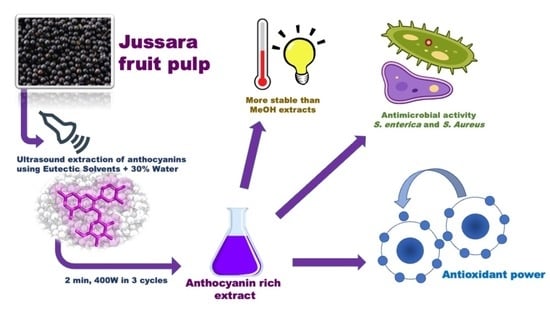
High-Performance Extraction Process of Anthocyanins from Jussara (Euterpe edulis) Using Deep Eutectic Solvents
Abstract
:1. Introduction
2. Material and Methods
2.1. Fruit Gathering
2.2. Eutectic Solvent Synthesis
2.3. Experimental Design: Response Surface Methodology
2.4. Identification and Quantification of Anthocyanins
2.5. Antioxidant Activity
2.6. Anthocyanin Thermal Stability
2.7. Anthocyanin Photostability
2.8. Disc-Diffusion Antimicrobial Sensitivity Test
3. Results and Discussion
3.1. Screening Process
3.2. Fractional Factorial Experimental Design (FFED)
3.3. Central Composite Rotatable Design (CCRD)
3.4. Antioxidant Activity
3.5. Determination of Anthocyanins Thermal Stability
3.6. Determination of Anthocyanins Photostability
3.7. Antimicrobial Activity
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.C.; Hopkins, J.R.; Carslaw, D.C.; Hamilton, J.F.; Nelson, B.S.; Stewart, G.; Dernie, J.; Passant, N.; Murrells, T. An increasing role for solvent emissions and implications for future measurements of volatile organic compounds: Solvent emissions of VOCs. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2020, 378, 20190328. [Google Scholar] [CrossRef] [PubMed]
- Beach, E.S.; Cui, Z.; Anastas, P.T. Green Chemistry: A design framework for sustainability. Energy Environ. Sci. 2009, 2, 1038. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Freitag, R.A.; Dabdoub, M.J.; Ferreira Batista, A.C.; Da Cruz Silveira, C. “Green chemistry” Os 12 princípios da química verde e sua inserção nas atividades de ensino e pesquisa. Quim. Nova 2003, 26, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.A. Green solvents for sustainable organic synthesis: State of the art. Green Chem. 2005, 7, 267. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; de Mejia, E.G. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef]
- Radošević, K.; Cvjetko Bubalo, M.; Gaurina Srček, V.; Grgas, D.; Dragičević, T.L.; Redovniković, R.I. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Pinheiro, M.C.D.O.; Abrantes, S.D.M.P. Avaliaçao da exposiçao aos corantes artificiais em balas e chicletes por crianças entre 3 e 9 anos estudantes de escolas particulares da Tijuca/Rio de Janeiro. 2012. Available online: http://www.revistaanalytica.com.br/artigos/8.pdf (accessed on 11 July 2014).
- Ramesh, M.; Muthuraman, A. Flavoring and Coloring Agents: Health Risks and Potential Problems. In Natural and Artificial Flavoring Agents and Food Dyes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–28. [Google Scholar] [CrossRef]
- Blumberg, P.O. Rainbow Risks: 6 Artificial Food Colors You Need to Know about—Reader’s Digest. Published 2010. Available online: https://www.rd.com/health/conditions/rainbow-risks-6-artificial-food-colors-you-need-to-know-about (accessed on 17 March 2022).
- da Silva, J.Z.; dos Reis, M.S. Consumption of Euterpe edulis fruit by wildlife: Implications for conservation and management of the southern Brazilian atlantic forest. An. Acad. Bras. Cienc. 2019, 91, e20180537. [Google Scholar] [CrossRef] [Green Version]
- Reis, M.S.; Fantini, A.C.; Nodari, R.O.; Reis, A.; Guerra, M.P.; Mantovani, A. Management and Conservation of Natural Populations in Atlantic Rain Forest: The Case Study of Palm Heart (Euterpe edulis Martius). Biotropica 2000, 32, 894–902. [Google Scholar] [CrossRef]
- Silva, P.; Carmo, L.; Silva, G.; Silveira-Diniz, M.; Casemiro, R.; Spoto, M. Physical, chemical, and lipid composition of juçara (Euterpe edulis Mart.) pulp. Alim. Nutr. Braz. J. Food Nutr. 2013, 24, 7–13. Available online: https://www.researchgate.net/publication/284267543_Physical_chemical_and_lipid_composition_of_jucara_Euterpe_edulis_Mart_pulp (accessed on 17 March 2022).
- Vannuchi, N.; Jamar, G.; Pisani, L.; Braga, A.R.C.; de Rosso, V.V. Chemical composition, bioactive compounds extraction, and observed biological activities from jussara (Euterpe edulis): The exotic and endangered Brazilian superfruit. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3192–3224. [Google Scholar] [CrossRef]
- Brito, E.S.; de Araújo, M.C.P.; Alves, E.E.; Carkeet, C.; Clevidence, B.A.; Novotny, J.A. Anthocyanins Present in Selected Tropical Fruits: Acerola, Jambolão, Jussara, and Guajiru. J. Agric. Food Chem. 2007, 55, 9389–9394. [Google Scholar] [CrossRef] [PubMed]
- Favaro, L.; Balcão, V.; Rocha, L.; Silva, E.; Oliveira, J.J.; Vila, M.; Tubino, M.; Favaro, L.I.L.; Balcão, V.M.; Rocha, L.K.H.; et al. Physicochemical characterization of a crude anthocyanin extract from the fruits of jussara (Euterpe edulis Martius): Potential for food and pharmaceutical applications. J. Braz. Chem. Soc. 2018, 29, 2072–2088. [Google Scholar] [CrossRef]
- Da Silva, N.A.; Rodrigues, E.; Mercadante, A.Z.; De Rosso, V.V. Phenolic Compounds and Carotenoids from Four Fruits Native from the Brazilian Atlantic Forest. Agric. Food Chem. 2014, 62, 5072–5084. [Google Scholar] [CrossRef]
- Cardoso, L.M.; Dias Novaes, R.; De Castro, C.A. Chemical composition, characterization of anthocyanins and antioxidant potential of Euterpe edulis fruits: Applicability on genetic dyslipidemia and hepatic steatosis in mice. Nutr. Hosp. 2015, 32, 702–709. [Google Scholar] [CrossRef]
- Borges, G.D.S.C.; Vieira, F.G.K.; Copetti, C.; Gonzaga, L.V.; Zambiazi, R.C.; Filho, J.M.; Fett, R. Chemical characterization, bioactive compounds, and antioxidant capacity of jussara (Euterpe edulis) fruit from the Atlantic Forest in southern Brazil. Food Res. Int. 2011, 44, 2128–2133. [Google Scholar] [CrossRef]
- Vieira, G.S.; Cavalcanti, R.N.; Meireles, M.A.A.; Hubinger, M.D. Chemical and economic evaluation of natural antioxidant extracts obtained by ultrasound-assisted and agitated bed extraction from jussara pulp (Euterpe edulis). J. Food Eng. 2013, 119, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, A.G.A.; Silva, K.A.; Silva, L.O.; Costa, A.M.M.; Akil, E.; Coelho, M.A.Z.; Torres, A.G. Jussara berry (Euterpe edulis M.) oil-in-water emulsions are highly stable: The role of natural antioxidants in the fruit oil. J. Sci. Food Agric. 2019, 99, 90–99. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed.; The Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Gutiérrez, M.C.; Ferrer, M.L.; Mateo, C.R.; del Monte, F. Freeze-drying of aqueous solutions of deep eutectic solvents: A suitable approach to deep eutectic suspensions of self-assembled structures. Langmuir 2009, 25, 5509–5515. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J. Chromatogr. A 2013, 1285, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Zhuang, B.; Dou, L.L.; Li, P.; Liu, E.H. Deep eutectic solvents as green media for extraction of flavonoid glycosides and aglycones from Platycladi Cacumen. J. Pharm. Biomed. Anal. 2017, 134, 214–219. [Google Scholar] [CrossRef]
- Torskangerpoll, K.; Andersen, Ø.M. Colour stability of anthocyanins in aqueous solutions at various pH values. Food Chem. 2005, 89, 427–440. [Google Scholar] [CrossRef]
- De Rosso, V.V.; Mercadante, A.Z. Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef]
- Do, M.; Rufino, S.M.; Alves, R.E.; de Brito, E.S. Metodologia Científica: Determinação Da Atividade Antioxidante Total Em Frutas Pela Captura Do Radical Livre ABTS+; Embrapa Agroindústria Tropical: Fortaleza, Brazil, 2007; Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/426954/metodologia-cientifica-determinacao-da-atividade-antioxidante-total-em-frutas-pela-captura-do-radical-livre-abts (accessed on 17 March 2021).
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- de Rosso, V.V.; Mercadante, A.Z. Evaluation of colour and stability of anthocyanins from tropical fruits in an isotonic soft drink system. Innov. Food Sci. Emerg. Technol. 2007, 8, 347–352. [Google Scholar] [CrossRef]
- Schulz, M.; Biluca, F.C.; Gonzaga, L.V.; Borges, G.; Vitali, L.; Micke, G.A.; de Gois, J.S.; de Almeida, T.S.; Borges, D.L.G.; Miller, P.R.M.; et al. Bioaccessibility of bioactive compounds and antioxidant potential of juçara fruits (Euterpe edulis Martius) subjected to in vitro gastrointestinal digestion. Food Chem. 2017, 228, 447–454. [Google Scholar] [CrossRef]
- Rogez, H.; Pompeu, D.R.; Akwie, S.N.T.; Larondelle, Y. Sigmoidal kinetics of anthocyanin accumulation during fruit ripening: A comparison between açai fruits (Euterpe oleracea) and other anthocyanin-rich fruits. J. Food Compos. Anal. 2011, 24, 796–800. [Google Scholar] [CrossRef] [Green Version]
- Gordon, A.; Gil Cruz, A.P.; Cabral, L.M.C.; de Freitas, S.C.; Taxi, C.M.A.D.; Donangelo, C.M.; Mattietto, R.D.A.; Friedrich, M.; da Matta, V.M.; Marx, F. Chemical characterization and evaluation of antioxidant properties of Açaí fruits (Euterpe oleraceae Mart.) during ripening. Food Chem. 2012, 133, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiao, J.; Gai, Q.-Y.; Wang, P.; Guo, N.; Niu, L.-L.; Fu, Y.-J. Enhanced and green extraction polyphenols and furanocoumarins from Fig (Ficus carica L.) leaves using deep eutectic solvents. J. Pharm. Biomed. Anal. 2017, 145, 339–345. [Google Scholar] [CrossRef]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Guo, N.; Kou, P.; Jiang, Y.-W.; Wang, L.-T.; Niu, L.-J.; Liu, Z.-M.; Fu, Y.-J. Natural deep eutectic solvents couple with integrative extraction technique as an effective approach for mulberry anthocyanin extraction. Food Chem. 2019, 296, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- López, N.; Delso, I.; Matute, D.; Lafuente, C.; Artal, M. Characterization of xylitol or citric acid:choline chloride:water mixtures: Structure, thermophysical properties, and quercetin solubility. Food Chem. 2020, 306, 125610. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K.; Redovnikovic, I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Triolo, A.; Lo Celso, F.; Brehm, M.; Di Lisio, V.; Russina, O. Liquid structure of a choline chloride-water natural deep eutectic solvent: A molecular dynamics characterization. J. Mol. Liq. 2021, 331, 115750. [Google Scholar] [CrossRef]
- Schulz, M.; Borges, G.; Gonzaga, L.V.; Seraglio, S.K.T.; Olivo, I.S.; Azevedo, M.S.; Nehring, P.; de Gois, J.S.; de Almeida, T.S.; Vitali, L.; et al. Chemical composition, bioactive compounds and antioxidant capacity of juçara fruit (Euterpe edulis Martius) during ripening. Food Res. Int. 2015, 77, 125–131. [Google Scholar] [CrossRef]
- Madalão, M.C.M.; Lima, E.M.F.; Benincá, D.B.; Saraiva, S.H.; de Carvalho, R.V.; Silva, P.I. Extraction of bioactive compounds from juçara pulp (Euterpe edulis M.) is affected by ultrasonic power and temperature. Ciência Agrotecnologia 2021, 45, 2021. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Murador, D.C.; de Souza Mesquita, L.M.; de Rosso, V.V. Bioavailability of anthocyanins: Gaps in knowledge, challenges and future research. J. Food Compos. Anal. 2017, 68, 31–40. [Google Scholar] [CrossRef]
- de Souza, M.O.; Silva, M.; Silva, M.E.; Oliveira, R.D.P.; Pedrosa, M.L. Diet supplementation with acai (Euterpe oleracea Mart.) pulp improves biomarkers of oxidative stress and the serum lipid profile in rats. Nutrition 2017, 26, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Berrios, J.D.J.; Powers, J.R.; Tang, J. Thermal Degradation of Anthocyanins from Purple Potato (Cv. Purple Majesty) and Impact on Antioxidant Capacity. J. Agric. Food Chem. 2011, 59, 11040–11049. [Google Scholar] [CrossRef] [PubMed]
- Inada, K.O.P.; Oliveira, A.A.; Revorêdo, T.B.; Martins, A.B.N.; Lacerda, E.C.Q.; Freire, A.S.; Braz, B.F.; Santelli, R.; Torres, A.G.; Perrone, D.; et al. Screening of the chemical composition and occurring antioxidants in jabuticaba (Myrciaria jaboticaba) and jussara (Euterpe edulis) fruits and their fractions. J. Funct. Foods 2015, 17, 422–433. [Google Scholar] [CrossRef] [Green Version]
- Bicudo, M.O.P.; Ribani, R.H. Anthocyanins, Phenolic Acids and Antioxidant Properties of Juçara Fruits (Euterpe edulis M.) Along the On-tree Ripening Process. Plant Foods Hum. Nutr. 2014, 69, 142–147. [Google Scholar] [CrossRef]
- Martins, P.L.G.; de Rosso, V.V.; Larangeira, P.; Martins, G.; De Rosso, V.V. Thermal and light stabilities and antioxidant activity of carotenoids from tomatoes extracted using an ultrasound-assisted completely solvent-free method. Food Res. Int. 2016, 82, 156–164. [Google Scholar] [CrossRef]
- Murador, D.C.; Braga, A.R.C.; Martins, P.L.G.; Mercadante, A.Z.; de Rosso, V.V. Ionic liquid associated with ultrasonic-assisted extraction: A new approach to obtain carotenoids from orange peel. Food Res. Int. 2019, 126, 108653. [Google Scholar] [CrossRef]
- De Souza Mesquita, L.M.; Ventura, S.P.M.; Braga, A.R.C.; Pisani, L.P.; Dias, A.C.R.V.; De Rosso, V.V. Ionic liquid-high performance extractive approach to recover carotenoids from: Bactris gasipaes fruits. Green Chem. 2019, 21, 2380–2391. [Google Scholar] [CrossRef]
- Türker, D.A.; Doğan, M. Ultrasound-assisted natural deep eutectic solvent extraction of anthocyanin from black carrots: Optimization, cytotoxicity, in-vitro bioavailability and stability. Food Bioprod. Process. 2022, 132, 99–113. [Google Scholar] [CrossRef]
- Furtado, P.; Figueiredo, P.; das Neves, H.C.; Pina, F. Photochemical and thermal degradation of anthocyanidins. J. Photochem. Photobiol. A Chem. 1993, 75, 113–118. [Google Scholar] [CrossRef]
- Chen, J.; Ma, X.H.; Yao, G.L.; Zhang, W.T.; Zhao, Y. Microemulsion-based anthocyanin systems: Effect of surfactants, cosurfactants, and its stability. Int. J. Food Prop. 2018, 21, 1152–1165. [Google Scholar] [CrossRef]
- Chen, C.C.; Lin, C.; Chen, M.H.; Chiang, P.Y. Stability and quality of anthocyanin in purple sweet potato extracts. Foods 2019, 8, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sipahli, S.; Mohanlall, V.; Mellem, J.J. Stability and degradation kinetics of crude anthocyanin extracts from H. sabdariffa. Food Sci. Technol. 2017, 37, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Liu, C.; Gao, W.; Li, Z.; Qin, G.; Qi, S.; Jiang, H.; Li, X.; Liu, M.; Yan, F.; et al. Anthocyanins Are Converted into Anthocyanidins and Phenolic Acids and Effectively Absorbed in the Jejunum and Ileum. J. Agric. Food Chem. 2021, 69, 992–1002. [Google Scholar] [CrossRef]
- Hensel, M. Evolution of pathogenicity islands of Salmonella enterica. Int. J. Med. Microbiol. 2004, 294, 95–102. [Google Scholar] [CrossRef]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as antimicrobial agents of natural plant origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Lima, M.C.; Paiva de Sousa, C.; Fernandez-Prada, C.; Harel, J.; Dubreuil, J.D.; de Souza, E.L. A review of the current evidence of fruit phenolic compounds as potential antimicrobials against pathogenic bacteria. Microb Pathog. 2019, 130, 259–270. [Google Scholar] [CrossRef]





| Compound | Chemical Structure | Molar Mass (g/mol) | Density (g/mL) at 20 °C | Chemical Characteristic |
|---|---|---|---|---|
| Chorine chloride |  | 139.62 | 1.10 (aq) | Hydrogen bond acceptor |
| L-proline |  | 115.13 | 1.35 | Hydrogen bond acceptor |
| Levulinic acid |  | 116.116 | 1.14 | Hydrogen bond donor |
| Butane-1,4-diol |  | 90.121 | 1.02 | Hydrogen bond donor |
| Glycerol |  | 92.0776 | 1.26 | Hydrogen bond donor |
| Xylitol |  | 152.12 | 0.77 | Hydrogen bond donor |
| Assay | DES | R(S/L) | Extraction Repetitions | Time (min) | R(CoSolvt%) |
|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | |
| 1 | DES1 | 1:15 | 1 | 2 | 40 |
| 2 | DES2 | 1:15 | 1 | 2 | 20 |
| 3 | DES1 | 1:25 | 1 | 2 | 20 |
| 4 | DES2 | 1:25 | 1 | 2 | 40 |
| 5 | DES1 | 1:15 | 5 | 2 | 20 |
| 6 | DES2 | 1:15 | 5 | 2 | 40 |
| 7 | DES1 | 1:25 | 5 | 2 | 40 |
| 8 | DES2 | 1:25 | 5 | 2 | 20 |
| 9 | DES1 | 1:15 | 1 | 6 | 20 |
| 10 | DES2 | 1:15 | 1 | 6 | 40 |
| 11 | DES1 | 1:25 | 1 | 6 | 40 |
| 12 | DES2 | 1:25 | 1 | 6 | 20 |
| 13 | DES1 | 1:15 | 5 | 6 | 40 |
| 14 | DES2 | 1:15 | 5 | 6 | 20 |
| 15 | DES1 | 1:25 | 5 | 6 | 20 |
| 16 | DES2 | 1:25 | 5 | 6 | 40 |
| 17 | DES1/DES2 | 1:20 | 3 | 4 | 30 |
| 18 | DES1/DES2 | 1:20 | 3 | 4 | 30 |
| 19 | DES1/DES2 | 1:20 | 3 | 4 | 30 |
| Assay | R (CoSolvt%) | Extraction Repetitions |
|---|---|---|
| X1 | X2 | |
| 1 | 16 | 2 |
| 2 | 44 | 2 |
| 3 | 16 | 6 |
| 4 | 44 | 6 |
| 5 | 10.3 | 4 |
| 6 | 49.7 | 4 |
| 7 | 30 | 1 |
| 8 | 30 | 7 |
| 9 | 30 | 4 |
| 10 | 30 | 4 |
| 11 | 30 | 4 |
| Assay | Total C3G Content (mg/100g Dry) | Total C3R (mg/100 g Dry) | TAL (mg/100 g Dry) |
|---|---|---|---|
| Y1actual | Y2actual | Y3actual | |
| 1 | 110.85 | 988.37 | 1099.21 |
| 2 | 81.82 | 747.47 | 829.29 |
| 3 | 101.52 | 933.65 | 1035.17 |
| 4 | 101.65 | 913.29 | 1014.93 |
| 5 | 133.67 | 1224.93 | 1358.60 |
| 6 | 116.76 | 1122.11 | 1238.87 |
| 7 | 118.17 | 1092.79 | 1210.96 |
| 8 | 110.04 | 997.73 | 1107.77 |
| 9 | 97.23 | 891.86 | 989.09 |
| 10 | 97.67 | 1059.43 | 1157.09 |
| 11 | 130.74 | 1264.24 | 1394.97 |
| 12 | 111.77 | 1063.85 | 1175.61 |
| 13 | 124.15 | 1174.53 | 1298.68 |
| 14 | 98.54 | 921.33 | 1019.87 |
| 15 | 100.84 | 961.75 | 1062.59 |
| 16 | 77.40 | 769.40 | 846.80 |
| 17 | 92.46 | 891.40 | 983.86 |
| 18 | 94.89 | 924.33 | 1019.22 |
| 19 | 95.94 | 908.96 | 1004.90 |
| Assay | C3G (μg/mL) | C3R (μg/mL) | TAL (μg/mL) | Relative Deviation (%) |
|---|---|---|---|---|
| Y1 | Y2 | Y3 | Y3 | |
| 1 | 167.4752 | 1959.889 | 2127.364 | −3.38 |
| 2 | 142.7458 | 1804.298 | 1947.044 | −12.95 |
| 3 | 175.5200 | 2050.806 | 2226.326 | 20.31 |
| 4 | 182.2589 | 2016.332 | 2198.591 | 19.31 |
| 5 | 160.5043 | 1860.417 | 2020.922 | −6.15 |
| 6 | 165.3153 | 1826.223 | 1991.538 | −7.72 |
| 7 | 84.00578 | 1131.545 | 1215.551 | −27.34 |
| 8 | 156.6489 | 2012.959 | 2169.608 | 29.40 |
| 9 | 179.7469 | 2078.261 | 2258.008 | −0.83 |
| 10 | 186.2938 | 2071.040 | 2257.333 | −0.86 |
| 11 | 172.7000 | 2145.786 | 2318.486 | 1.80 |
| Solvent | T (°C) | T (K) | Kd (min−1) | t1/2 (min) | Ea (kJ·mol−1) |
|---|---|---|---|---|---|
| MeOH | 60 | 333.15 | 0.0002 | 3465.7 | 55.94 |
| 90 | 363.15 | 0.001 | 693.1 | ||
| Ch-Xyl | 60 | 333.15 | 0.00008 | 8664.3 | 115.35 |
| 90 | 363.15 | 0.0025 | 277.3 |
| Solvent | Conditions | Kd (h−1) | t1/2 (h) |
|---|---|---|---|
| MeOH | Light | 0.0015 | 462.1 |
| Dark | 0.0005 | 1286.3 | |
| Ch-Xyl | Light | 0.0008 | 866.4 |
| Dark | 0.0003 | 2310.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vannuchi, N.; Braga, A.R.C.; De Rosso, V.V. High-Performance Extraction Process of Anthocyanins from Jussara (Euterpe edulis) Using Deep Eutectic Solvents. Processes 2022, 10, 615. https://doi.org/10.3390/pr10030615
Vannuchi N, Braga ARC, De Rosso VV. High-Performance Extraction Process of Anthocyanins from Jussara (Euterpe edulis) Using Deep Eutectic Solvents. Processes. 2022; 10(3):615. https://doi.org/10.3390/pr10030615
Chicago/Turabian StyleVannuchi, Nicholas, Anna Rafaela Cavalcate Braga, and Veridiana Vera De Rosso. 2022. "High-Performance Extraction Process of Anthocyanins from Jussara (Euterpe edulis) Using Deep Eutectic Solvents" Processes 10, no. 3: 615. https://doi.org/10.3390/pr10030615