High-Level Expression of Nitrile Hydratase in Escherichia coli for 2-Amino-2,3-Dimethylbutyramide Synthesis
Abstract
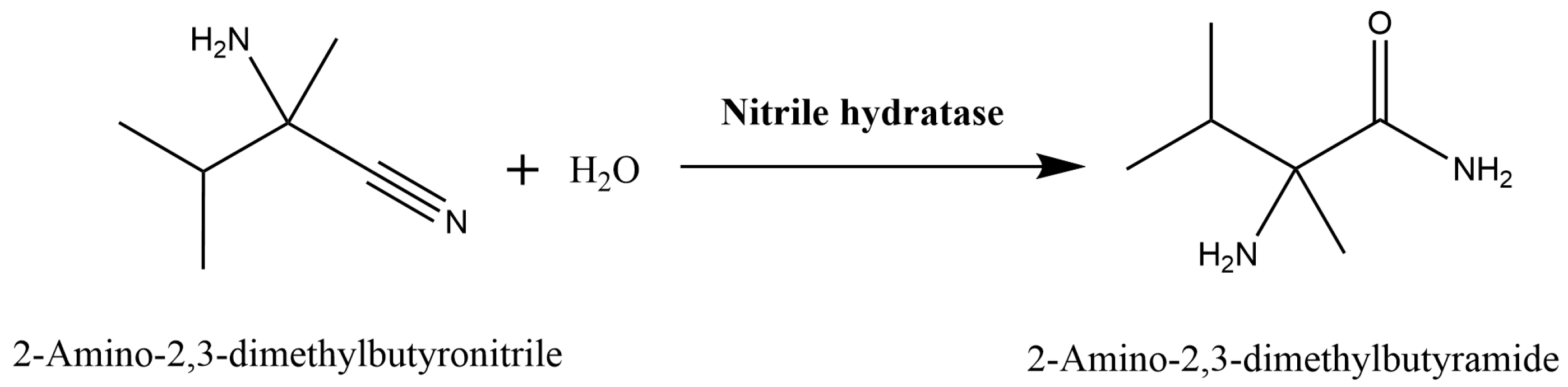
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Recombinant NHase Library
2.3. Cultivation Conditions of Recombinant NHase
2.4. Screening of the Recombinant NHase Library
2.5. Construction of His-Tag Fused NHase
2.6. Recombinant NHase Preparation and Purification
2.7. GC Analysis and NHase Activity Assay
2.8. Characterization of Purified Recombinant NHase
2.9. Selection of Recombinant Strain
2.10. Optimization of Fermentation Conditions
3. Results and Discussion
3.1. Screening of the NHase Library
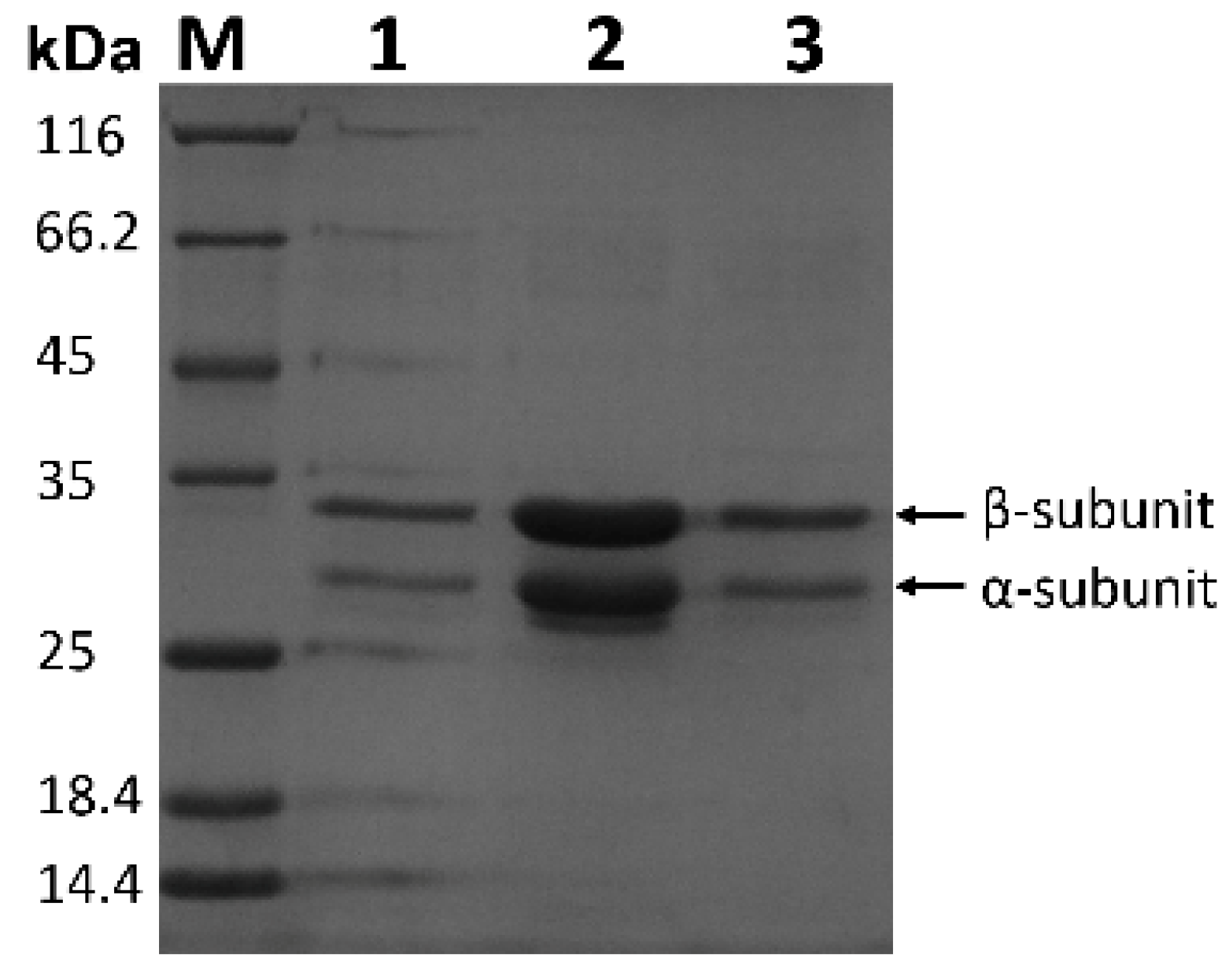
3.2. Purification of Recombinant NHase
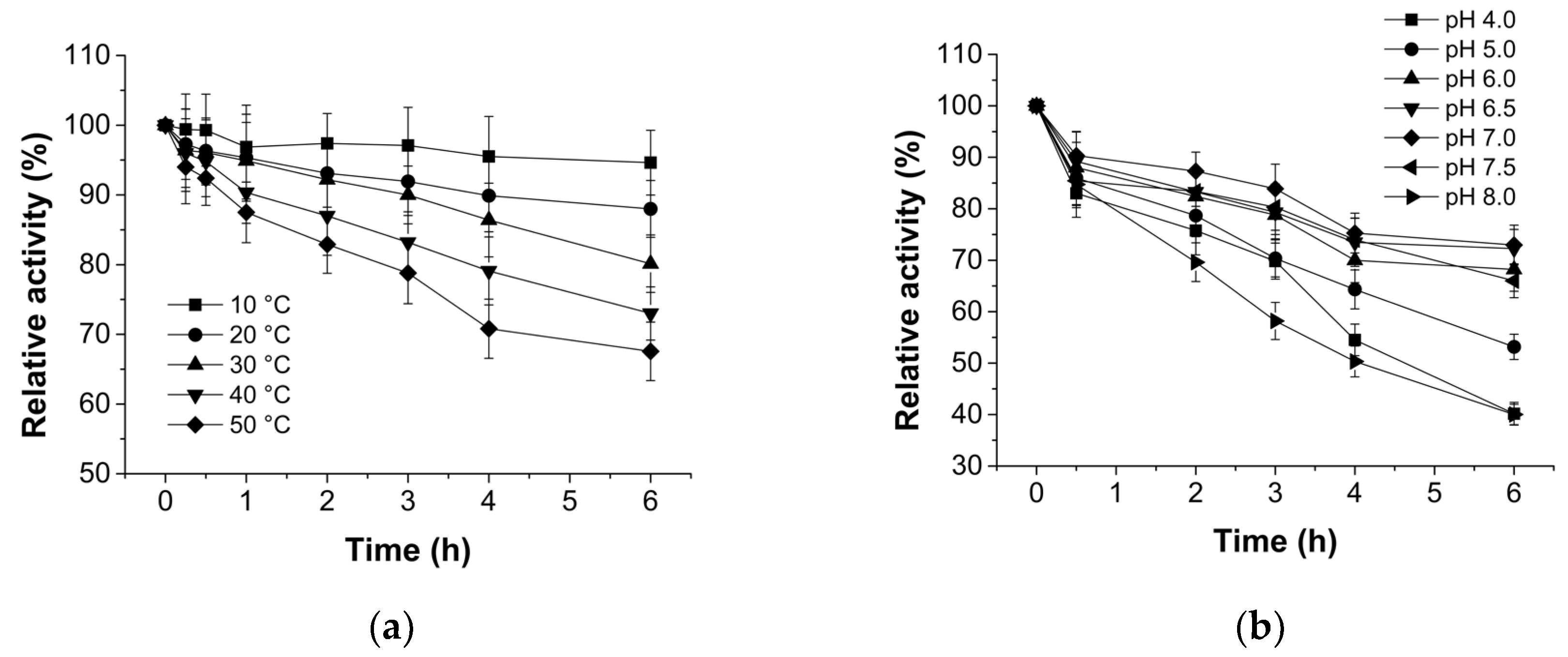
3.3. Characterization of Recombinant NHase
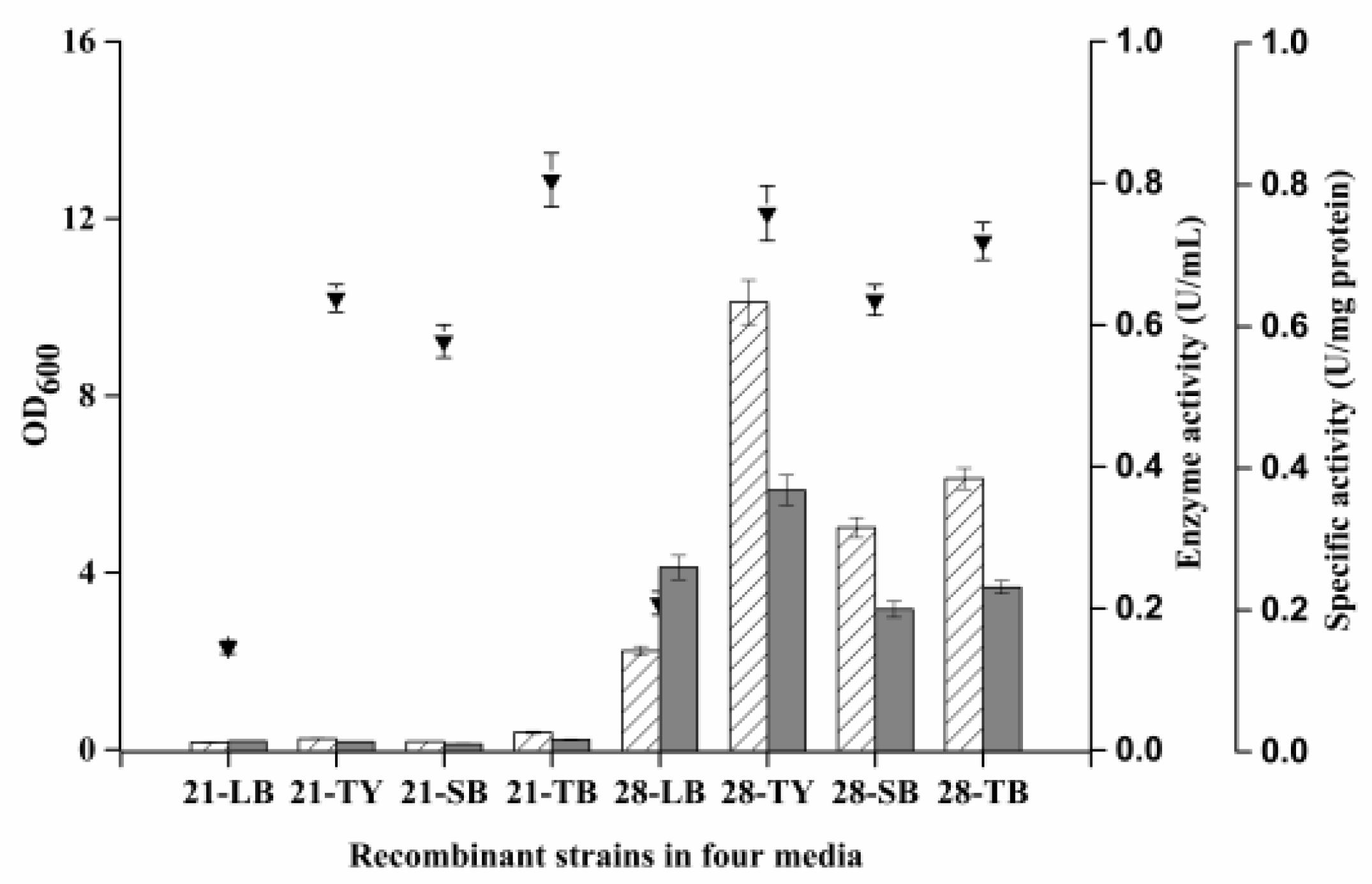
3.4. Selection of Recombinant Strain
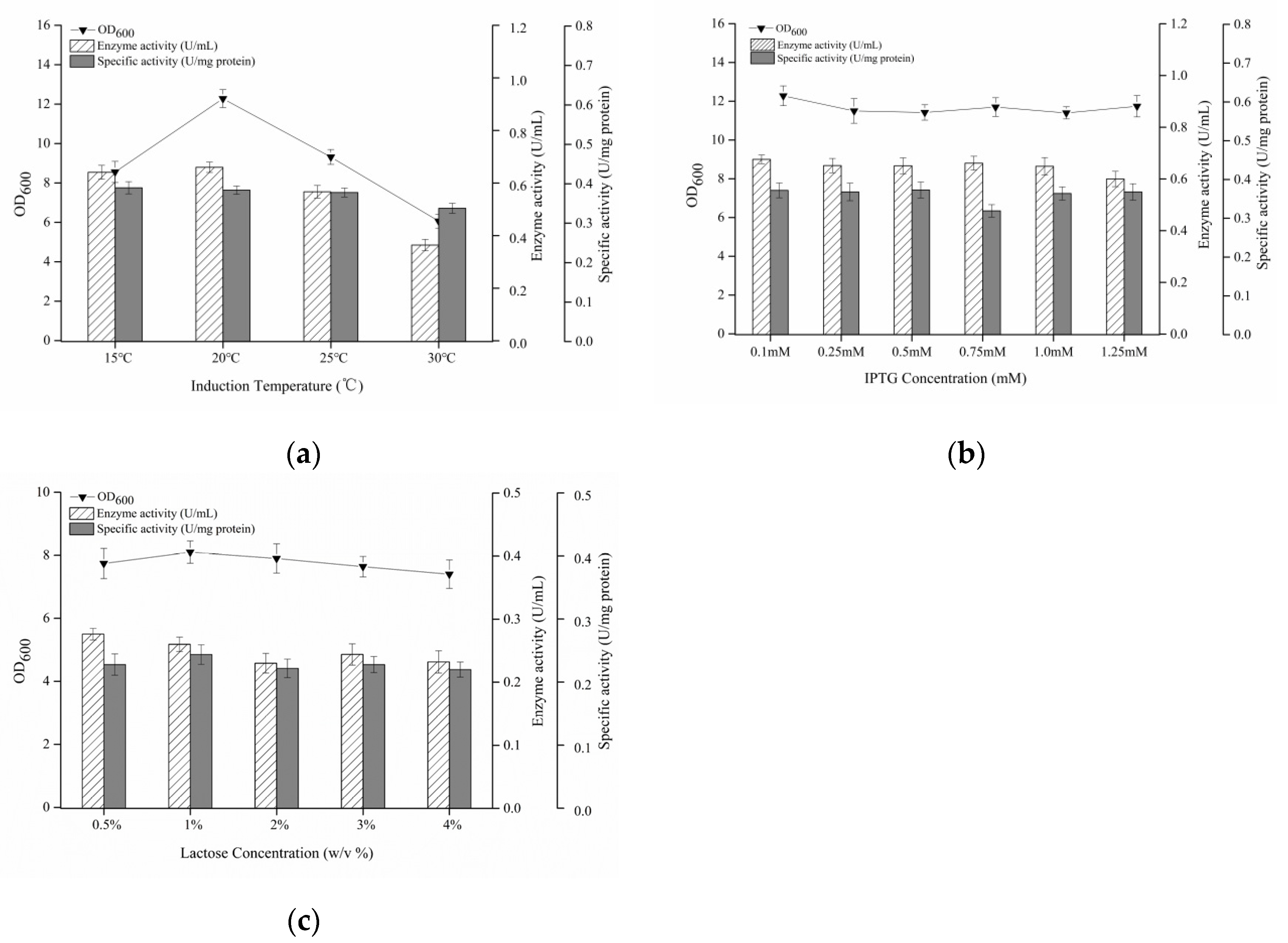
3.5. Optimization of Induction Temperature and Inducers
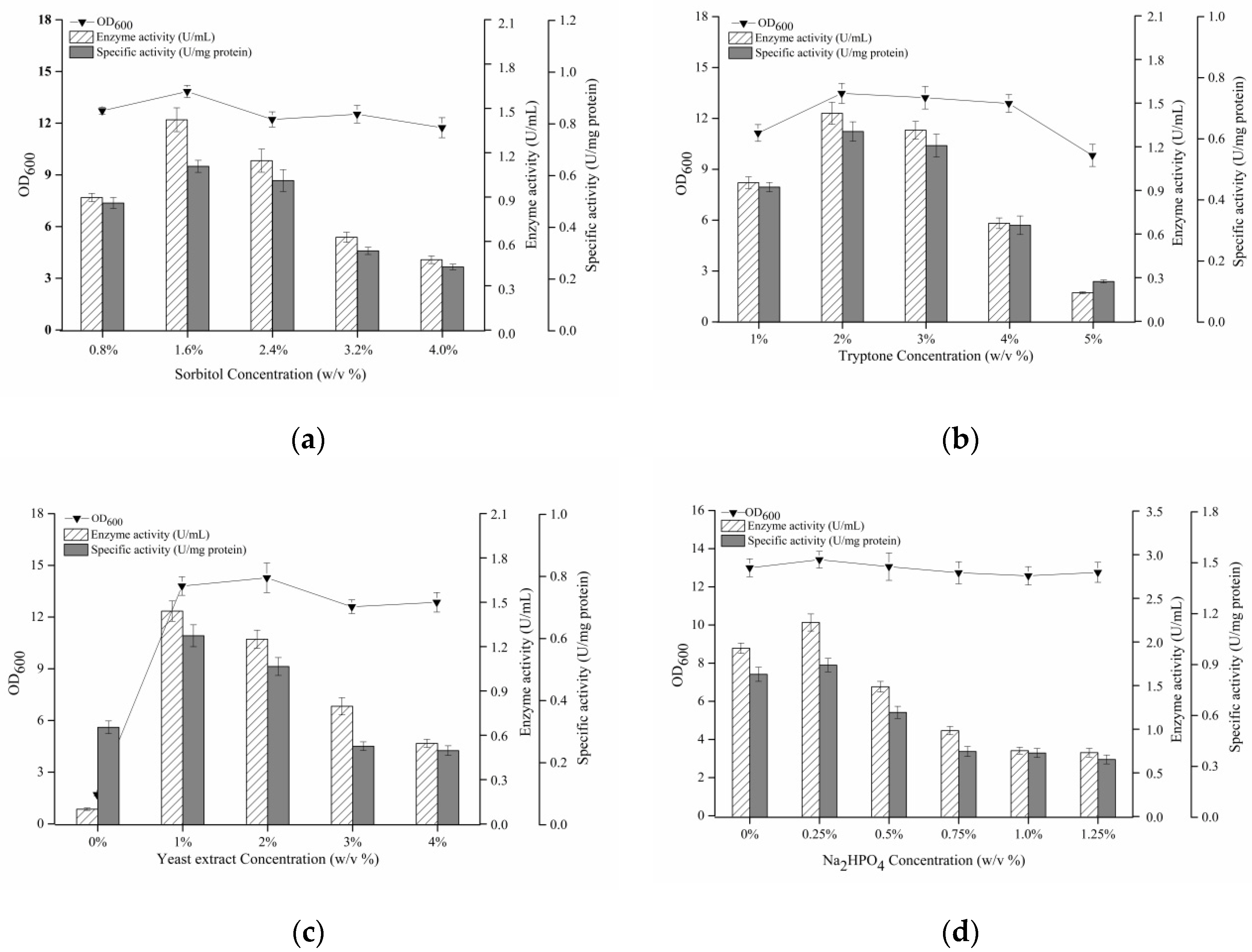
3.6. Optimization of Medium Components
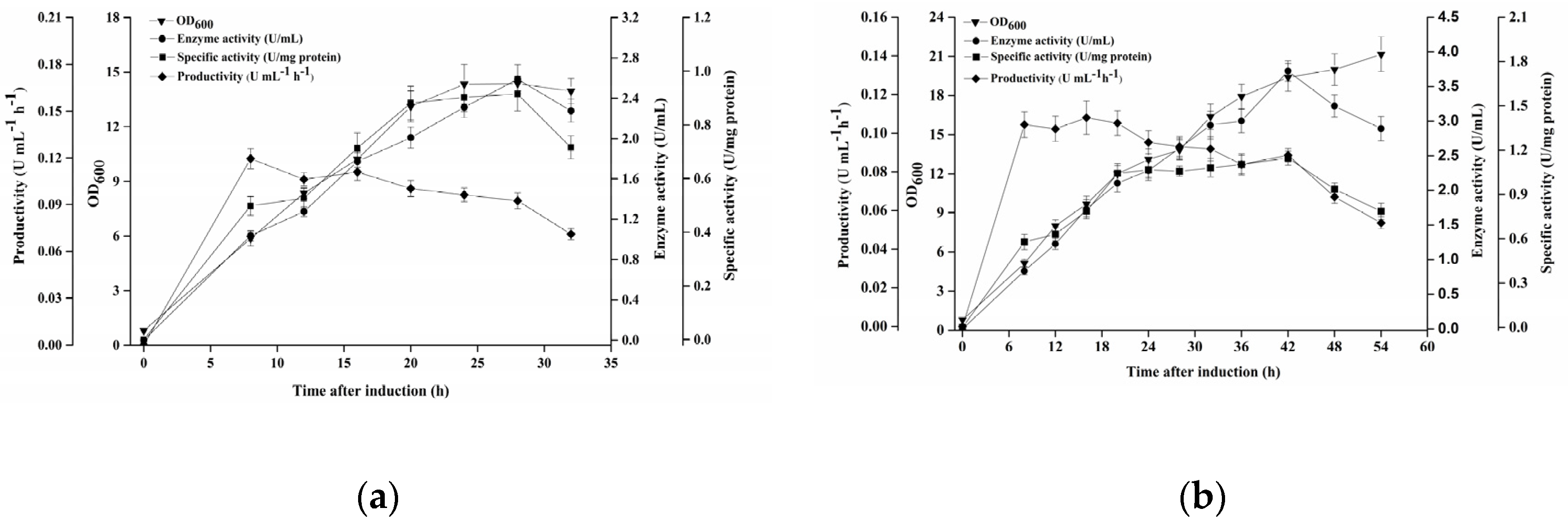
3.7. Optimization of Feeding Strategy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Lu, D.; Yang, J.; Xu, Y.; Gong, C.; Li, Z. Enantioselective phytotoxicity and the relative mechanism of current chiral herbicides. Curr. Protein Pept. Sci. 2017, 18, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Balabanova, D.A.; Paunov, M.; Goltsev, V.; Cuypers, A.; Vangronsveld, J.; Vassilev, A. Photosynthetic performance of the imidazolinone resistant sunflower exposed to single and combined treatment by the herbicide imazamox and an amino acid extract. Front. Plant Sci. 2016, 7, 1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamberth, C. Heterocyclic chemistry in crop protection. Pest Manag. Sci. 2013, 69, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Wepplo, P. Imidazolinone herbicides: Synthesis and novel chemistry. Pest Manag. Sci. 1990, 29, 293–315. [Google Scholar] [CrossRef]
- Hess, F.G.; Harris, J.E.; Pendino, K.; Ponnock, K. Imidazolinones. In Hayes’ Handbook of Pesticide Toxicology; Academic Press: Burlington MA, USA, 2010; pp. 1853–1863. [Google Scholar]
- Boesten, W.H.J.; Kamphuis, J. Process for the Preparation of Alpha-amino-alpha-methylcarboxylic Acid Amides and Alpha-amino-alpha-cycloalkylcarboxylic Acid Amides. EP 0231546 (A1), 14 November 1989. [Google Scholar]
- Cheng, Z.; Xia, Y.; Zhou, Z. Recent advances and promises in nitrile hydratase: From mechanism to industrial applications. Front. Bioeng. Biotechnol. 2020, 8, 352. [Google Scholar] [CrossRef]
- Faisal, M. Green fluorosolvents for biocatalysis. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 143–162. [Google Scholar]
- Martínková, L.; Rucká, L.; Nešvera, J.; Pátek, M. Recent advances and challenges in the heterologous production of microbial nitrilases for biocatalytic applications. World J. Microb. Biot. 2017, 33, 1–11. [Google Scholar] [CrossRef]
- Lin, Z.J.; Zheng, R.C.; Wang, Y.J.; Zheng, Y.G.; Shen, Y.C. Enzymatic production of 2-amino-2,3-dimethylbutyramide by cyanide-resistant nitrile hydratase. J. Ind. Microbiol. Biot. 2012, 39, 133–141. [Google Scholar] [CrossRef]
- Lin, Z.J.; Zheng, R.C.; Lei, L.H.; Zheng, Y.G.; Shen, Y.C. Ferrous and ferric ions-based high-throughput screening strategy for nitrile hydratase and amidase. J. Microbiol. Meth. 2011, 85, 214–220. [Google Scholar] [CrossRef]
- Lin, Z.J.; Zheng, R.C.; Zheng, Y.G.; Shen, Y.C. Biosynthesis of 2-amino-2,3-dimethylbutyramide by nitrile hydratase from a newly isolated cyanide-resistant strain of Rhodococcus qingshengii. Biotechnol. Lett. 2011, 33, 1809–1813. [Google Scholar] [CrossRef]
- Kubáč, D.; Kaplan, O.; Elišáková, V.; Patek, M.; Vejvoda, V.; Slámová, K.; Tothova, A.; Lemaire, M.; Gallienne, E.; Lutz-Wahl, S.; et al. Biotransformation of nitriles to amides using soluble and immobilized nitrile hydratase from Rhodococcus erythropolis A4. J. Mol. Catal. B—Enzym. 2008, 50, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Pei, X.; Zhang, H.; Meng, L.; Xu, G.; Yang, L.; Wu, J. Efficient cloning and expression of a thermostable nitrile hydratase in Escherichia coli using an auto-induction fed-batch strategy. Process Biochem. 2013, 48, 1921–1927. [Google Scholar] [CrossRef]
- Supreetha, K.; Rao, S.N.; Srividya, D.; Anil, H.S.; Kiran, S. Advances in cloning, structural and bioremediation aspects of nitrile hydratases. Mol. Biol. Rep. 2019, 46, 4661–4673. [Google Scholar] [CrossRef] [PubMed]
- Grill, B.; Glänzer, M.; Schwab, H.; Steiner, K.; Pienaar, D.; Brady, D.; Donsbach, K.; Winkler, M. Functional Expression and Characterization of a Panel of Cobalt and Iron-Dependent Nitrile Hydratases. Molecules 2020, 25, 2521. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yamaki, T.; Oikawa, T.; Ito, K.; Nakamura, T. Cloning and sequencing of a nitrile hydratase gene from Pseudonocardia thermophila JCM3095. J. Ferment. Bioeng. 1997, 83, 474–477. [Google Scholar] [CrossRef]
- Peplowski, L.; Kubiak, K.; Nowak, W. Mechanical aspects of nitrile hydratase enzymatic activity. Steered molecular dynamics simulations of Pseudonocardia thermophila JCM 3095. Chem. Phys. Lett. 2008, 467, 144–149. [Google Scholar] [CrossRef]
- Brandão, P.F.; Verseck, S.; Syldatk, C. Bioconversion of D, L-tert-leucine Nitrile to D-tert-leucine by recombinant cells expressing nitrile hydratase and D-selective amidase. Eng. Life Sci. 2004, 4, 547–556. [Google Scholar] [CrossRef]
- Van Pelt, S.; van Rantwijk, F.; Sheldon, R.A. Synthesis of aliphatic (S)-α-hydroxycarboxylic amides using a one-pot bienzymatic cascade of immobilised oxynitrilase and nitrile hydratase. Adv. Synth. Catal. 2009, 351, 397–404. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shimizu, S. Nitrile hydrolases. Curr. Opin. Chem. Biol. 2000, 4, 95–102. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry; Pearson Prentice Hall: New York, NY, USA, 2005. [Google Scholar]
- Petrillo, K.L.; Wu, S.; Hann, E.C.; Cooling, F.B.; Ben-Bassat, A.; Gavagan, J.E.; DiCosimo, R.; Payne, M.S. Over-expression in Escherichia coli of a thermally stable and regio-selective nitrile hydratase from Comamonas testosteroni 5-MGAM-4D. Appl. Microbiol. Biot. 2005, 67, 664–670. [Google Scholar] [CrossRef]
- Pereira, R.A.; Graham, D.; Rainey, F.A.; Cowan, D.A. A novel thermostable nitrile hydratase. Extremophiles 1998, 2, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Su, E.; Ma, X.; Yang, S.; Wei, D. High-level soluble and functional expression of Trigonopsis variabilis D-amino acid oxidase in Escherichia coli. Bioproc. Biosyst. Eng. 2014, 37, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Su, E.Z.; Wang, H.L.; Cai, W.W.; Wang, W.; Wei, D.Z. Cloning, overexpression, and characterization of a high enantioselective nitrilase from Sphingomonas wittichii RW1 for asymmetric synthesis of (R)-phenylglycine. Appl. Biochem. Biotech. 2014, 173, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Su, E.; Zhu, Y.; Deng, S.; Wei, D. High-level expression of glutaryl-7-aminocephalosporanic acid acylase from Pseudomonas diminuta NK703 in Escherichia coli by combined optimization strategies. J. Biotechnol. 2013, 168, 607–615. [Google Scholar] [CrossRef]
- Fischer-Colbrie, G.; Herrmann, M.; Heumann, S.; Puolakka, A.; Wirth, A.; Cavaco-Paulo, A.; Guebitz, G.M. Surface modification of polyacrylonitrile with nitrile hydratase and amidase from Agrobacterium tumefaciens. Biocatal. Biotransformation 2006, 24, 419–425. [Google Scholar] [CrossRef]
- Zhu, S.; Ma, X.; Su, E.; Wei, D. Efficient hydration of 2-amino-2,3-dimethylbutyronitrile to 2-amino-2,3-dimethylbutyramide in a biphasic system via an easily prepared whole-cell biocatalyst. Green Chem. 2015, 17, 3992–3999. [Google Scholar] [CrossRef]

| Organic Solvent | Relative Activity (%) | ||
|---|---|---|---|
| 5% (v/v) | 15% (v/v) | 50% (v/v) | |
| Isooctane | 102.59 ± 5.31 | 101.61 ± 5.26 | 100.22 ± 4.86 |
| Heptane | 95.24 ± 5.45 | 81.20 ± 3.66 | 80.88 ± 4.74 |
| Hexane | 77.92 ± 4.67 | 77.05 ± 5.23 | 75.05 ± 3.93 |
| Pentane | 97.00 ± 6.06 | 95.00 ± 4.87 | 94.13 ± 5.29 |
| Butyl acetate | 72.63 ± 3.86 | 58.84 ± 3.30 | nd 1 |
| Octanol | 84.00 ± 4.20 | 59.84 ± 3.13 | 57.87 ± 3.16 |
| Dimethyl Sulfoxide | 71.13 ± 3.65 | 59.46 ± 3.10 | nd |
| Cyclohexane | 84.88 ± 4.51 | 83.09 ± 3.89 | 74.35 ± 4.45 |
| Toluene | 81.66 ± 3.70 | 79.23 ± 4.51 | 73.22 ± 3.83 |
| Dichloromethane | 87.55 ± 4.04 | 69.28 ± 4.16 | 54.51 ± 3.33 |
| Isopropyl ether | 94.00 ± 5.78 | 90.00 ± 5.06 | 74.30 ± 3.63 |
| Ethyl acetate | 74.60 ± 3.70 | nd | nd |
| Benzene | 87.70 ± 5.54 | 86.30 ± 4.85 | 81.00 ± 4.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, S.; Zhu, S.; Zhang, X.; Sun, X.; Ma, X.; Su, E. High-Level Expression of Nitrile Hydratase in Escherichia coli for 2-Amino-2,3-Dimethylbutyramide Synthesis. Processes 2022, 10, 544. https://doi.org/10.3390/pr10030544
Deng S, Zhu S, Zhang X, Sun X, Ma X, Su E. High-Level Expression of Nitrile Hydratase in Escherichia coli for 2-Amino-2,3-Dimethylbutyramide Synthesis. Processes. 2022; 10(3):544. https://doi.org/10.3390/pr10030544
Chicago/Turabian StyleDeng, Senwen, Shujing Zhu, Xinyi Zhang, Xi Sun, Xiaoqiang Ma, and Erzheng Su. 2022. "High-Level Expression of Nitrile Hydratase in Escherichia coli for 2-Amino-2,3-Dimethylbutyramide Synthesis" Processes 10, no. 3: 544. https://doi.org/10.3390/pr10030544
APA StyleDeng, S., Zhu, S., Zhang, X., Sun, X., Ma, X., & Su, E. (2022). High-Level Expression of Nitrile Hydratase in Escherichia coli for 2-Amino-2,3-Dimethylbutyramide Synthesis. Processes, 10(3), 544. https://doi.org/10.3390/pr10030544






