Rapid Quantitative Determination of Multiple Pesticide Residues in Mango Fruits by Surface-Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
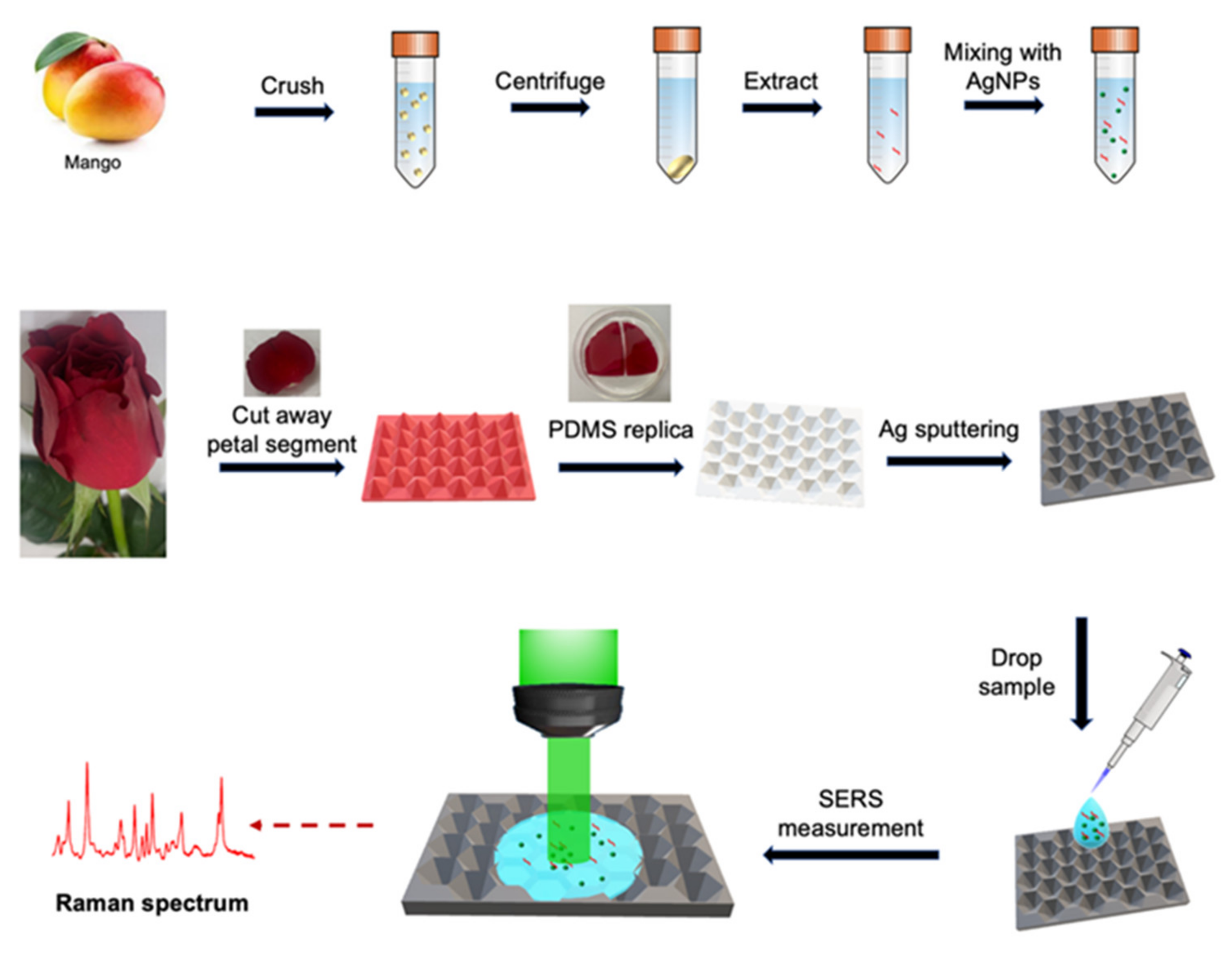
2.1. Material
2.2. Synthesis of Silver Nanoparticles
2.3. Replication of Rose Petals by PDMS Elastomer
2.4. Screening of SERS Substrate by Crystal Violet
2.5. Preparation of Standard Pesticide Samples
2.6. Preparation of Mango Samples with Tested Pesticides
2.7. Data Analysis
3. Results
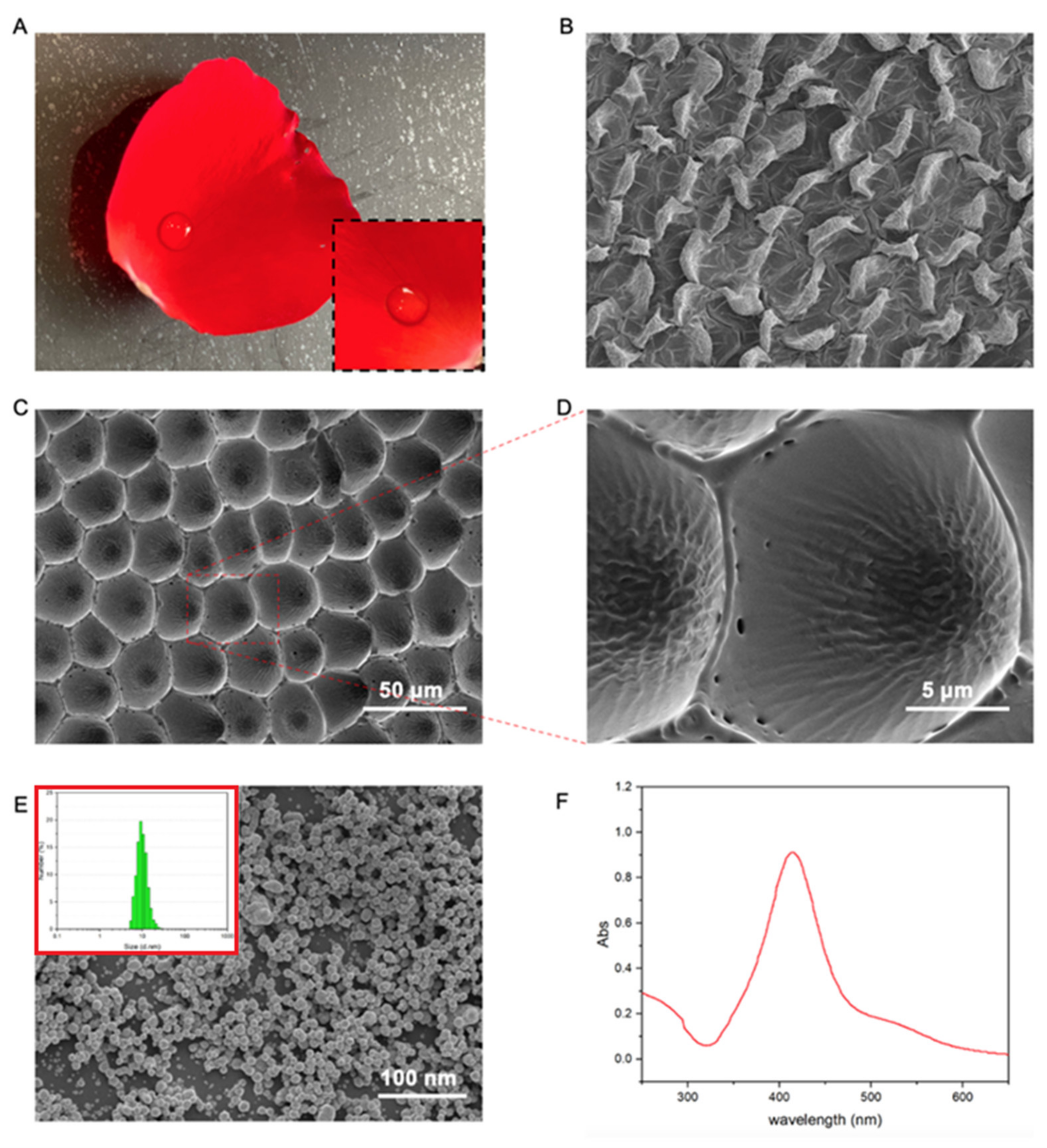
3.1. Rose Petal Simulated PDMS/Ag Microstructure
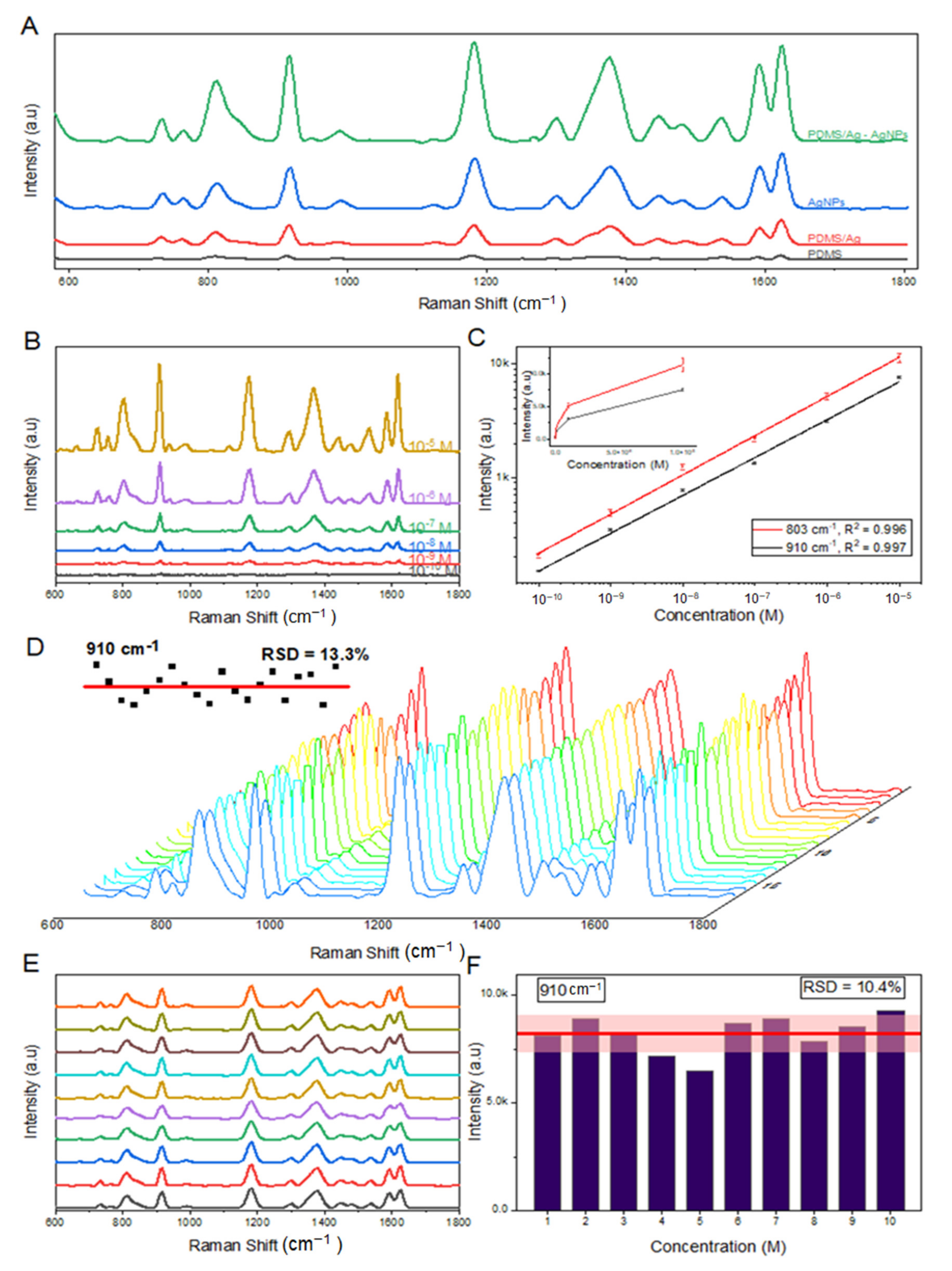
3.2. Efficiency of SERS Substrates on the SERS Measurement of Crystal Violet
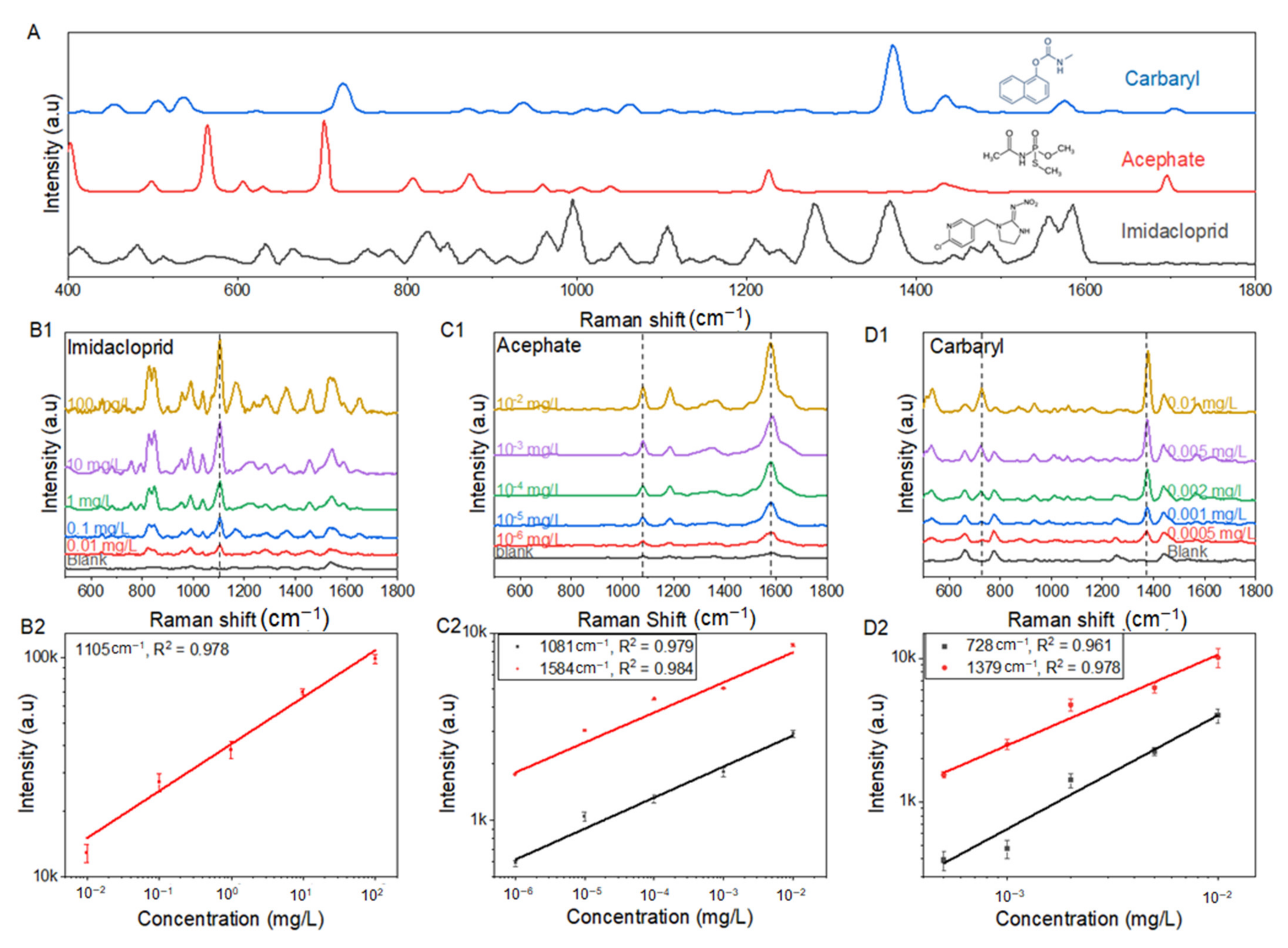
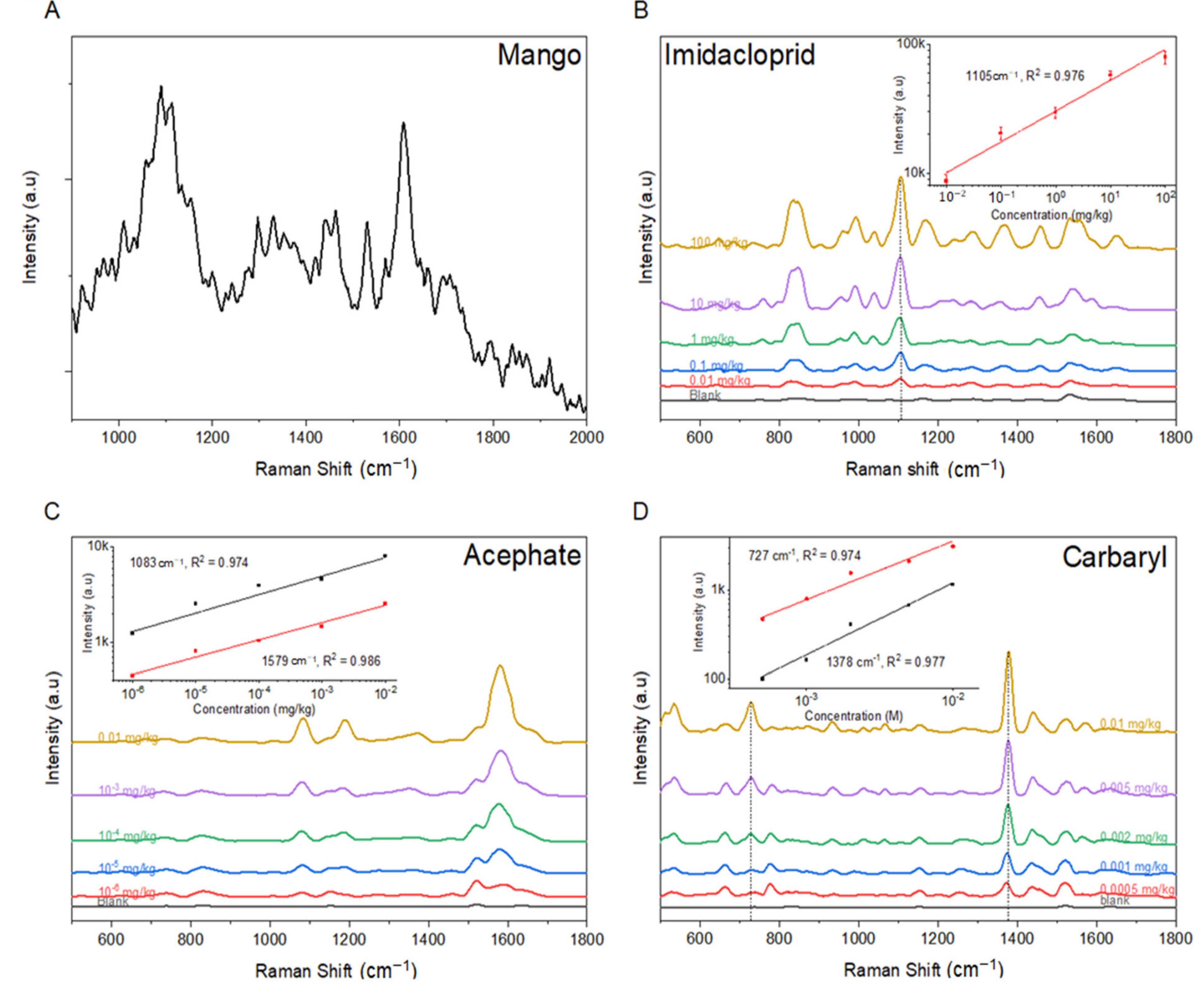
3.3. Raman Spectrum and Quantitative Analysis of Imidacloprid, Acephate, and Carbaryl
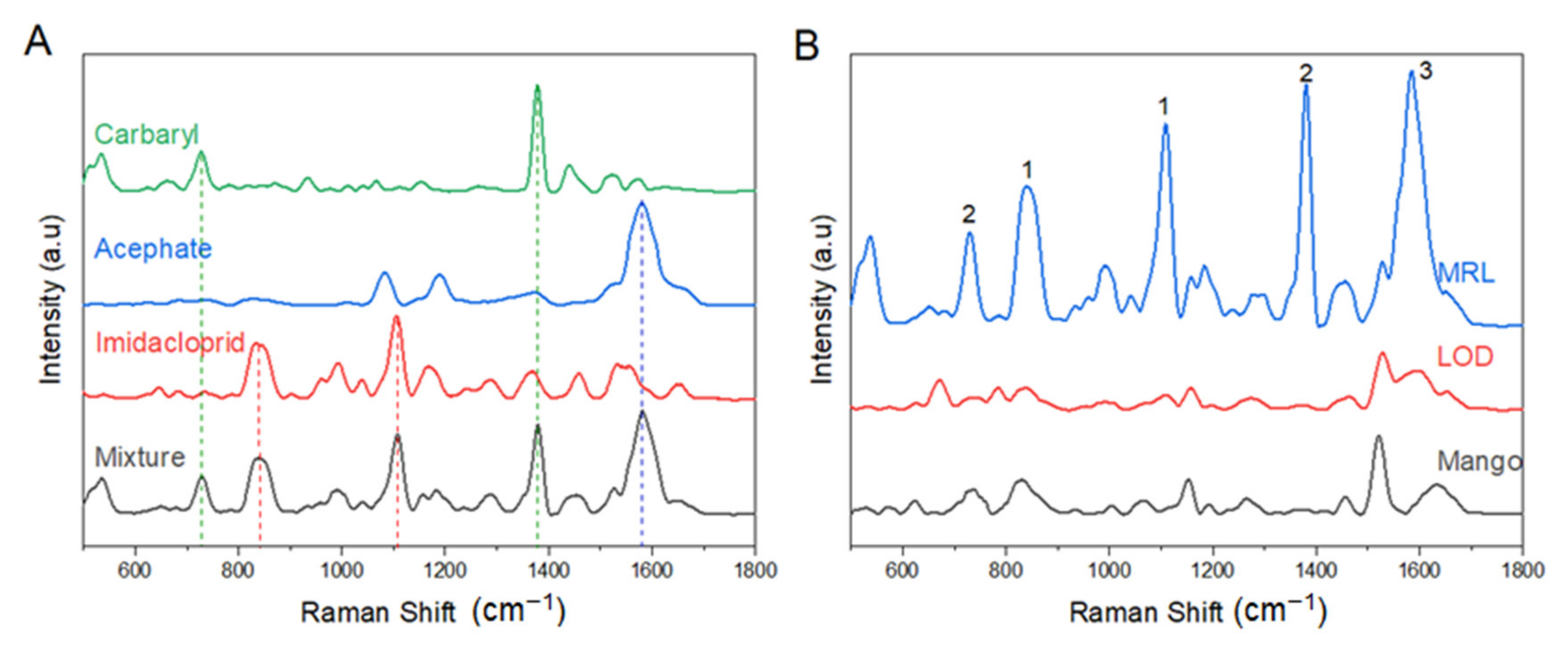
3.4. SERS Analysis of Pesticides in Mango Fruit
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sivakumar, D.; Jiang, Y.; Yahia, E.M. Maintaining mango (Mangifera indica L.) fruit quality during the export chain. Food Res. Int. 2011, 44, 1254–1263. [Google Scholar] [CrossRef]
- Ntsoane, M.L.; Zude-Sasse, M.; Mahajan, P.; Sivakumar, D. Quality assesment and postharvest technology of mango: A review of its current status and future perspectives. Sci. Hortic. 2019, 249, 77–85. [Google Scholar] [CrossRef]
- Godase, S.K.; Bhole, S.R.; Shivpuje, P.R.; Patil, B.P. Assessment of yield loss in mango (Mangifera indica) due to mango hopper (Idioscopus niveosparsus) (Homoptera: Cicadelidae). Indian J. Agric. Sci. 2004, 74, 370–372. [Google Scholar]
- Kapoor, U.; Srivastava, M.; Srivastava, L. Toxicological impact of technical imidacloprid on ovarian morphology, hormones and antioxidant enzymes in female rats. Food Chem. Toxicol. 2011, 49, 3086–3089. [Google Scholar] [CrossRef]
- Feng, S.; Kong, Z.; Wang, X.; Peng, P.; Zeng, E.Y. Assessing the genotoxicity of imidacloprid and RH-5849 in human peripheral blood lymphocytes in vitro with comet assay and cytogenetic tests. Ecotoxicol. Environ. Saf. 2005, 61, 239–246. [Google Scholar] [CrossRef]
- Brutti, M.; Blasco, C.; Picó, Y. Determination of benzoylurea insecticides in food by pressurized liquid extraction and LC-MS. J. Sep. Sci. 2010, 33, 1–10. [Google Scholar] [CrossRef]
- Banerjee, K.; Oulkar, D.P.; Dasgupta, S.; Patil, S.B.; Patil, S.H.; Savant, R.; Adsule, P.G. Validation and uncertainty analysis of a multi-residue method for pesticides in grapes using ethyl acetate extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2007, 1173, 98–109. [Google Scholar] [CrossRef]
- Stachniuk, A.; Fornal, E. Liquid Chromatography-Mass Spectrometry in the Analysis of Pesticide Residues in Food. Food Anal. Methods 2016, 9, 1654–1665. [Google Scholar] [CrossRef] [Green Version]
- Filho, A.M.; dos Santos, F.N.; de Paula Pereira, P.A. Multi-residue analysis of pesticide residues in mangoes using solid-phase microextraction coupled to liquid chromatography and UV–Vis detection. J. Sep. Sci. 2011, 34, 2960–2966. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Chae, S.S.; Jang, S.; Choi, W.J.; Chang, H.; Lee, J.-O.; Lee, T.I. “Atomic Force Masking” Induced Formation of Effective Hot Spots along Grain Boundaries of Metal Thin Films. ACS Appl. Mater. Interfaces 2016, 8, 32094–32101. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, T.; Sun, D.-W.; Cheng, J.-H. Raman imaging for food quality and safety evaluation: Fundamentals and applications. Trends Food Sci. Technol. 2017, 62, 177–189. [Google Scholar] [CrossRef]
- Morey, R.; Ermolenkov, A.; Payne, W.Z.; Scheuring, D.C.; Koym, J.W.; Vales, M.I.; Kurouski, D. Non-invasive identification of potato varieties and prediction of the origin of tuber cultivation using spatially offset Raman spectroscopy. Anal. Bioanal. Chem. 2020, 412, 4585–4594. [Google Scholar] [CrossRef]
- Zhang, D.; Pu, H.; Huang, L.; Sun, D.-W. Advances in flexible surface-enhanced Raman scattering (SERS) substrates for nondestructive food detection: Fundamentals and recent applications. Trends Food Sci. Technol. 2021, 109, 690–701. [Google Scholar] [CrossRef]
- Zhai, C.; Peng, Y.; Li, Y.; Chao, K. Extraction and identification of mixed pesticides’ Raman signal and establishment of their prediction models. J. Raman Spectrosc. 2017, 48, 494–500. [Google Scholar] [CrossRef]
- Wang, K.; Sun, D.-W.; Pu, H.; Wei, Q. Surface-enhanced Raman scattering of core-shell Au@Ag nanoparticles aggregates for rapid detection of difenoconazole in grapes. Talanta 2019, 191, 449–456. [Google Scholar] [CrossRef]
- Shi, G.; Wang, M.; Zhu, Y.; Wang, Y.; Xu, H. A novel natural SERS system for crystal violet detection based on graphene oxide wrapped Ag micro-islands substrate fabricated from Lotus leaf as a template. Appl. Surf. Sci. 2018, 459, 802–811. [Google Scholar] [CrossRef]
- Xi, J.; Jiang, L. Biomimic Superhydrophobic Surface with High Adhesive Forces. Ind. Eng. Chem. Res. 2008, 47, 6354–6357. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Cheng, S.; Li, B.; Li, A.; Du, G.; Hu, W. Bio-inspired nanostructures for enhanced light management. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2017, 35, 06GJ02. [Google Scholar] [CrossRef]
- Kumar, P.; Khosla, R.; Soni, M.; Deva, D.; Sharma, S.K. A highly sensitive, flexible SERS sensor for malachite green detection based on Ag decorated microstructured PDMS substrate fabricated from Taro leaf as template. Sens. Actuators B Chem. 2017, 246, 477–486. [Google Scholar] [CrossRef]
- Wang, M.; Shi, G.; Zhu, J.; Zhu, Y.; Sun, X.; Wang, P.; Jiao, T.; Li, R. Preparation of a Novel SERS Platform Based on Mantis Wing with High-Density and Multi-Level “Hot Spots”. Nanomaterials 2019, 9, 672. [Google Scholar] [CrossRef] [Green Version]
- Mu, Z.; Zhao, X.; Xie, Z.; Zhao, Y.; Zhong, Q.; Bo, L.; Gu, Z. In situ synthesis of gold nanoparticles (AuNPs) in butterfly wings for surface enhanced Raman spectroscopy (SERS). J. Mater. Chem. B 2013, 1, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Ting, Y.-S.; Chen, B.-Y.; Cheng, Y.-W.; Liu, T.-Y. Bionic shark skin replica and zwitterionic polymer brushes functionalized PDMS membrane for anti-fouling and wound dressing applications. Surf. Coat. Technol. 2020, 391, 125663. [Google Scholar] [CrossRef]
- Pencheva, D.; Bryaskova, R.; Kantardjiev, T. Polyvinyl alcohol/silver nanoparticles (PVA/AgNps) as a model for testing the biological activity of hybrid materials with included silver nanoparticles. Mater. Sci. Eng. C 2012, 32, 2048–2051. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, A.F.; Jiang, L. Petal Effect: A Superhydrophobic State with High Adhesive Force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Z.; Liu, W. Adhesion behaviors on four special wettable surfaces: Natural sources, mechanisms, fabrications and applications. Soft Matter 2021, 17, 4895–4928. [Google Scholar] [CrossRef]
- Xiao, G.; He, Y.; Liu, S.; Yi, H.; Du, L. Laser processing of micro/nano biomimetic structures. Micro Nano Lett. 2021, 16, 327–335. [Google Scholar] [CrossRef]
- Lee, C.; Robertson, C.S.; Nguyen, A.H.; Kahraman, M.; Wachsmann-Hogiu, S. Thickness of a metallic film, in addition to its roughness, plays a significant role in SERS activity. Sci. Rep. 2015, 5, 11644. [Google Scholar] [CrossRef] [Green Version]
- Arifin, E.; Lee, J.-K. The Distance-Dependent Fluorescence Enhancement Phenomena in Uniform Size Ag@SiO2@SiO2(dye) Nanocomposites. Bull. Korean Chem. Soc. 2013, 34, 539–544. [Google Scholar] [CrossRef] [Green Version]
- Mao, A.; Jin, X.; Gu, X.; Wei, X.; Yang, G. Rapid, green synthesis and surface-enhanced Raman scattering effect of single-crystal silver nanocubes. J. Mol. Struct. 2012, 1021, 158–161. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef] [Green Version]
- Herrera, G.M.; Padilla, A.C.; Hernandez-Rivera, S.P. Surface enhanced Raman scattering (SERS) studies of gold and silver nanoparticles prepared by laser ablation. Nanomaterials 2013, 3, 158–172. [Google Scholar] [CrossRef]
- Wei, W.; Huang, Q. Rapid fabrication of silver nanoparticle-coated filter paper as SERS substrate for low-abundance molecules detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 179, 211–215. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, X.; Cui, Q.; Yang, Y.; Li, L. Synthesis and application of bifunctional gold/gelatin nanocomposites with enhanced fluorescence and Raman scattering. Colloids Surf. A Physicochem. Eng. Asp. 2017, 514, 117–125. [Google Scholar] [CrossRef]
- Cyrankiewicz, M.; Wybranowski, T.; Kruszewski, S. Silver Nanoparticles as Enhancing Substrates for Raman and Fluorescence Spectroscopy. Acta Phys. Pol. A 2014, 125, A-11–A-15. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface enhanced Raman scattering enhancement factors: A comprehensive study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Meng, W.; Hu, F.; Jiang, X.; Lu, L. Preparation of silver colloids with improveduniformity and stable surface-enhanced Ramanscattering. Nanoscale Res. Lett. 2015, 10, 34. [Google Scholar]
- Harraz, F.A.; Ismail, A.A.; Bouzid, H.; Al-Sayari, S.A.; Al-Hajry, A.; Al-Assiri, M.S. Surface-enhanced Raman scattering (SERS)-active substrates from silver plated-porous silicon for detection of crystal violet. Appl. Surf. Sci. 2015, 331, 241–247. [Google Scholar] [CrossRef]
- Zhao, X.; Li, C.; Li, Z.; Yu, J.; Pan, J.; Si, H.; Yang, C.; Jiang, S.; Zhang, C.; Man, B. In-situ electrospun aligned and maize-like AgNPs/PVA@ Ag nanofibers for surface-enhanced Raman scattering on arbitrary surface. Nanophotonics 2019, 8, 1719–1729. [Google Scholar] [CrossRef]
- European Food Safety Authority. The 2014 European Union report on pesticide residues in food. EFSA J. 2016, 14, e04611. [Google Scholar] [CrossRef]
- Dries, M.A. Vietnam MOH Revises MRLs for Pesticides in Foods; USDA Foreign Agricultural Service: Washington, DC, USA, 2017; pp. 1–4.
| SERS Substrate | Enhancement Factor (EF) | References |
|---|---|---|
| Rose petal replica from PDMS/Ag-AgNPs substrate | 4.7 × 107 | This work |
| Mixing with silver colloid | 9.5 × 103 | [36] |
| AgNPs-meso-PSi hybrid structures | 1.3 × 106 | [37] |
| Graphene oxide (GO) on the Ag micro-islands substrate using micro/nanostructured Lotus leaf (L.l.) as a template | 1.52 × 106 | [16] |
| Chemical Compounds | NR Raman Bands (cm−1) | SERS Bands (cm−1) | Band Assignment |
|---|---|---|---|
| Imidacloprid | 660 | 661 | C-Cl stretching |
| 830 | 829 | C-C-C symmetric stretching | |
| 989 | 993 | C-N stretching | |
| 1110 | 1105 | C-C-C bending | |
| 1351 | 1374 | C-N stretching | |
| 1580 | 1564 | C-N asym | |
| Acephate | 400 | 404 | P pyramidalization mode |
| 565 | 544 | P-S-C stretching | |
| 710 | 678 | P-O-C stretching | |
| 871 | 883 | P-O-C stretching | |
| 1081 | |||
| 1223 | 1227 | P=O stretching | |
| 1692 | 1584 | ketone | |
| Carbaryl | 453 | 453 | C-C bending |
| 534 | - | C-C bending | |
| 723 | 728 | N-C-O-C bending | |
| 1374 | 1379 | symmetric ring vibration | |
| 1432 | 1435 | C-H wagging | |
| 1576 | 1575 | (C=C) phenyl stretch |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, U.T.; Phan, Q.H.T.; Nguyen, L.P.; Luu, P.D.; Doan, T.D.; Trinh, H.T.; Dinh, C.T.; Nguyen, T.V.; Tran, T.Q.; Le, D.X.; et al. Rapid Quantitative Determination of Multiple Pesticide Residues in Mango Fruits by Surface-Enhanced Raman Spectroscopy. Processes 2022, 10, 442. https://doi.org/10.3390/pr10030442
Pham UT, Phan QHT, Nguyen LP, Luu PD, Doan TD, Trinh HT, Dinh CT, Nguyen TV, Tran TQ, Le DX, et al. Rapid Quantitative Determination of Multiple Pesticide Residues in Mango Fruits by Surface-Enhanced Raman Spectroscopy. Processes. 2022; 10(3):442. https://doi.org/10.3390/pr10030442
Chicago/Turabian StylePham, Uyen Thu, Quynh Huong Thi Phan, Linh Phuong Nguyen, Phuong Duc Luu, Tien Duy Doan, Ha Thu Trinh, Cuc Thi Dinh, Tai Van Nguyen, Toan Quoc Tran, Duy Xuan Le, and et al. 2022. "Rapid Quantitative Determination of Multiple Pesticide Residues in Mango Fruits by Surface-Enhanced Raman Spectroscopy" Processes 10, no. 3: 442. https://doi.org/10.3390/pr10030442