1. Introduction
There are multiple advantages and possible applications of food 3D printing. Beyond the possibility of creating complex structures, the technique can also be used to supply personalised nutrition and food formulations for consumers with different preferences and needs [
1]. It can be used to enhance children’s curiosity towards vegetable-based foods, supply more appealing foods for the elderly and people with swallowing difficulties and produce delivery systems for the controllable release of nutrients and medication [
2]. One example of a nutritious and healthy 3D-printed food that has been studied is fibre- and/or protein-rich snacks [
3]. The 3D printing technique could also be used to create novel textures, such as plant-based steaks with textural properties resembling those of meat [
4].
One of the greatest challenges in food 3D printing is finding inks that have good printing precision and shape stability [
5,
6]. Inks used for extrusion-based 3D printing must be able to flow through a nozzle and retain their shape after printing. Rheological characterisation of inks for 3D printing has been used in efforts to predict the printability of a material and its dimensional stability after printing [
3,
7,
8]. Examples of parameters investigated include storage modulus (G′), loss modulus (G″), tan (δ) and yield stress. Storage modulus is a measure of the elasticity of a material and its ability to store deformational energy and can be seen as a measure of the structural strength and mechanical rigidity of a material at rest [
9]. Loss modulus represents the viscous response of the material and is a measure of the energy dissipated as heat during deformation [
9]. Tan(δ), or loss tangent, is the ratio between G″ and G′, i.e., the ratio of energy lost to energy stored during cyclic deformation. Yield stress relates to the force that can be applied before the structure of the material starts to break and flow is initiated [
3].
A correlation between shear modulus and the deformation behaviour of methylcellulose gels has been reported [
10], indicating that this parameter can be used to predict printability. Multiple studies have observed a correlation between storage modulus and/or yield stress of the ink with the degree of deformation after printing [
5,
11,
12]. Furthermore, it has been shown that the loss modulus value can supply additional information important for predicting the dimensional stability of 3D-printed food structures [
8].
One advantage of 3D food printing compared with the use of moulds or forms is the possibility of using different infill patterns for the interior of the printed object in order to alter its textural properties. The effect of the infill pattern of 3D-printed foods such as air-fried potato snacks and chocolate has been investigated in two previous studies, both of which showed that the infill pattern can influence the texture of printed foods [
13,
14].
Few studies have so far investigated the possibility of using plant-based materials for food printing without the inclusion of thickeners such as sodium alginate, xanthan gum or methylcellulose. Chen et al. [
15] found that not including thickeners for samples 3D-printed using soy protein isolates reduced the objects’ shape stability and hardness. Another study showed that 3D printing quality was satisfactory, although the samples tended to swell, when using either oat or faba bean protein concentrates without additional thickeners at solid contents of 45% and 35%, respectively [
5]. In the same study, 3D objects were produced from rye bran inks with a solid content of 30%. The starch present in the plant materials was assumed to act as a natural thickening agent through gelatinisation. Other factors affecting the viscosity and printability of the inks include composition, size and shape of insoluble particles and response to heating and shear between the materials [
5].
In this study, the following two current trends—3D printing and plant-based foods—are combined. The objective was to produce 100% faba bean-based 3D-printed food prototypes. Edible inks composed of different fractions (faba bean fractions rich in protein, starch or fibre) will be compared on rheological properties and printability. The influence of the composition and infill pattern on the texture and microstructure of the 3D-printed samples will also be investigated. This study provides new insight into how the proportion of faba bean fractions influences the properties of 3D-printed foods.
2. Materials and Methods
The protein-, starch- and fibre-rich fractions used in this study were extracted from dehulled and milled faba beans (
Vicia faba var. Gloria) kindly provided by RISE (Research Institutes of Sweden). A complete characterisation of the fractions can be found in [
16]. A brief summary of the composition of the fractions are the following protein-(protein 77.3%; starch 0.3%; fibre 2.3%; fat 3.4%; ash 8%), starch-(protein 0.5%; starch 94.5%; fibre 3.6%; fat 0.3%; ash 0.2%), and fibre-rich (protein 5.3%; starch 22.5%; fibre 73.1%; fat 0.4%; ash 3.5%). The beans were grown in central Sweden, harvested and dried in 2016. Sodium hydroxide (NaOH) and hydrochloric acid (HCl), purchased from Merck KGaA (Darmstadt, Germany), were used for extraction.
2.1. Extraction
To separate the cotyledon and hull, the faba beans were dehulled (Hi-Tech Machinery Manufacturing Co. Ltd., Heze, Shandong, China) and then milled (Ultra-Centrifugal Mill ZM-1, Retsch, Haan, Germany) into flours with a mesh size of 0.5 mm. Only the cotyledon flour was used for further extraction. The extraction was performed as described previously, with some slight modifications [
16]. In brief, faba bean flour was dispersed at a distilled water:flour ratio of 10:1 (
v/w) and the pH was adjusted to 9.0 using 2 M NaOH. The protein was separated by centrifugation (Thermo Scientific, Sorvall Lynx 4000, Waltham, MA, USA) at 3700×
g (20 °C, 30 min) and precipitated at pH 4 using 1 M HCl. The protein was then washed once, the pH adjusted to 7 and the protein freeze-dried (Martin Christ, Epsilon 2-6D LSC Plus, Osterode am Harz, Germany). For starch and fibre extraction, the pellet from the first centrifugation step was re-dispersed in distilled water, the pH adjusted to 9.5 and the mixture stirred at room temperature for 24 h. Thereafter, the mixture was left to stand without stirring at 4 °C for 24 h before being centrifuged (3700×
g, 20 °C, 5 min). The supernatant was discarded and the pellet re-dispersed in distilled water. This centrifugation step was repeated until pH 7 was reached. To separate the starch from the fibre, the final pellet was dispersed in distilled water and filtered through a 70 μm nylon filter. This filtration step was repeated 12 times, followed by drying of the starch-rich filtrate at 40 °C for 48 h and freeze-drying of the fibre-rich filter cake. After drying, all fractions were sieved (Retsch, AS200 basic, Haan, Germany) through a 150 μm (protein and starch) or 250 μm (fibre) mesh (Retsch, Testsieve, Haan, Germany).
2.2. Swelling Power and Water Soluble Index
The swelling power (SP) of the samples was determined in triplicate following a modified version of existing methods by Muñoz et al. and Schoch [
17,
18]. In brief, 0.5 g of sample was weighed into a 15 mL centrifuge tube with a screw cap and 10 mL of distilled water was added. The tubes were placed in a shaking water bath at 60 °C for 10 min. The tubes were removed and left to cool to room temperature in an ice water bath before centrifugation at 7000 g for 20 min. The supernatant was decanted and left to dry at 105 °C overnight. The dried sample was used to calculate the water soluble index (WSI) and the mass of the sediment was used to determine the swelling power. These were calculated using the following equations:
2.3. Preparation of Inks
The composition of the different inks (fibre-rich, protein-rich, starch-rich, protein- and starch-rich; see
Table 1) were chosen based on a pre-study, providing printable inks covering a relatively wide range of composition. The mixture of flours to water ratio was adjusted for each ink. The criteria for the inks were that they should be printable and produce a standing object. The pastes used for 3D printing and stress sweep measurements were prepared by first mixing the starch with 70% of the total amount of diH
2O used. The starch dispersion was then heated in a water bath at 60 °C for 10 min. Thereafter, the mixture was removed from the water bath, the fibre and protein added and the mixture mixed thoroughly by hand using a spatula. For rheological characterisation, the fibre was added and the sample mixed before addition of the protein and additional mixing. After the addition of fibre and protein, the final 30% of diH
2O was added before mixing to a homogenous paste. To further homogenise the sample and eliminate larger air bubbles, the paste was extruded three times through a 5-mL Luer syringe without a needle before being added to the 3-mL cartridges used for printing. The cartridges were sealed and left at room temperature for 1 h before printing and rheological measurements.
2.4. Rheological Characterisation of Inks
The viscoelastic properties of the inks were analysed by stress sweep measurements using a Discovery HR-3 rheometer (TA Instruments, New Castle, DE, USA) equipped with a 40 mm aluminium plate. The stress was increased logarithmically from 0.001 to 10,000 Pa at a frequency of 0.1 Hz and a temperature of 22 °C. The edges of the sample were covered with paraffin oil to limit evaporation and a 300 s resting time was applied before starting the measurements. The yield stress was defined as the stress at which 5% loss of the original storage modulus was observed. The analysis was performed in duplicate.
2.5. 3-D Printing
The model of the cube (14 × 14 × 14 mm) used for printing was created using the digital design tool Tinkercad (Autodesk Inc., San Rafael, CA, USA) and exported as an STL file to the bioprinter. The cubes were printed at room temperature using two different infill patterns (honeycomb, grid). For the cubes with a honeycomb pattern, the pattern available in the bioprinter software was used. The grid pattern was created using Cellink Heartware (Version 2.1.6, Cellink, Gothenburg, Sweden, 2021) combined with the open source slicing software Slic3r (Version 1.3.0, 2021).
Samples were printed on petri dishes using a BIO X bioprinter (Cellink, Gothenburg, Sweden). Pressure and pre-flow were adjusted for each ink individually. The number of layers was 24, the infill density was 25% and the tip diameter of the nozzle was 580 μm. After printing, the samples were left for 5 min at room temperature and then stored at −18 °C until freeze-drying.
2.6. Freeze Drying
Frozen 3D-printed objects were further frozen at −50 °C for 48 h, followed by freeze-drying (Martin Christ, Epsilon 2-6D LSC Plus, Osterode am Harz, Germany).
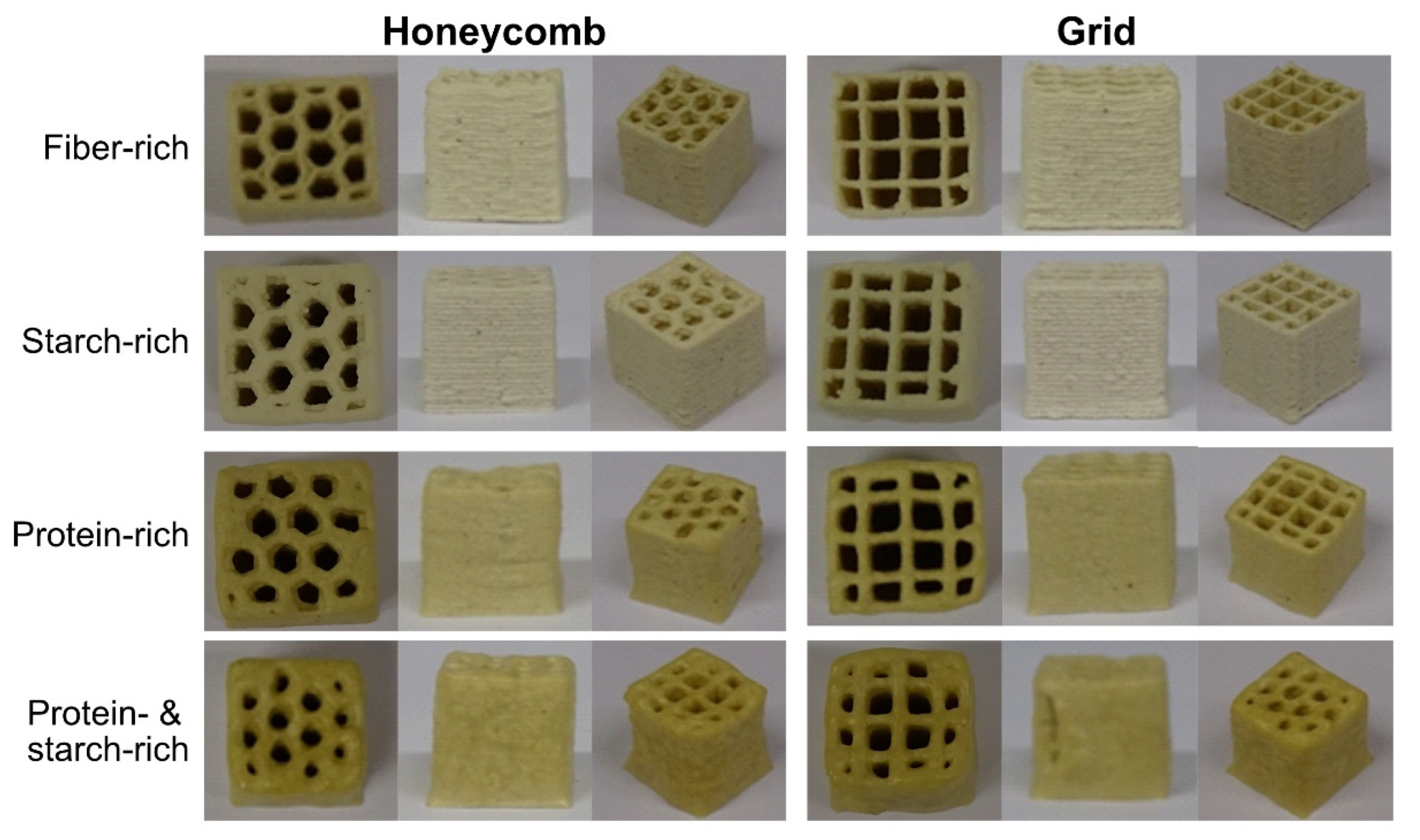
2.7. Visual Inspection
The freeze-dried cubes were evaluated and compared using six criteria; colour, infill pattern, wall straightness, wall texture, layer distinction and uniformity.
2.8. Colour Measurements
Cube colour was measured with L*a*b colour space using a colorimeter (CR-300, Minolta, Japan). The built-in light source was carefully placed on the wall of the cube, on the side without the infill pattern, until the end of measurements.
2.9. Texture Analysis
Compression tests were performed on the freeze-dried samples using a texture analyser (Stable Micro Systems, TA-HDi, Surrey, UK) equipped with a 500 N load cell and a 36 mm cylindrical aluminium probe. The samples were compressed to 60% at a rate of 1 mm/s. The compression tests were performed in two directions, from the top (infill pattern facing the direction of compression) and from the side (infill pattern facing 90° from the direction of compression). The compression tests were performed in triplicate.
2.10. Image Analysis for Particle Size after Compression
Compressed matter collected from the texture analysis was spread out on a black background to emphasise contrast and images (1800 × 4000 pixels) of the compressed cube particles were taken at a height of 30 cm. Fiji-Image J was used for image analysis. Using the software, the individual crushed pieces were counted and measured in terms of length, width and area. A ruler was used to standardise the scale bar (0.095 mm per pixel).
2.11. Scanning Electron Microscopy
Freeze-dried samples were fractured, sputter-coated with gold (Au) (Cressington Scientific Instruments, Sputter coater-108 auto, Watford, UK) and examined using a scanning electron microscope (Hitachi, FlexSEM 1000II, Tokyo, Japan) at 5 kV. Images (1280 × 960 pixels) were recorded digitally at two different magnifications, giving a pixel size of 0.992 μm/pixel and 0.198 μm/pixel.
2.12. Statistical Analysis
Results from the rheology and texture analysis were analysed by analysis of variance (ANOVA) and pairwise comparison (Tukey) using R studio (Version 1.2.5033, RStudio Inc., MA, USA). The size distribution of the fragments after compression in the texture analysis was visualised with ggplot2, with geometry density and log scale, using R studio (Version 1.2.5033, RStudio Inc., Boston, MA, USA). Principal component analysis (PCA), loading and score plot, were created using Simca17, Satorious Stedim Data Analytics AB.
4. Discussion
The different compositions of inks were adjusted to create printable inks with good flow and shape stability properties. The water content of the inks was increased with fibre and starch content, which correlated well with the swelling power of the fractions. Based on visual inspection, the infill pattern was least distinct for the protein- and starch-rich samples, with irregular size and shape of the holes within the pattern. The protein- and starch-rich sample also had the highest percentage of ink-failure due to unstable flow and low shape stability. This suggests that the fibre might have a stabilising effect, contributing to the shape stability of the cubes. During ink production, the starch is heated to 60 °C, which is below the gelatinisation temperature of faba bean starch (67–72 °C [
20]). Heating the starch to gelatinisation has been found to enhance the structural function of starch in cookie systems [
21]. Structure and shape instability, particularly for the protein- and starch-rich cubes (with no fibre), may have been caused by the starch not fully gelatinising because of insufficient heating temperature during the production of the bio-inks.
Due to difficulties such as wall slip and material escaping the gap during viscosity measurements of highly viscous or semi-solid materials, oscillatory tests at small deformations were used rather than a rotational rheometer [
22]. All inks showed predominantly solid-like behaviour (G′ > G″), with G′ values in the approximate range 2–30 kPa. A similar range of G′ values has previously been reported for printable pastes, and significantly lower G′ values (down to 10 Pa) for pastes forming self-standing cylinders [
5,
8]. The filaments of the samples with the lowest G′ (protein- and starch-rich, protein-rich) seemed to swell more and showed a slight tendency to collapse. These two samples also had the highest loss tangent values, which could potentially explain their lower dimensional stability. The starch-rich samples showed higher yield stress than the other inks. Similarly, the pressure needed to print the starch-rich cubes was significantly higher than for the other inks. A correlation between high yield stress and high printing pressure has been observed by others [
5].
High yield stress or high storage modulus alone is not always sufficient to create a good ink, e.g., a previous study found that two inks with similar G′ and phase angles showed different shape stability after printing [
5]. Hence, G′ and phase angle alone are not always sufficient to determine the suitability of a material for 3D printing. Combining these results with the rheological characterisation of our inks, it appears that a combination of high G′, high yield stress and low phase angle is required to provide an ink with good shape stability after printing.
After printing and freeze-drying, the texture of the cubes was analysed by compression tests performed along two different axes of the cubes. The effect of orientation was investigated on the cubes standing, with the infill pattern facing upwards (compressed from the top), and lying on their side, with the infill pattern facing 90° from the axis of compression (compressed from the side). A significantly higher peak force (3- to 7-fold higher) was recorded as the cubes were compressed from the top compared with from the side. The higher force required for top compression is likely due to the wall layers being stacked directly on top of each other, creating a denser wall structure that requires more force to compress. For compression from the side, there are gaps between the layers because of the infill pattern, which will reduce the force required for compression until fracture. The multiple peaks observed from the force vs. strain graphs for samples compressed from the side, but not the top, was a result of the infill pattern. The multiple peaks also related to the size and spreading of pieces after compression, as seen in
Figure 3. The samples compressed from the side spread out in a linear fashion as multiple larger pieces that likely relate to the multiple peaks observed in the force vs. strain graphs (
Figures S1 and S2).
Scanning electron microscopy of the freeze-dried samples revealed that microstructure was strongly affected by the freezing and freeze-drying. The microstructure consisted of cavities with an approximate diameter of 30–70 μm. A similar microstructure in freeze-dried gelatin products has been observed by others [
23]. The porous structure was more irregular for the samples with more fibre and less protein and these samples also had less smooth surfaces. The more irregular structure could potentially explain the lower peak force seen for the starch-rich and fibre-rich samples. Fracture is generally believed to occur by the propagation of cracks, which form at or close to defects and weak spots acting as stress concentrators during deformation [
19,
24]. Hence, it could be hypothesised that the increased heterogeneity of the starch-rich and fibre-rich samples contributed to their lower peak force.
The SEM micrographs also indicated that pore walls were thicker for the protein-rich and protein- and starch-rich samples than for the starch-rich and fibre-rich samples. Further studies, e.g., by X-ray tomography, would have been needed to confirm this. Nonetheless, increased cell diameter and cell wall thickness have previously been correlated to reduced crushing/breaking stress and compression modulus of cellular corn starch and corn-based extrudates [
25,
26,
27].
Crispiness and crunchiness are textural properties directly related to microstructure and macrostructure that influence the mechanical and fracture properties of solid food [
19]. A higher force was required for compression from the top in this study, indicating that the cubes would be perceived as harder. A previous combination of sensory evaluation and texture analysis of almonds showed that almonds perceived as more brittle and less hard by sensory evaluation required less force for compression and displayed more deformation peaks in the textural analysis [
28]. In this study, the cubes compressed from the side required less force, with more deformation peaks present, indicating that side compression of the cube may enhance the perceived crispness and brittleness of the products and perhaps be a preferred characteristic in a future food product.
In a laboratory taste test on the cubes of the four different recipes, the fibre-rich and starch-rich samples were perceived as more neutral in flavour and with a crispier texture. The protein-rich and protein- and starch-rich samples were rated similar to each other and were less preferred than the other samples, both in terms of flavour and texture. The protein-rich cubes were perceived by the tasters to have a hard texture that became clayey after chewing. None of the different recipes produced cubes that were perceived as beany in flavour.