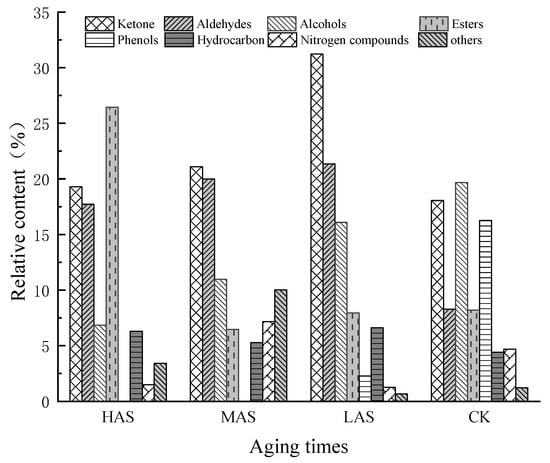
Figure 2.
Relative contents (%) of eight types of volatile compounds detected in samples of An–tea with different aging times.
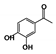
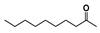
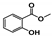
The relative contents of the volatile compounds in samples with different aging times were significantly different from each other, disregarding those of CK. The relative contents of ketones in the HAS, MAS, and LAS samples were 19.29%, 21.09%, and 31.22%, respectively, while those of aldehydes were 17.72%, 20.00%, and 21.35%, respectively, with the content of both compounds tending to decrease with increasing aging time. This was consistent with the decreasing trend in the aldehyde content reported for aged Pu’er tea [18]. The relative contents of ketones and aldehydes in the CK samples were 18.06% and 8.30%, respectively. During the early stages of aging, the relative contents of these two types of compounds increased by 13.16% and 13.05%, respectively, significantly increasing their accumulation. The HAS, MAS, LAS, and CK samples had relative alcohol contents of 6.85%, 10.99%, 16.08%, and 19.68%, respectively, indicating a decreasing trend with increasing aging time, while, in contrast, the ester contents were 26.45%, 6.47%, 7.96%, and 8.22%, respectively, indicating an increasing trend with increasing aging time. The ester content of HAS was significantly higher, with those of MAS and LAS being similar to that of CK. The hydrocarbon contents of HAS, MAS, LAS, and CK samples were similar at 6.29%, 5.28%, 6.61%, and 4.22%, respectively, while the contents of nitrogen-containing compounds were 1.48%, 7.16%, 1.27%, and 4.69%, respectively. The contents of other compounds were 3.42%, 10.03%, 0.67%, and 1.23%, respectively, with the MAS samples showing the highest contents. Phenols were only present in LAS and CK with relative contents of 2.28% and 16.25%, respectively, indicating that phenols underwent a rapid transformation during the early stages of aging.
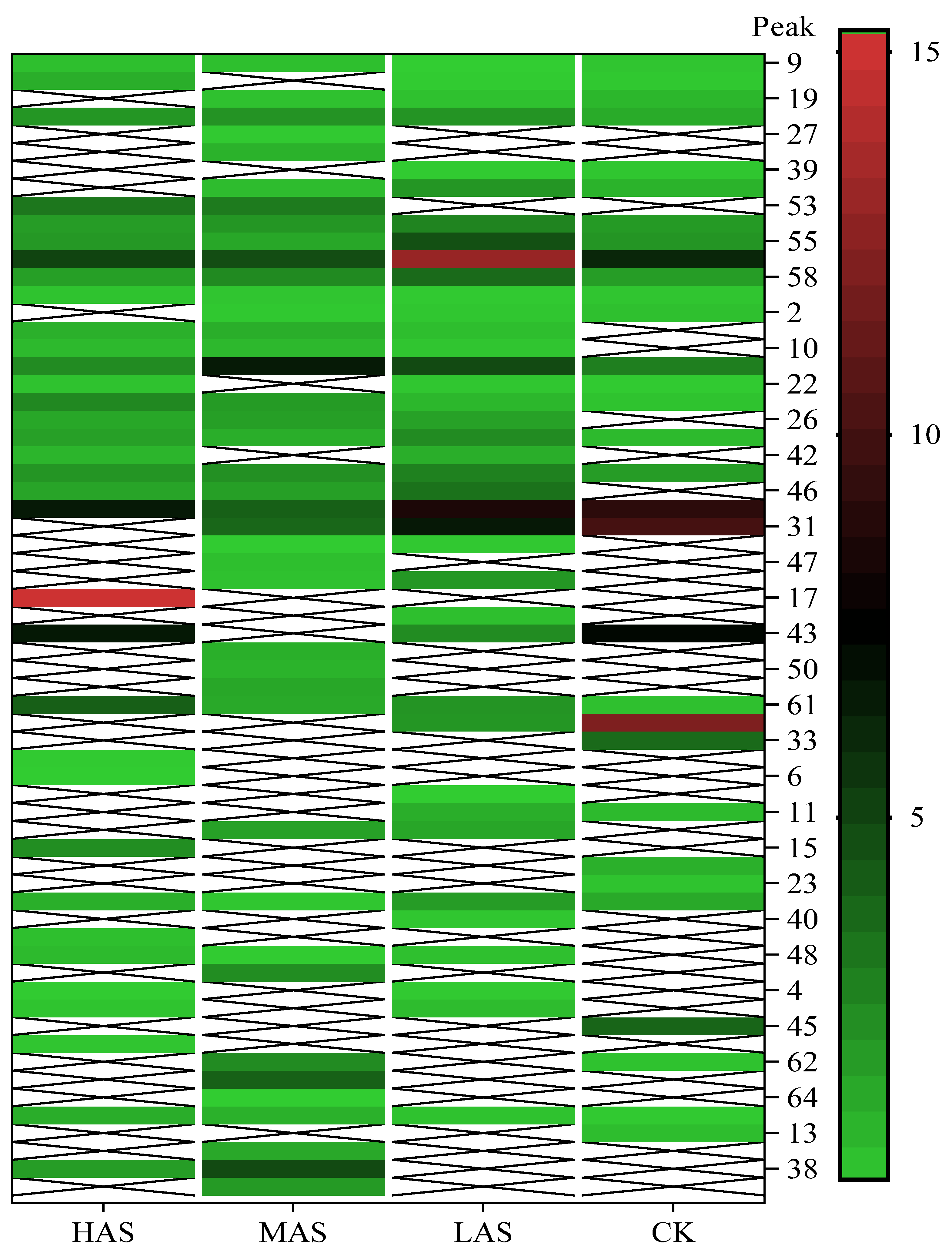
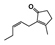
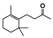
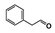
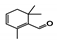
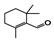
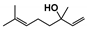
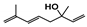
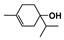
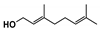
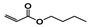
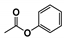
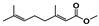
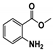
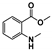
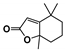
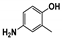
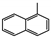
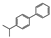
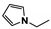
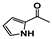
The main volatile compounds and their relative contents also differed in samples with different degrees of aging (Figure 3). The compounds with the highest contents in HAS were phenyl acetate (15.28%), methyl salicylate (6.88%), linalool (6.85%), β-ionone (5.27%), and dihydroactinidiolide (4.29%); those in MAS were benzaldehyde (6.81%), dimethoxybenzene (5.04%), β-ionone (4.92%), and linalool (4.24%); those in LAS were β-ionone (13.30%), linalool (8.80%), 3,7-dimethyl-1,5,7-octatriene-3-ol (6.86%), and benzaldehyde (5.04%); and those in CK were 4-amino-o-cresol (12.38%), 3,7-dimethyl-1,5,7-octatriene-3-ol (10.30%), linalool (9.38%), salicylic acid methyl ester (7.51%), and β-ionone (6.37%). In general, high contents of β-ionone and linalool were present in all four samples, with relative contents ranging from 4.92% to 13.30% and from 4.24% to 9.38%, respectively. Thus. these compounds were the key volatile components, so they would probably also provide the characteristics of the An–tea aroma.
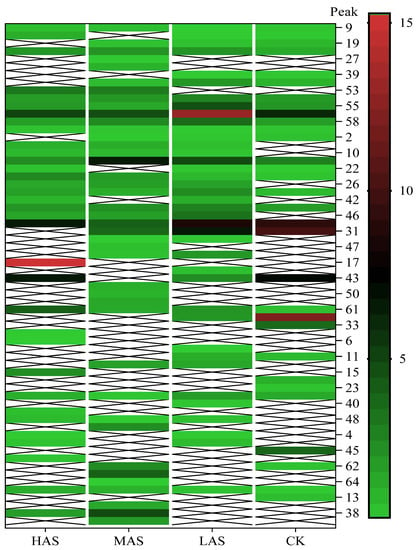
Figure 3.
The heatmap of compounds in four An–tea samples at different aging times.
3.3. Principal Component Analysis of Volatile Substances
Principal component analysis (PCA) is a method used for dimensionality reduction and data simplification [19]. PCA has been widely used in the identification and quality evaluation of volatile compounds of tea [20].
The PCA of the volatile compounds in the four An–tea samples was based on eight categories. If the characteristic value was > 1, three principal components were obtained (Table 3) with contributions of 47.84%, 31.00%, and 21.16%, respectively, giving a total of 100%. According to Table 4, ketones and aldehydes showed a strong positive correlation, while alcohols and esters showed a strong negative correlation with the first principal component. Phenols, hydrocarbons, and esters had a strong negative correlation with the second principal component. Nitrogen-containing compounds had a large negative correlation with the third principal component. Table 5 shows that HAS, MAS, and LAS had the highest scores for the first, second, and third principal components, respectively, with the comprehensive score for the quality of the volatile substances denoted by the F score, which was highest for MAS and followed by LAS, HAS, and CK.

Table 3.
Eigenvalues and contribution rates of first three principal components.

Table 4.
Eigenvectors and loading matrix of first three principal components.

Table 5.
Overall scores of principal components after standardization.
4. Discussion
4.1. Aroma Components of Aged An–tea
More than 700 types of volatile compounds have been related to tea aroma. However, only some of these play key roles in the quality of tea aroma [7]. In this study, a total of 64 volatile compounds were detected in aged An–tea, with only 20 in common to the HAS, MAS, and LAS samples (Table 6)—with ketones and aldehydes being the most common. Owing to the homogeneity of the specific “aging aroma” of An–tea in samples with different degrees of aging, it can be speculated that the 20 common volatile compounds from above form the material basis of its formation, with some of them potentially being essential for the “aging aroma” of An–tea. Of the 20 common compounds, ketones provide fragrant, fruity, and woody aroma notes; aldehydes have fragrant, herbal, and medicinal notes; alcohols have fragrant and fruity notes; hydrocarbons and esters have woody notes; and furans have fruity and fragrant notes, all of which constitute the “background” to the An–tea “aging aroma”.

Table 6.
Aroma characteristics of the 20 volatile compounds found in all 4 samples of An–tea.
Of the common compounds, the relative contents of linalool, β-ionone, safranal, benzaldehyde, 6-methyl-5-hepten-2-one, and α-ionone were high. The threshold values of linalool [21], β-ionone [21], benzaldehyde [22], and α-ionone [22] are 0.6, 0.2, 0.35, and 0.4 g/L, respectively, while that of 6-methyl-5-hepten-2-one [23] is 50 g/L. The threshold value of safranal has not yet been determined. From these results, linalool, β-ionone, benzaldehyde, and α-ionone were considered the key compounds providing the aroma quality of aged An–tea. One study has used GC/MS to determine the volatile substances in three types of low-grade aged An–tea in Chizhou City [3]. According to this study, linolenic acid (10.15%), dibutyl phthalate (10.12%), styrene (9.43%), and linalool (9.33%) produced the characteristic aroma substances of An–tea, while β-ionone might also be related to the characteristics of aged tea. Therefore, it can be suggested that linalool and β-ionone are two key compounds in the “aging aroma” of An–tea.
As well as the endogenous compounds produced by various biochemical reactions in tea, the leaves of the checker-patterned Indocalamus and bamboo baskets used for packaging An–tea also produce volatile compounds. These exogenous volatile compounds could also be absorbed by An–tea during the aging process, thus changing the composition of the aroma and types of flavor of An–tea, a topic that is also worthy of research attention.
4.2. Medicinal Analysis of Volatile Compounds in An–tea
An–tea not only produces an excellent tasting beverage, but also has high medicinal value. In Guangdong and Southeast Asia, An–tea is known as “holy tea”, somewhat reflecting its medicinal value. Linalool has been found to have analgesic, anti-anxiety, sedative, hypnotic, anti-inflammatory, antitumor, antibacterial, and other pharmacological activities among common volatile substances [24]. β-Ionone also has a wide range of biological activities, especially some anticancer cell activity. In vitro experiments have shown that α-ionone can inhibit the proliferation of breast cancer cells, colorectal adenocarcinoma cells, and human colorectal fibroblasts [25].
Furthermore, many medicinal compounds were present as volatile substances in An–tea of different aging times. For example, phenyl acetate in HAS is an important pharmaceutical intermediate that can be converted to diphenyl carbonate by dimethyl carbonate to treat acute and chronic jaundice, hepatitis, cholecystitis, and other diseases [26]. Geraniol can inhibit cell oxidative stress and reduce vascular endothelial inflammation, resulting in anti-atherosclerosis effects [27]. However, owing to the low extraction rate and the high cost of tea, chemical synthesis remains the main method for obtaining volatile pharmaceutical compounds. Increasing the extraction rate of volatile compounds from An–tea through technological improvement would help to develop and utilize the medicinal value of An–tea.
5. Conclusions
Of the An–tea samples, HAS, the most aged, contained the smallest number of volatile components, while MAS contained the greatest number and LAS contained an intermediate number. The number of volatile substances in the aged samples increased to different degrees with aging time. The volatile compounds in LAS were similar to those in CK but this similarity decreased with increasing aging time. The number of common substances shared by MAS and HAS with CK decreased, while the numbers shared by the aged samples were highly similar. Furthermore, the relative contents of ketones, aldehydes, and alcohols in the three aged samples were generally higher than those of esters, hydrocarbons, nitrogenous compounds, and other compounds, except for esters in HAS and other compounds in MAS. The contents of β-ionone and linalool were high in all four types of An–tea samples, with their relative contents varying from 4.92% to 13.30% and from 4.24% to 9.38%, respectively. These two compounds were considered the key volatile compounds in An–tea and are probably responsible for the characteristic aroma of An–tea. Finally, the comprehensive score for the quality of the volatile substances was highest for MAS and followed by the HAS, LAS, and CK tea samples.
Author Contributions
Conceptualization, T.Y. and Z.G.; methodology, T.Y.; software, S.W.; validation, S.W. and X.S.; formal analysis, D.Z.; investigation, Z.G.; writing—original draft preparation, S.W.; writing—review and editing, T.Y.; project administration, Z.G.; funding acquisition, Z.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Major Projects of Natural Science in Colleges and Universities of Anhui Province (grant number KJ2020ZD60), the Open Research Project of Anhui Simulation Design and Modern Manufacture Engineering Technology Research Center(Huangshan University, grant number SGCZXZD1902) and the Innovation Center of Characteristic Biological Resources Development and Great Health Technology (Huangshan University, grant number kypt202101).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shen, S.; Huang, J.; Li, T.; Wei, Y.; Xu, S.; Wang, Y.; Ning, J. Untargeted and targeted metabolomics reveals potential marker compounds of an tea during storage. LWT 2022, 154, 112791. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y. Studies on the quality and chemical constituents of An–tea. China Tea Process. 2009, 29–31. (In Chinese) [Google Scholar] [CrossRef]
- Han, A.; Li, L.; Xu, X. Analysis of quality and chemical components of An–tea. J. Tea Bus. 2016, 38, 79–83. (In Chinese) [Google Scholar] [CrossRef]
- She, X.S.; Gan, Z.T.; Li, K.; Yao, T.; Wang, S.Q.; Zhang, B. In Determination and difference analysis of aroma compounds in An–Tea with different aging time. In Proceedings of the 5th International Conference on Advances in Energy, Environment and Chemical Engineering (AEECE), Shanghai, China, 16–18 August 2019. [Google Scholar]
- Gan, Z.; Wang, S.; Zhang, P.; Yao, T. The content and spatial distribution of soil heavy metals in the typical tea gardens of An–tea production in southern Anhui Province. J. Shaanxi Norm. Univ. (Nat. Sci. Ed.) 2018, 46, 112–119. (In Chinese) [Google Scholar] [CrossRef]
- State Administration for Market Regulation of China. Announcement of the General Administration of Quality Supervision, Inspection and Quarantine on Approving the Protection of Geographical Indication Products for Leijiadian Thin-Skinned Walnuts and Other Products (No. 190 of 2013). 2013. Available online: https://m.cqn.com.cn/zj/content/2014-01/21/content_2109718.htm (accessed on 19 January 2022). (In Chinese)
- Wang, M.Q.; Zhu, Y.; Zhang, Y.; Jiang, S.; Zhi, L.; Peng, L.H. A Review of Recent Research on Key Aroma Compounds in Tea. Food Sci. 2019, 40, 341–349. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Z.; Zhao, C.; Kong, H.; Lu, X.; Xu, G. A comparative study of volatile components in green, oolong and black teas by using comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry and multivariate data analysis. J. Chromatogr. A 2013, 1313, 245–252. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Wang, C.; Li, C.W.; Liu, S.H.; Zhang, C.X.; Li, L.W.; Jiang, D.H. Characterization of Aroma-Active Compounds of Pu-erh Tea by Headspace Solid-Phase Microextraction (HS-SPME) and Simultaneous Distillation-Extraction (SDE) Coupled with GC-Olfactometry and GC-MS. Food Anal. Methods 2016, 9, 1188–1198. [Google Scholar] [CrossRef]
- Xu, S.; Zeng, X.; Wu, H.; Shen, S.; Yang, X.; Deng, W.-W.; Ning, J. Characterizing volatile metabolites in raw Pu’er tea stored in wet-hot or dry-cold environments by performing metabolomic analysis and using the molecular sensory science approach. Food Chem. 2021, 350, 129186. [Google Scholar] [CrossRef]
- Ou, Q.H.; Li, J.M.; Yang, X.E.; Yang, W.Y.; Liu, G.; Shi, Y.M. Identification of Pu’er raw tea with different storage years by infrared spectroscopy. J. Food Process. Preserv. 2021, 45, 16103. [Google Scholar] [CrossRef]
- Tian, X.; Wang, J.; Deng, Y.; Luo, L.; Liang, Z. Characteristic aroma components analysis of raw Pu’er tea at different storage time. Food Ferment. Ind. 2016, 42, 194–202. (In Chinese) [Google Scholar] [CrossRef]
- Wei, L.; Su, M.; Chen, S.; Wu, Y. Research on Quality Changes of Liubao Tea in Different Storage Time. Southwest China J. Agric. Sci. 2015, 28, 376–380. (In Chinese) [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Liu, Z.; Mao, Q. Analysis of aroma components in green brick tea. Food Sci. Tech. 2015, 40, 68–72. (In Chinese) [Google Scholar] [CrossRef]
- Lv, S.D.; Wu, Y.S.; Li, C.W.; Xu, Y.Q.; Liu, L.; Meng, Q.X. Comparative Analysis of Pu-erh and Fuzhuan Teas by Fully Automatic Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry and Chemometric Methods. J. Agric. Food Chem. 2014, 62, 1810–1818. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.P.; Zhong, Q.S.; Lin, Z.; Wang, L.; Tan, J.F.; Guo, L. Aroma characterisation of Pu-erh tea using headspace-solid phase microextraction combined with GC/MS and GC-olfactometry. Food Chem. 2012, 130, 1074–1081. [Google Scholar] [CrossRef]
- Lin, J.; Dai, Y.; Guo, Y.N.; Xu, H.R.; Wang, X.C. Volatile profile analysis and quality prediction of Longjing tea (Camellia sinensis) by HS-SPME/GC-MS. J. Zhejiang Univ. Sci. B 2012, 13, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.D.; Meng, Q.X.; Xu, Y.Q.; Liu, S.H. Recent progress in aroma analysis methods and aroma active compounds in Pu-erh tea. Food Sci. 2014, 34, 292–298. (In Chinese) [Google Scholar] [CrossRef]
- Guo, Q.; Wu, W.; Massart, D.L.; Boucon, C.; de Jong, S. Feature selection in principal component analysis of analytical data. Chemom. Intell. Lab. Syst. 2002, 61, 123–132. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Liu, T.; Dong, M.; Yu, Z. Modeling for Quality Evaluation of Dongting Biluochun Tea Based on Principal Component Analysis. Food Res. Dev. 2018, 39, 15–22. (In Chinese) [Google Scholar] [CrossRef]
- Zheng, P.C.; Liu, P.P.; Gong, Z.M.; Wang, S.P.; Teng, J.; Wang, X.P.; Ye, F. Analysis of Characteristic Aroma Components of Hubei Black Tea. J. Tea Sci. 2017, 37, 465–475. (In Chinese) [Google Scholar] [CrossRef]
- Sun, B.G.; Chen, H.T. The Technology of Food Flavoring, 3rd ed.; Chemical Industry Press: Beijing, China, 2017. [Google Scholar]
- Li, W.N.; Guo, C.F.; Zhang, Y.X.; Wei, J.P.; Li, Y.T. GC-MS Analysis of Aroma Components of Apple Juice Fermented with Lactic Acid Bacteria. Food Sci. 2017, 38, 146–154. (In Chinese) [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Hăncianu, M.; Costache, I.-I.; Miron, A. Linalool: A review on a key odorant molecule with valuable biological properties. Flavour Fragr. J. 2014, 29, 193–219. [Google Scholar] [CrossRef]
- Sun, X.R.; Liu, J.R.; Chen, B.Q. Advances in bioactivity of β-ionone. J. Toxicol. 2008, 22, 477–480. (In Chinese) [Google Scholar] [CrossRef]
- Cao, P.; Yang, J.; Yang, X.G.; Yao, J.; Wang, Y.; Wang, G.Y. Organotitanium Compound Catalysts for Transesterification of Dimethyl Carbonate and Phenyl Acetate to Diphenyl Carbonate. Chin. J. Catal. 2009, 30, 65–68. [Google Scholar] [CrossRef]
- Sun, L.H.; Sun, L.M. The research progress of geraniol. Northwest Pharm. J. 2009, 24, 428–430. (In Chinese) [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).