Investigating the Trade-Off between Design and Operational Flexibility in Continuous Manufacturing of Pharmaceutical Tablets: A Case Study of the Fluid Bed Dryer
Abstract
:1. Introduction
- Factory and enterprise integration and plant-wide optimisation,
- Exploiting manufacturing intelligence,
- Creating disruptive business models.
2. Methodology
2.1. Description of the Mathematical Model
2.2. Deriving the Operational Envelopes
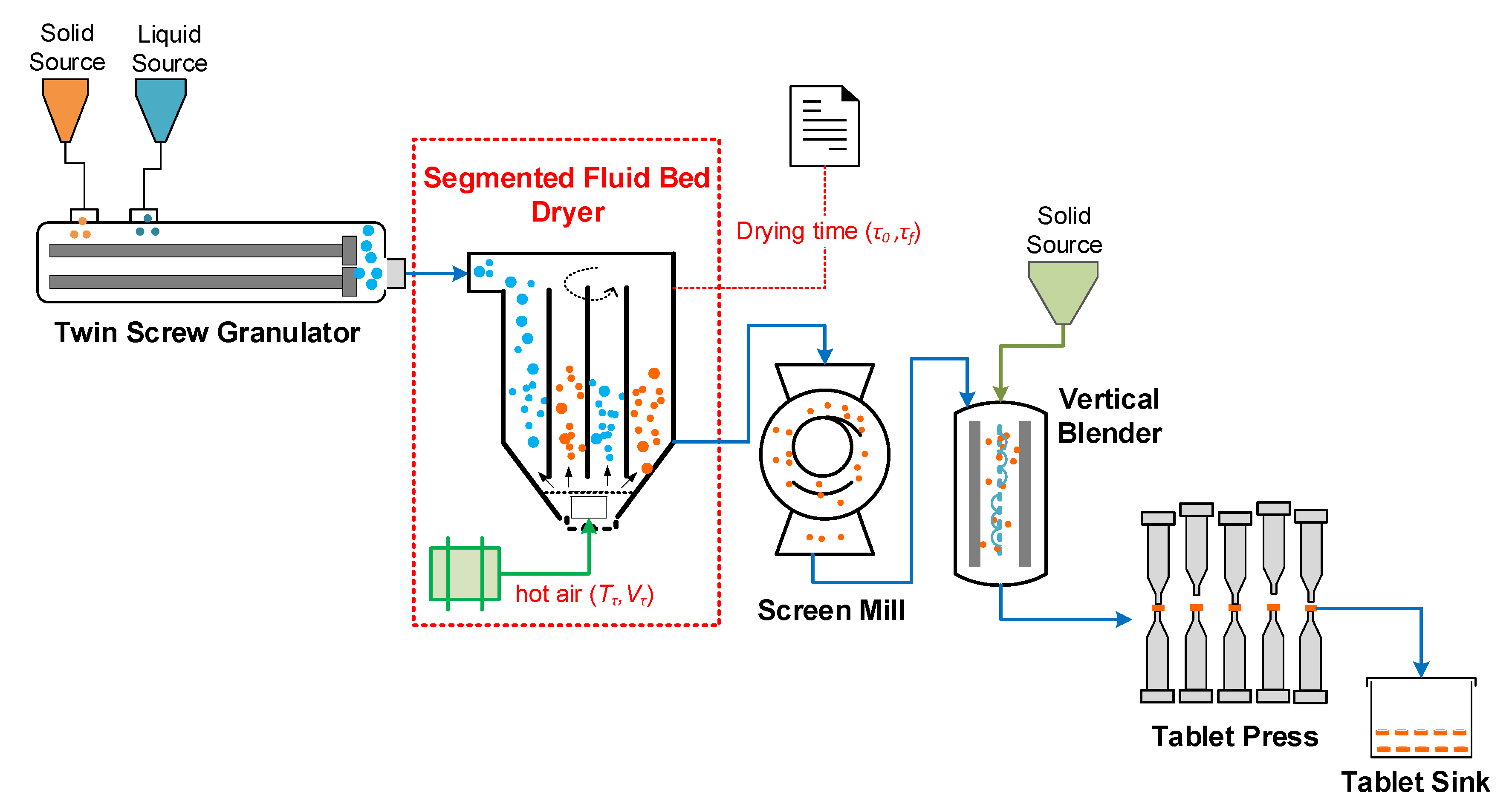
3. Case Study: Segmented Fluidised Bed Dryer
- (a)
- When and are allowed to vary freely we obtain the optimal operational envelope as shown in Figure 3 which maximises the area of the rectangle within the feasible boundary.
- (b)
- When we constrain the variation that T and V can have to the following range and , solving (M3) gives the optimal operational envelope as shown in Figure 4. This maximises the envelope size while also maintaining the maximal distance to the feasible boundary using model (M3).
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbols | Definition |
| time variables. | |
| differential state variables. | |
| derivatives of with respect to time . | |
| algebraic state variables. | |
| time-varying control and not bounded variables, which present the design decision variable in process. | |
| time-varying control and bounded variables, which present the operational variable in processes. | |
| sizes of the bound variables | |
| number of bounded variables | |
| processing time. |
References
- Davis, J.; Edgar, T.; Porter, J.; Bernaden, J.; Sarli, M. Smart manufacturing, manufacturing intelligence and demand-dynamic performance. Comput. Chem. Eng. 2012, 47, 145–156. [Google Scholar] [CrossRef]
- Bogle, I.D.L. A Perspective on Smart Process Manufacturing Research. Challenges for Process Systems Engineers. Engineering 2017, 3, 161–165. [Google Scholar] [CrossRef]
- Li, D. Perspective for smart factory in petrochemical industry. Comput. Chem. Eng. 2016, 91, 136–148. [Google Scholar] [CrossRef]
- Gamer, T.; Hoernicke, M.; Kloepper, B.; Bauer, R.; Isaksson, A.J. The autonomous industrial plant—Future of process engineering, operations and maintenance. J. Process Control 2020, 88, 101–110. [Google Scholar] [CrossRef]
- Kamble, S.S.; Gunasekaran, A.; Gawankar, S.A. Sustainable Industry 4.0 framework: A systematic literature review identifying the current trends and future perspectives. Process Saf. Environ. Prot. 2018, 117, 408–425. [Google Scholar] [CrossRef]
- Rafiei, M.; Ricardez-Sandoval, L.A. New frontiers, challenges, and opportunities in integration of design and control for enterprise-wide sustainability. Comput. Chem. Eng. 2020, 132, 106610. [Google Scholar] [CrossRef]
- Wang, Z.; Escotet-Espinoza, M.S.; Ierapetritou, M. Process analysis and optimization of continuous pharmaceutical manufacturing using flowsheet models. Comput. Chem. Eng. 2017, 107, 77–91. [Google Scholar] [CrossRef]
- Boukouvala, F.; Muzzio, F.J.; Ierapetritou, M.G. Design space of pharmaceutical processes using data-driven-based methods. J. Pharm. Innov. 2010, 5, 119–137. [Google Scholar] [CrossRef]
- Boukouvala, F.; Muzzio, F.J.; Ierapetritou, M.G. Dynamic data-driven modeling of pharmaceutical processes. Ind. Eng. Chem. Res. 2011, 50, 6743–6754. [Google Scholar] [CrossRef]
- Litster, J.; Bogle, I.D.L. Smart Process Manufacturing for Formulated Products. Engineering 2019, 5, 1003–1009. [Google Scholar] [CrossRef]
- Lakio, S.; Ervasti, T.; Tajarobi, P.; Wikström, H.; Fransson, M.; Karttunen, A.; Ketolainen, J.; Folestad, S.; Abrahmsén-Alami, S.; Korhonen, O. Provoking an end-to-end continuous direct compression line with raw materials prone to segregation. Eur. J. Pharm. Sci. 2017, 109, 514–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Muzzio, F.J.; Ierapetritou, M.; Ramachandran, R. A Combined feed-forward/feed-back control system for a QbD-based continuous tablet manufacturing process. Processes 2015, 3, 339–356. [Google Scholar] [CrossRef] [Green Version]
- Oka, S.; Sahay, A.; Meng, W.; Muzzio, F. Diminished segregation in continuous powder mixing. Powder Technol. 2017, 309, 79–88. [Google Scholar] [CrossRef]
- Diab, S.; Gerogiorgis, D.I. Process modelling, simulation and technoeconomic evaluation of crystallisation antisolvents for the continuous pharmaceutical manufacturing of rufinamide. Comput. Chem. Eng. 2018, 111, 102–114. [Google Scholar] [CrossRef] [Green Version]
- Diab, S.; McQuade, D.T.; Gupton, B.F.; Gerogiorgis, D.I. Process design and optimization for the continuous manufacturing of nevirapine, an active pharmaceutical ingredient for HIV treatment. Org. Process. Res. Dev. 2019, 23, 320–333. [Google Scholar] [CrossRef]
- Nagy, B.; Szilágyi, B.; Domokos, A.; Vészi, B.; Tacsi, K.; Rapi, Z.; Pataki, H.; Marosi, G.; Nagy, Z.K.; Nagy, Z.K. Dynamic flowsheet model development and digital design of continuous pharmaceutical manufacturing with dissolution modeling of the final product. Chem. Eng. J. 2021, 419, 129947. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, O.; Sampat, C.; Bhalode, P.; Ramachandran, R.; Ierapetritou, M. Digital twins in pharmaceutical and biopharmaceutical manufacturing: A literature review. Processes 2020, 8, 1088. [Google Scholar] [CrossRef]
- Food and Drug Administration. Pharmaceutical cGMPs for the 21st Century—A Risk-Based Approach; Technical Report; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER): Rockville, MD, USA, 2004.
- Food and Drug Administration. Guidance for Industry Q8 Pharmaceutical Development; Technical Report August; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER): Rockville, MD, USA, 2009.
- Huang, J.; Kaul, G.; Cai, C.; Chatlapalli, R.; Hernandez-Abad, P.; Ghosh, K.; Nagi, A. Quality by design case study: An integrated multivariate approach to drug product and process development. Int. J. Pharmaceut. 2009, 382, 23–32. [Google Scholar] [CrossRef]
- Garcia-Munoz, S.; Luciani, C.V.; Vaidyaraman, S.; Seibert, K.D. Definition of design spaces using mechanistic models and geometric projections of probability maps. Org. Process. Res. Dev. 2015, 19, 1012–1023. [Google Scholar] [CrossRef]
- Peterson, J.J. A Bayesian approach to the ICH Q8 definition of design space. J. Biopharm. Stat. 2008, 18, 959–975. [Google Scholar] [CrossRef]
- Laky, D.; Xu, S.; Rodriguez, J.S.; Vaidyaraman, S.; García Muñoz, S.; Laird, C. An optimization-based framework to define the probabilistic design space of pharmaceutical processes with model uncertainty. Processes 2019, 7, 96. [Google Scholar] [CrossRef] [Green Version]
- Swaney, R.E.; Grossmann, I.E. An index for operational flexibility in chemical process design. Part I: Formulation and theory. AIChE J. 1985, 31, 621–630. [Google Scholar] [CrossRef]
- Kusumo, K.P.; Gomoescu, L.; Paulen, R.; García Muñoz, S.; Pantelides, C.C.; Shah, N.; Chachuat, B. Bayesian approach to probabilistic design space characterization: A nested sampling strategy. Ind. Eng. Chem. Res. 2019, 59, 2396–2408. [Google Scholar] [CrossRef]
- Samsatli, N.J.; Sharif, M.; Shah, N.; Papageorgiou, L.G. Operational envelopes for batch processes. AIChE J. 2001, 47, 2277–2288. [Google Scholar] [CrossRef]
- Sharifian, S.; Sotudeh-Gharebagh, R.; Zarghami, R.; Tanguy, P.; Mostoufi, N. Uncertainty in chemical process systems engineering: A critical review. Rev. Chem. Eng. 2019, 37, 687–714. [Google Scholar] [CrossRef]
- Charitopoulos, V.M.; Aguirre, A.M.; Papageorgiou, L.G.; Dua, V. Uncertainty aware integration of planning, scheduling and multi-parametric control. Comput. Aid. Chem. Eng. 2018, 44, 1171–1176. [Google Scholar]
- Wang, Z.; Ierapetritou, M. Global sensitivity, feasibility, and flexibility analysis of continuous pharmaceutical manufacturing processes. Comput. Aid. Chem. Eng. 2018, 41, 189–213. [Google Scholar]
- von Stosch, M.; Schenkendorf, R.; Geldhof, G.; Varsakelis, C.; Mariti, M.; Dessoy, S.; Vandercammen, A.; Pysik, A.; Sanders, M. Working within the Design Space: Do Our Static Process Characterization Methods Suffice? Pharmaceutics 2020, 12, 562. [Google Scholar] [CrossRef]
- gPROMS; v.7.0.1; PSEnterprise: London, UK, 2021; Available online: https://www.psenterprise.com/products/gproms (accessed on 6 September 2021).
- Djuris, J.; Djuric, Z. Modeling in the quality by design environment: Regulatory requirements and recommendations for design space and control strategy appointment. Int. J. Pharm. 2017, 533, 346–356. [Google Scholar] [CrossRef]
- Su, Q.; Ganesh, S.; Moreno, M.; Bommireddy, Y.; Gonzalez, M.; Reklaitis, G.V.; Nagy, Z.K. A perspective on Quality-by-Control (QbC) in pharmaceutical continuous manufacturing. Comput. Chem. Eng. 2019, 125, 216–231. [Google Scholar] [CrossRef]
- Szilágyi, B.; Borsos, Á.; Pal, K.; Nagy, Z.K. Experimental implementation of a Quality-by-Control (QbC) framework using a mechanistic PBM-based non-linear model predictive control involving chord length distribution measurement for the batch cooling crystallization of l-ascorbic acid. Chem. Eng. Sci. 2019, 195, 335–346. [Google Scholar] [CrossRef] [Green Version]
- Burgschweiger, J.; Groenewold, H.; Hirschmann, C.; Tsotsas, E. From hygroscopic single particle to batch fluidized bed drying kinetics. Can. J. Chem. Eng. 1999, 77, 333–341. [Google Scholar] [CrossRef]
- Burgschweiger, J.; Tsotsas, E. Experimental investigation and modelling of continuous fluidized bed drying under steady-state and dynamic conditions. Chem. Eng. Sci. 2002, 57, 5021–5038. [Google Scholar] [CrossRef]
- Kucherenko, S.; Albrecht, D.; Saltelli, A. Exploring multi-dimensional spaces: A comparison of Latin hypercube and quasi Monte Carlo sampling techniques. arXiv 2015, arXiv:1505.02350. [Google Scholar]
- Saltelli, A.; Annoni, P.; Azzini, I.; Campolongo, F.; Ratto, M.; Tarantola, S. Variance based sensitivity analysis of model output. Design and estimator for the total sensitivity index. Comput. Phys. Com. 2010, 181, 259–270. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.-L.; Papageorgiou, L.G.; Bogle, I.D.L.; Charitopoulos, V.M. Investigating the Trade-Off between Design and Operational Flexibility in Continuous Manufacturing of Pharmaceutical Tablets: A Case Study of the Fluid Bed Dryer. Processes 2022, 10, 454. https://doi.org/10.3390/pr10030454
Jiang S-L, Papageorgiou LG, Bogle IDL, Charitopoulos VM. Investigating the Trade-Off between Design and Operational Flexibility in Continuous Manufacturing of Pharmaceutical Tablets: A Case Study of the Fluid Bed Dryer. Processes. 2022; 10(3):454. https://doi.org/10.3390/pr10030454
Chicago/Turabian StyleJiang, Sheng-Long, Lazaros G. Papageorgiou, Ian David L. Bogle, and Vassilis M. Charitopoulos. 2022. "Investigating the Trade-Off between Design and Operational Flexibility in Continuous Manufacturing of Pharmaceutical Tablets: A Case Study of the Fluid Bed Dryer" Processes 10, no. 3: 454. https://doi.org/10.3390/pr10030454





