Biosorption of Precious Metals Present at Dilute Concentrations on Fungal Pellets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture of Fungal Pellets
2.2. Determination of the Optimal pH for Ag and Au Biosorption on Fungal Pellets
2.3. Ag and Au Biosorption Kinetics by Fungal Pellets
2.4. Pseudo-Second-Order Kinetic Modeling
2.5. Data Statistical Analysis
3. Results and Discussion
3.1. Determination of the Optimal pH for Biosorption on Aspergillus tabacinus Pellets
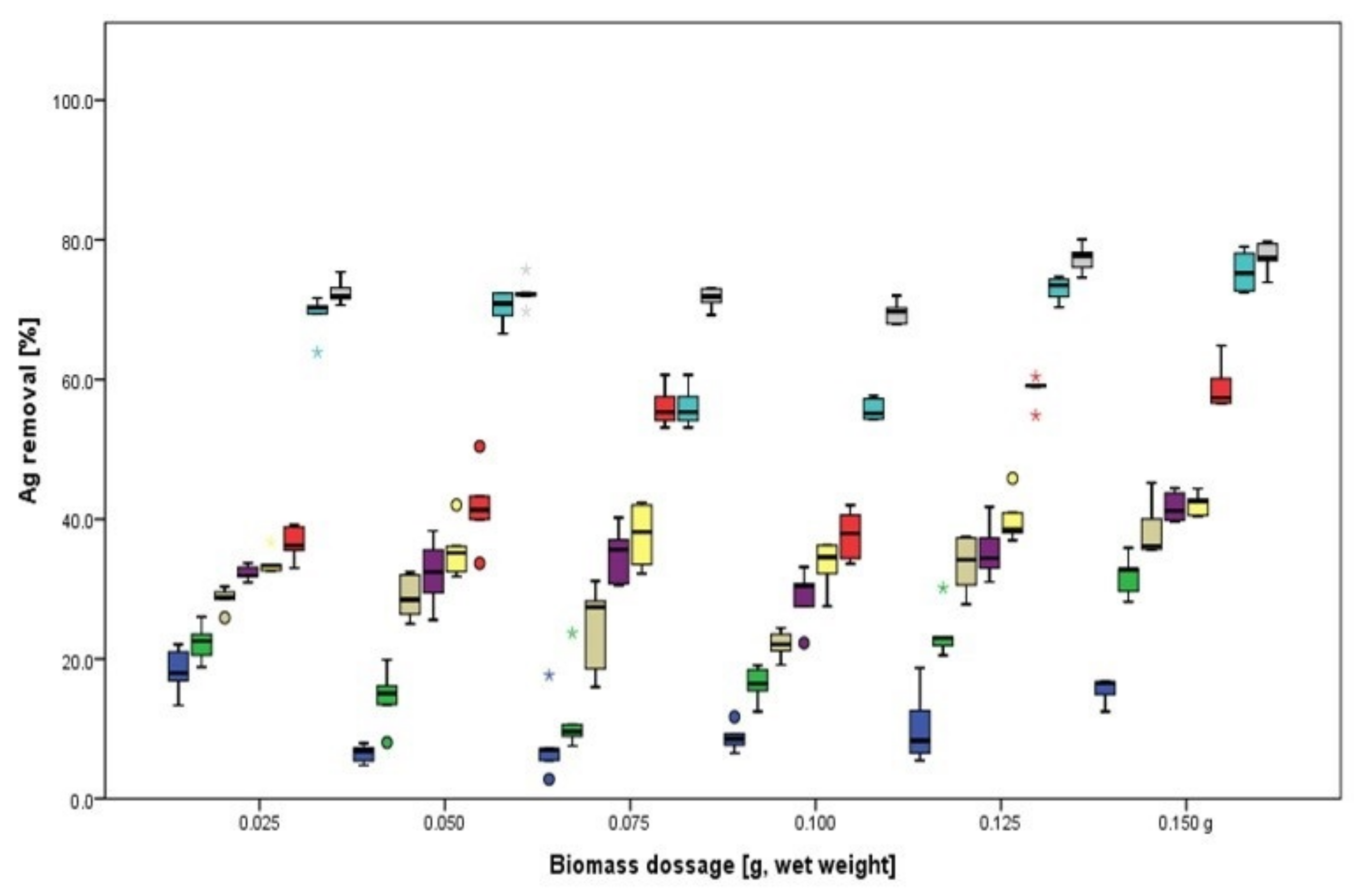
3.1.1. Biosorption of Ag
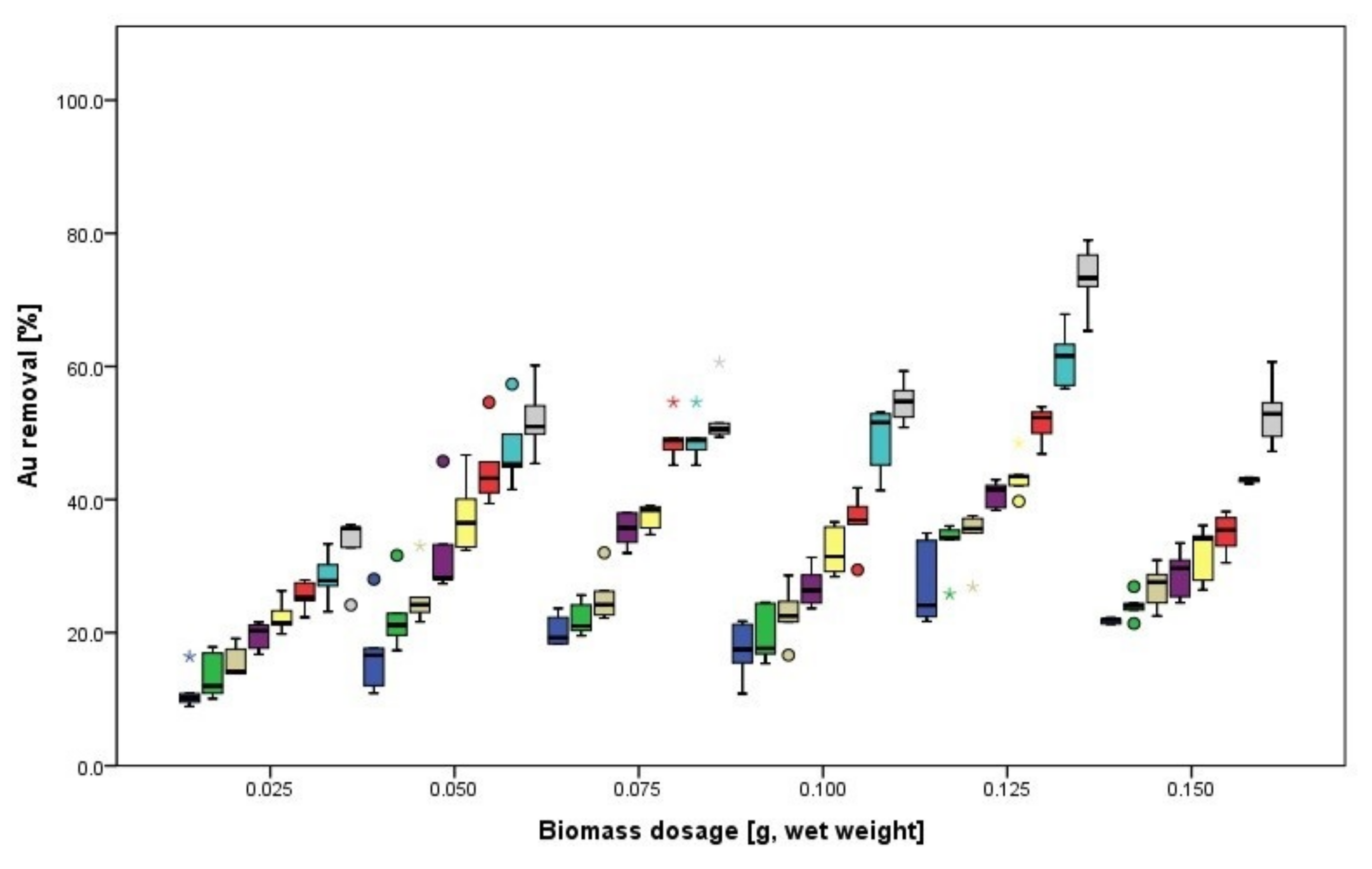
3.1.2. Biosorption of Au
3.2. Determination of the Optimal pH for Biosorption on Cladosporium cladosporioides Pellets
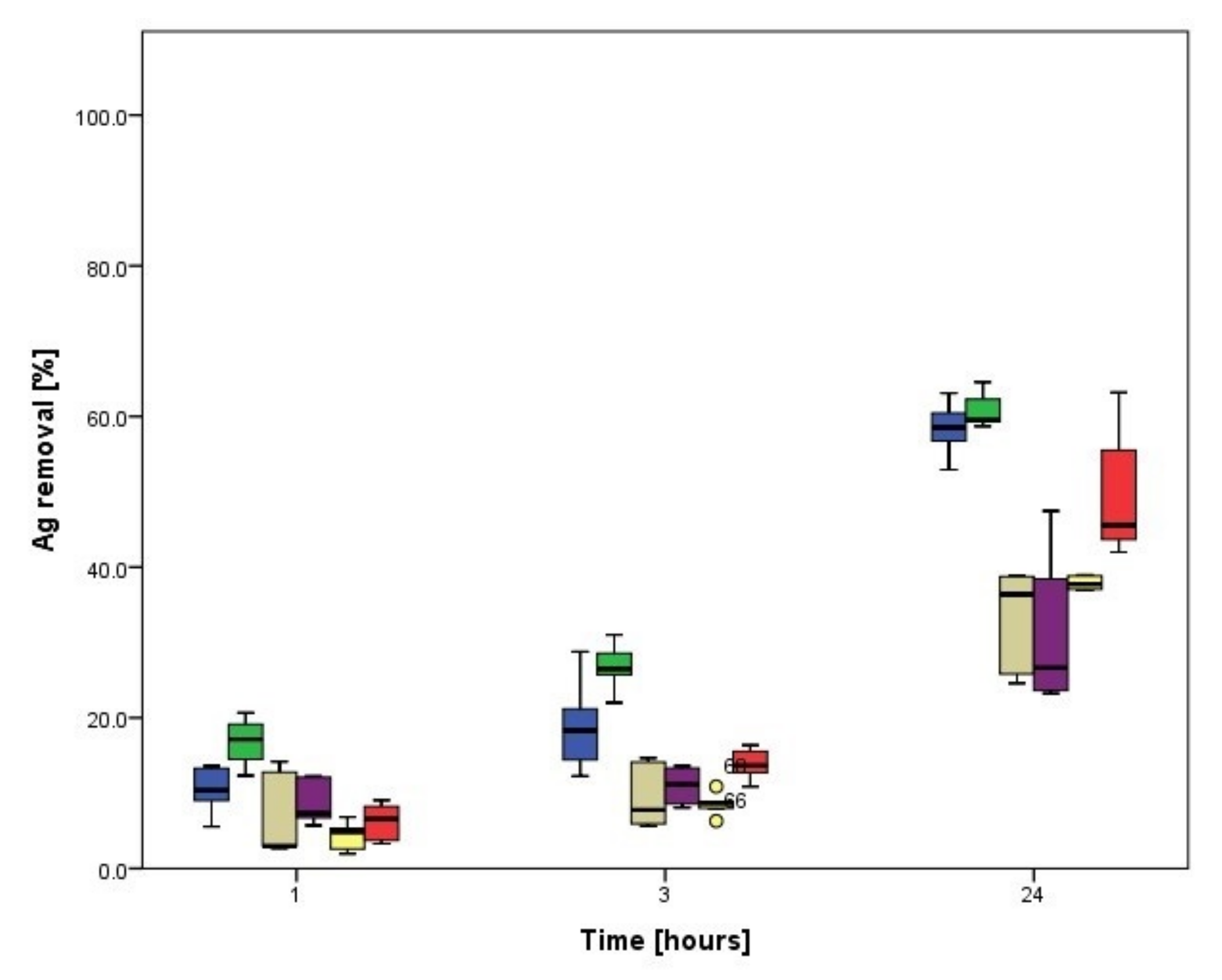
3.2.1. Biosorption of Ag
3.2.2. Biosorption of Au
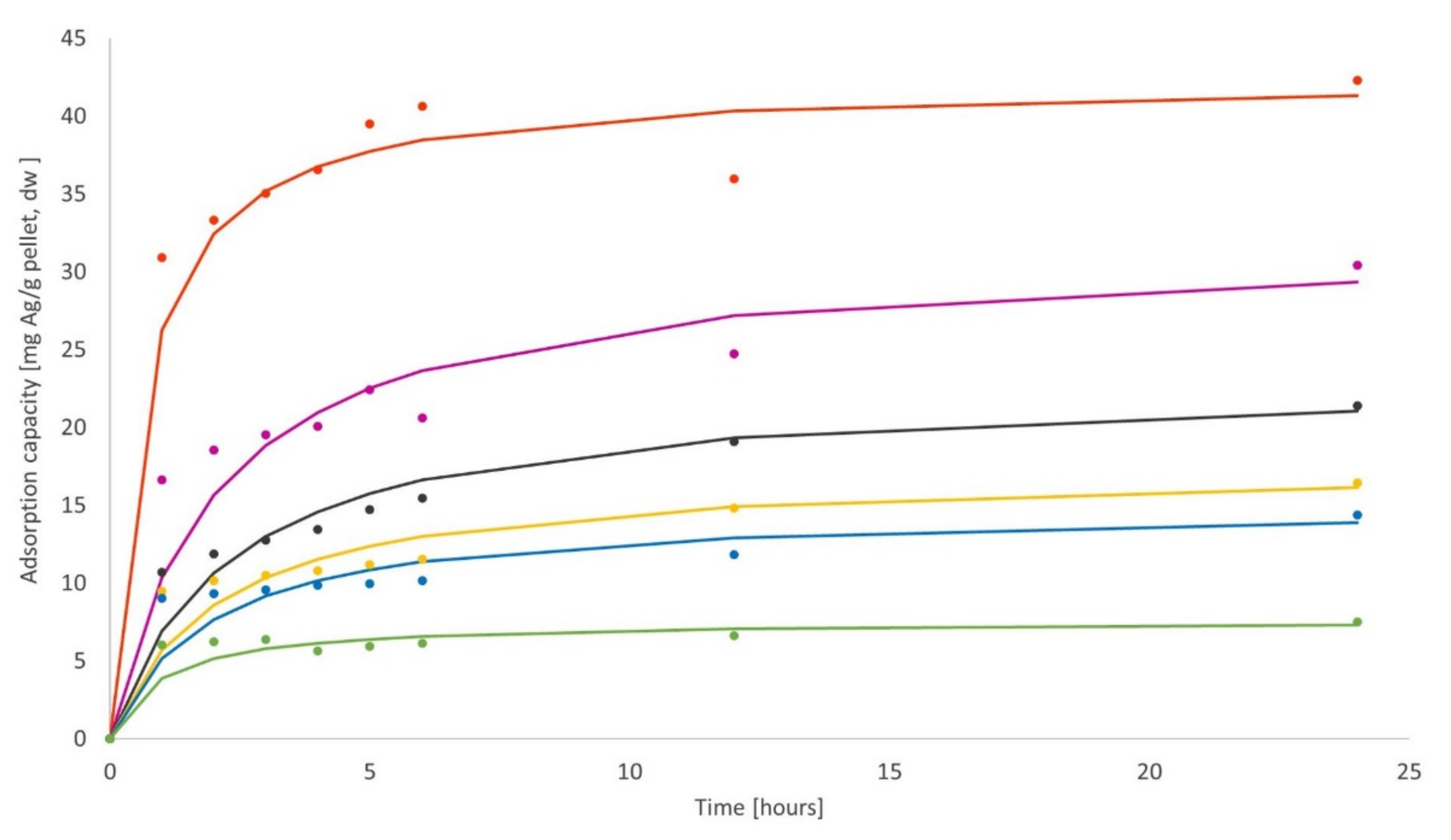
3.3. Biosorption Kinetics on Aspergillus tabacinus Pellets
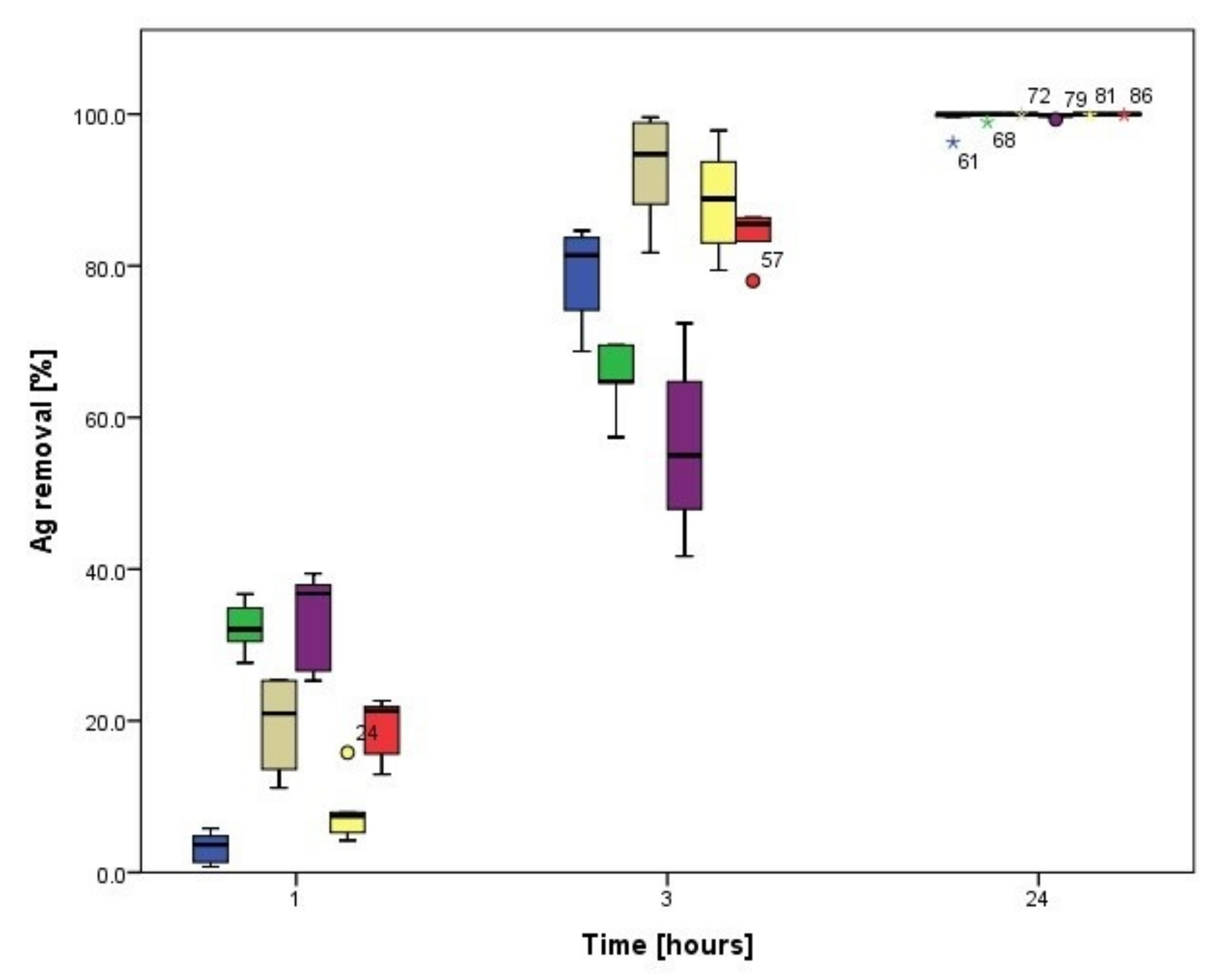
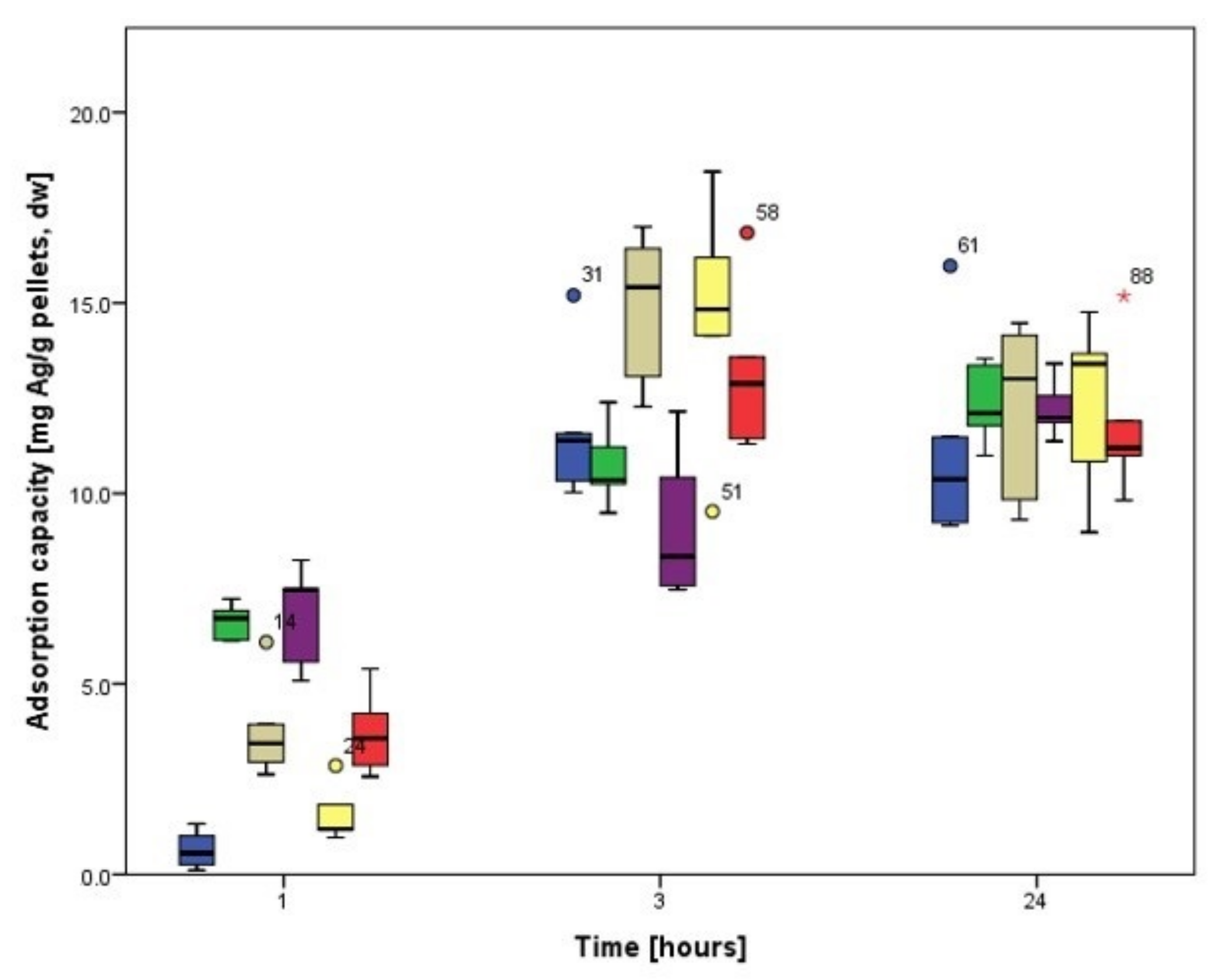
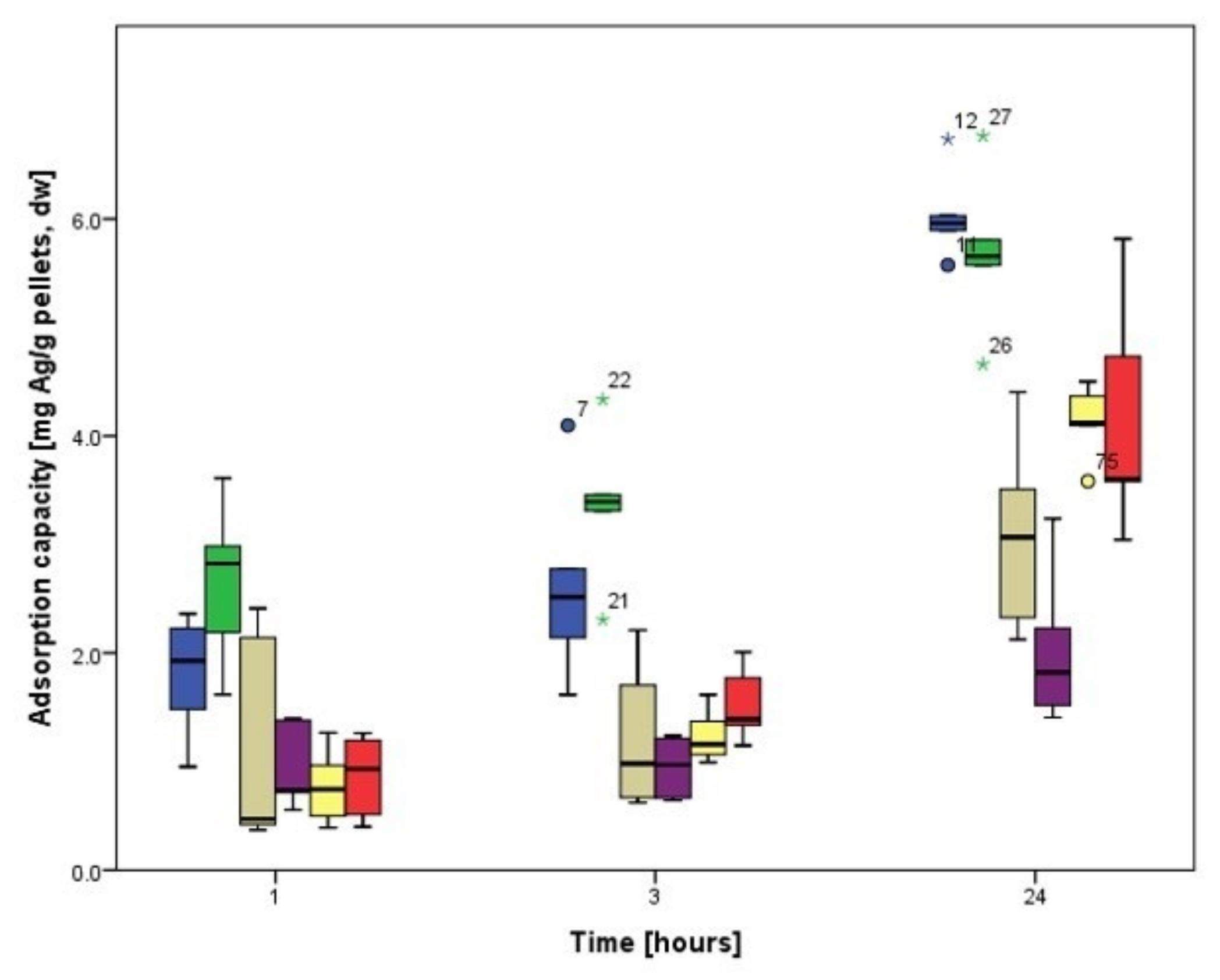
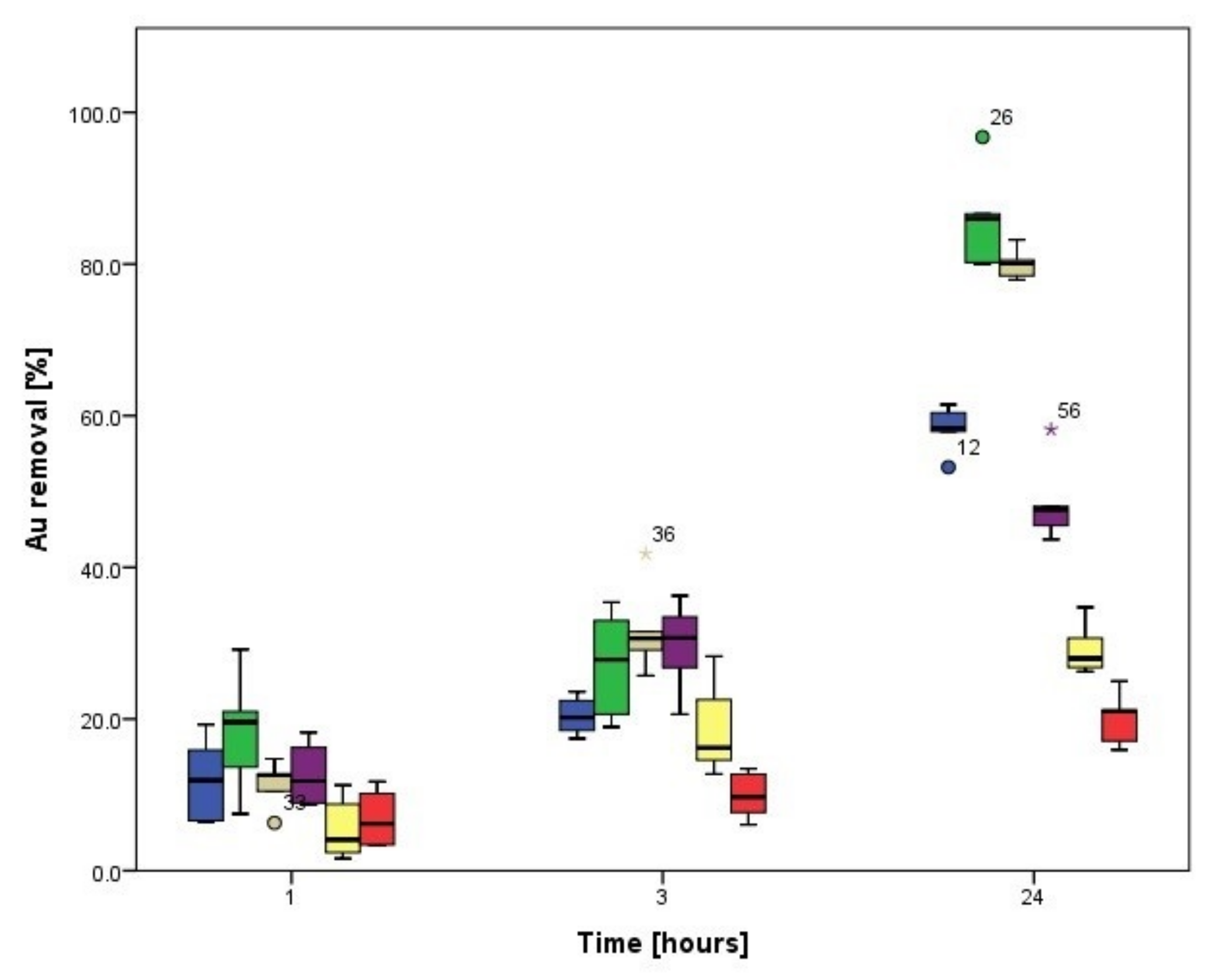
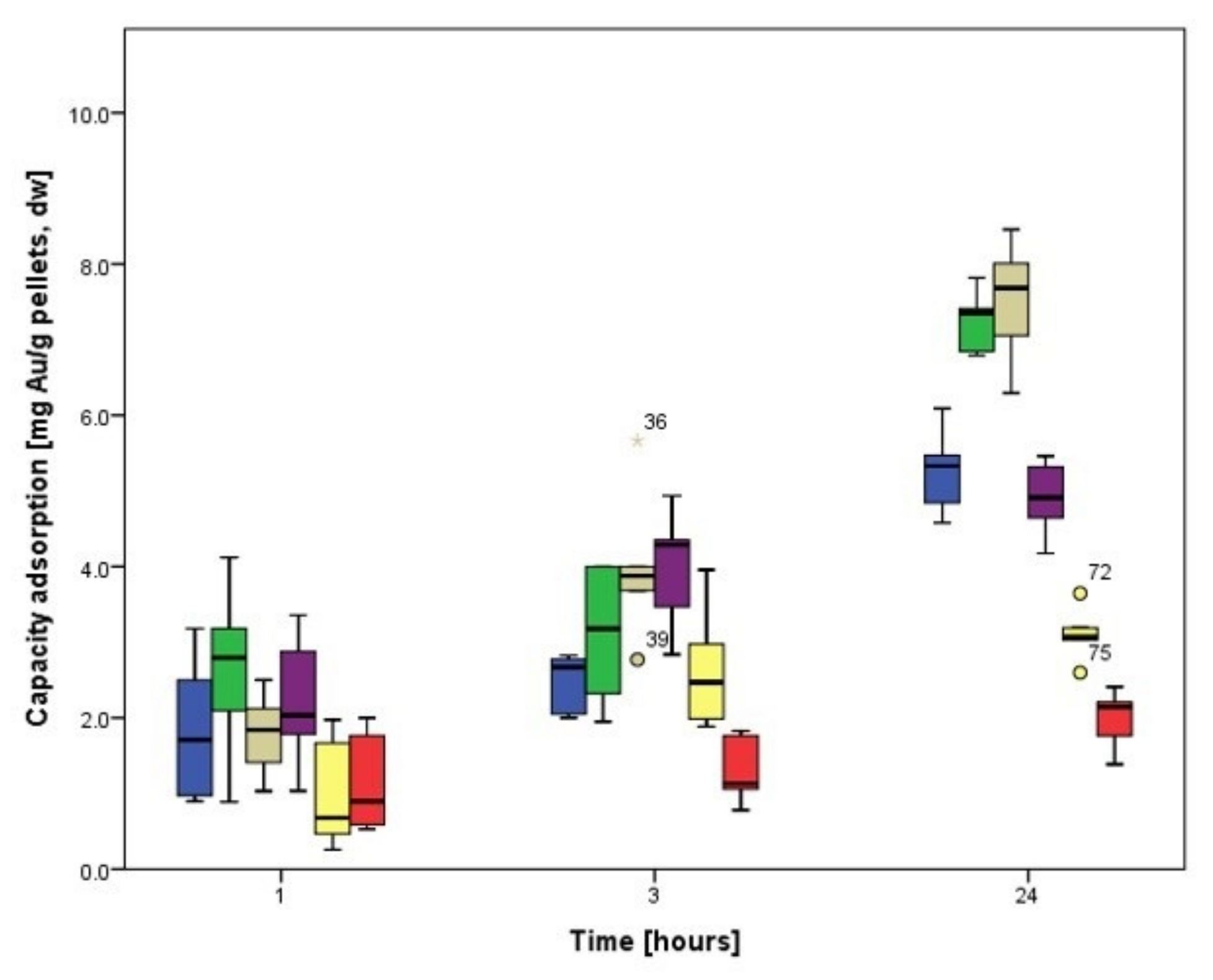
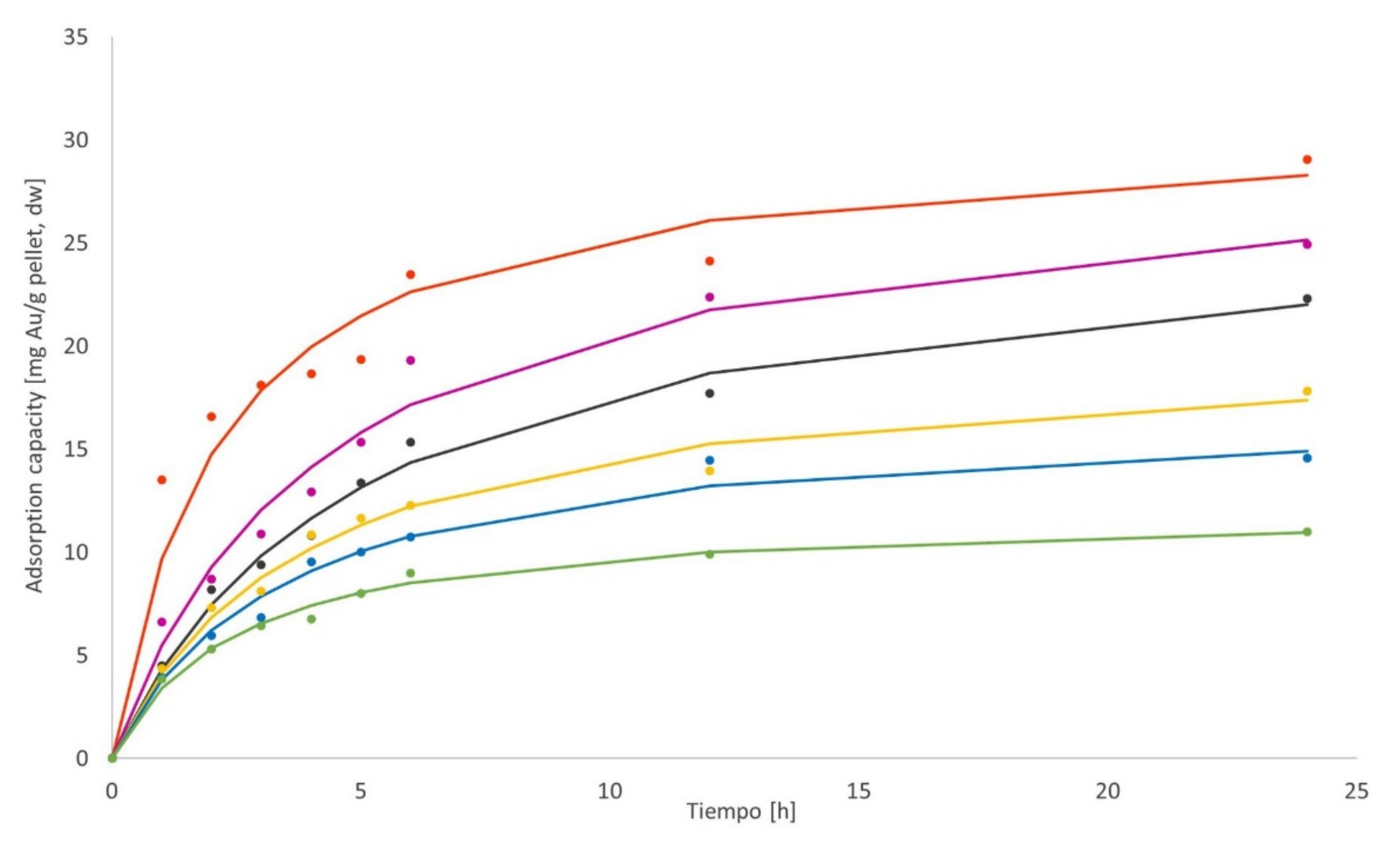
3.3.1. Biosorption Kinetics of Ag
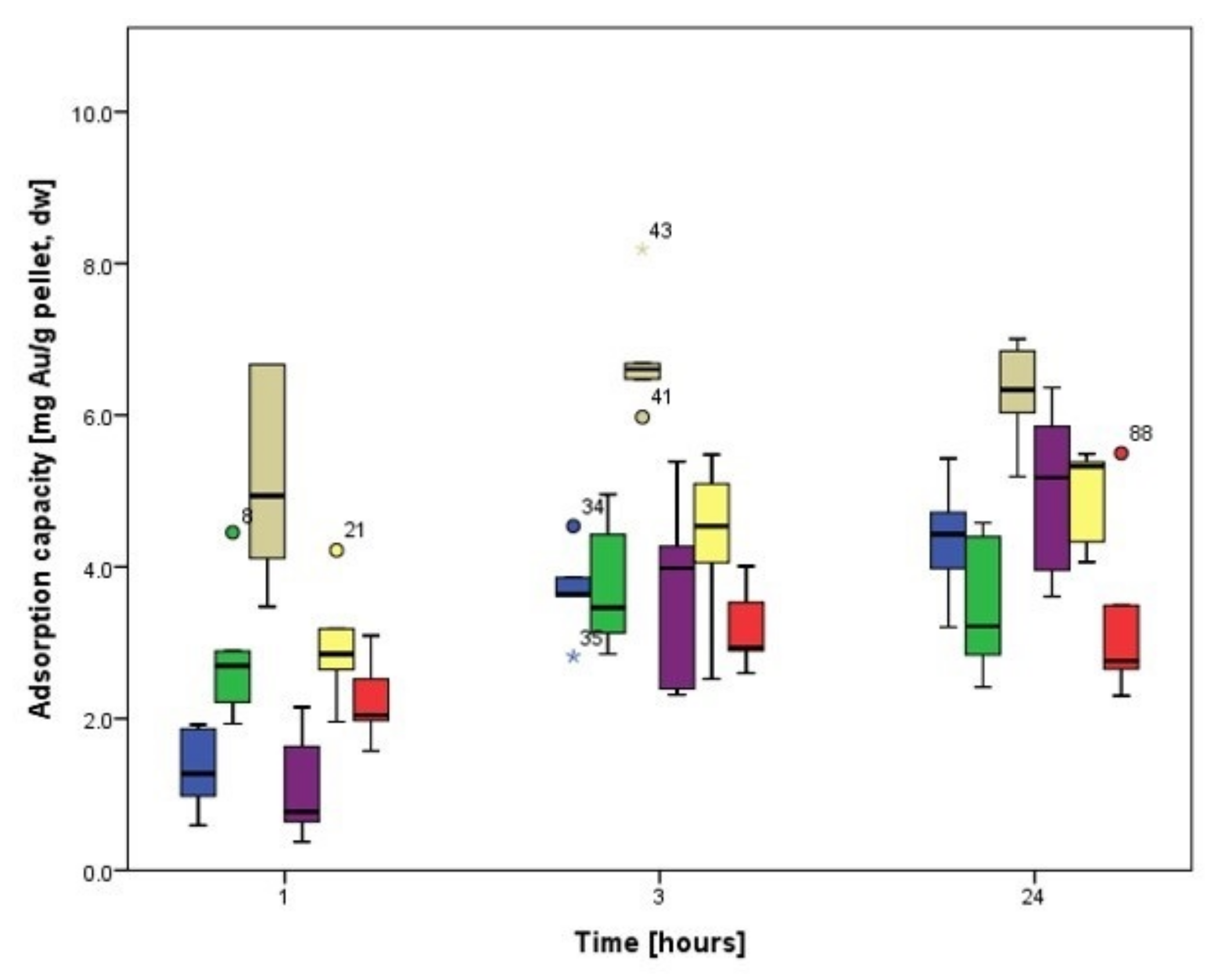
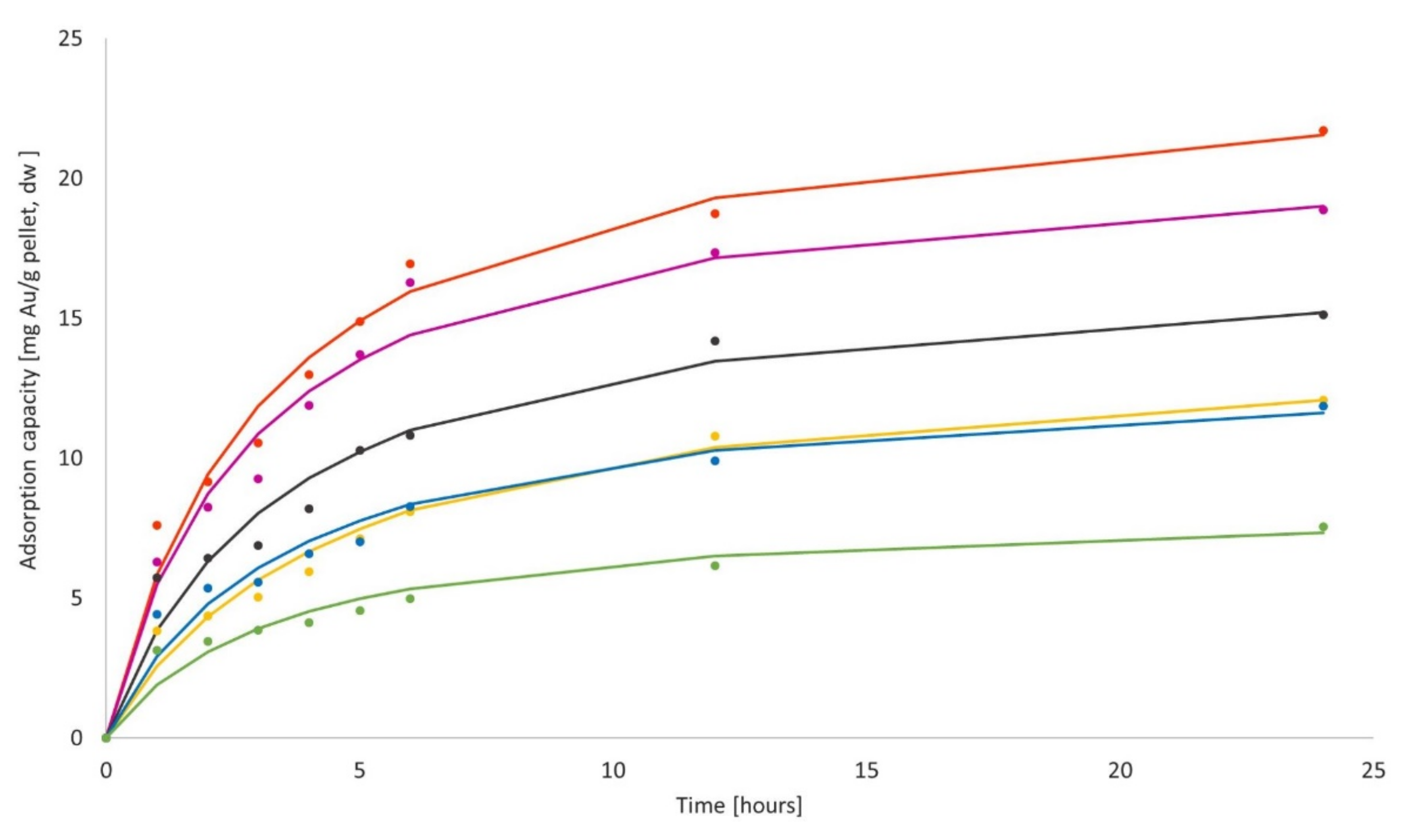
3.3.2. Biosorption Kinetics of Au
3.4. Biosorption Kinetics on Cladosporium cladosporioides Pellets
3.4.1. Biosorption Kinetics of Ag
3.4.2. Biosorption Kinetics of Au
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Legorreta-Castañeda, A.J.; Lucho-Constantino, C.A.; Beltrán-Hernández, R.I.; Coronel-Olivares, C.; Vázquez-Rodríguez, G.A. Biosorption of water pollutants by fungal pellets. Water 2020, 12, 1155. [Google Scholar] [CrossRef] [Green Version]
- Manguilimotan, L.C.; Bitacura, J.G. Biosorption of cadmium by filamentous fungi isolated from coastal water and sediments. J. Toxicol. 2018, 2018, 7170510. [Google Scholar] [CrossRef]
- Lo, Y.C.; Cheng, C.L.; Han, Y.L.; Chen, B.Y.; Chang, J.S. Recovery of high-value metals from geothermal sites by biosorption and bioaccumulation. Bioresour. Technol. 2014, 160, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Rozman, U.; Kalčíková, G.; Marolt, G.; Skalar, T.; Gotvajn, A.Ž. Potential of waste fungal biomass for lead and cadmium removal: Characterization, biosorption kinetic and isotherm studies. Environ. Technol. Innov. 2020, 18, 100742. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Adeniyi, A.G. Statistical modelling and optimisation of the biosorption of Cd (II) and Pb (II) onto dead biomass of Pseudomonas aeruginosa. Chem. Prod. Proc. Model. 2021, 16, 20190139. [Google Scholar] [CrossRef]
- Cox, P.W.; Paul, G.C.; Thomas, C.R. Image analysis of the morphology of filamentous micro-organisms. Microbiology 1998, 144, 817–827. [Google Scholar] [CrossRef] [Green Version]
- Veiter, L.; Rajamanickam, V.; Herwig, C. The filamentous fungal pellet-relationship between morphology and productivity. Appl. Microbiol. Biotechnol. 2018, 102, 2997–3006. [Google Scholar] [CrossRef] [Green Version]
- Espinosa-Ortiz, E.J.; Rene, E.R.; Pakshirajan, K.; van Hullebusch, E.D.; Lens, P.N. Fungal pelleted reactors in wastewater treatment: Applications and perspectives. Chem. Eng. J. 2016, 283, 553–571. [Google Scholar] [CrossRef]
- Shen, N.; Chirwa, E.M. Live and lyophilized fungi-algae pellets as novel biosorbents for gold recovery: Critical parameters, isotherm, kinetics and regeneration studies. Bioresour. Technol. 2020, 306, 123041. [Google Scholar] [CrossRef]
- Tyupa, D.V.; Kalenov, S.V.; Baurina, M.M.; Kabanov, O.V.; Skladnev, D.A.; Kuznetsov, A.Y. Optimization of silver biosorption by fungi forming granules from aqueous solutions of silver nitrate. Clean Technol. Environ. Policy 2017, 19, 53–62. [Google Scholar] [CrossRef]
- Bindschedler, S.; Bouquet, T.Q.T.V.; Job, D.; Joseph, E.; Junier, P. Fungal biorecovery of gold from e-waste. Adv. Appl. Microbiol. 2017, 99, 53–81. [Google Scholar] [PubMed]
- Fashola, M.O.; Ngole-Jeme, V.M.; Babalola, O.O. Heavy metal pollution from gold mines: Environmental effects and bacterial strategies for resistance. Int. J. Environ. Res. Public Health 2016, 13, 1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed, S. Recovery of gold from secondary sources—A review. Hydrometallurgy 2012, 115, 30–51. [Google Scholar] [CrossRef]
- Islam, M.A.; Jacob, M.V.; Antunes, E. A critical review on silver nanoparticles: From synthesis and applications to its mitigation through low-cost adsorption by biochar. J. Environ. Manag. 2021, 281, 111918. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Jabeen, F.; Qureshi, N.A.; Asghar, M.S.; Shakeel, M.; Noureen, A. Toxicity of silver nanoparticles in fish: A critical review. J. Biodivers. Environ. Sci. 2015, 6, 211–227. [Google Scholar]
- Maeda, I.; Tsuruta, T. Microbial Gold Biosorption and Biomineralization from Aqueous HAuCl4 Solution. Minerals 2020, 10, 285. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.W.; Iizuka, A.; Shibata, E. Gold recovery from dilute aqueous solution by a biosorbent derived from woody biomass. Chem. Eng. Commun. 2021, 208, 1711–1724. [Google Scholar] [CrossRef]
- Das, N. Recovery of precious metals through biosorption—A review. Hydrometallurgy 2010, 103, 180–189. [Google Scholar] [CrossRef]
- Soundararajan, R.K.V.; Varjani, S.; Chandrasekaran, R.; Kandasamy, D. Recovery of Gold and Other Precious Metals by Biosorption. In Sustainable Resource Management; Guo, W., Ngo, H.H., Surampalli, R.Y., Zhang, T.C., Eds.; John Wiley & Sons: Weinheim, Germany, 2021; Volume 2, pp. 463–488. [Google Scholar]
- Legorreta-Castañeda, A.J. Biominería de Au y Ag por Gránulos Fúngicos en Efluentes de la Industria Minera. Ph.D. Thesis, Mineral de la Reforma, Universidad Autónoma del Estado de Hidalgo, Pachuca de Soto, Mexico, 2021. (In Spanish). [Google Scholar]
- Stavart, A.; Leroy, C.; Van Lierde, A. Potential use of carbon felt in gold hydrometallurgy. Miner. Eng. 1999, 12, 545–558. [Google Scholar] [CrossRef]
- Reyes-Cruz, V.; Oropeza, M.T.; González, I.; Ponce-De-León, C. Electrochemical recovery of silver from cyanide leaching solutions. J. Appl. Electrochem. 2002, 32, 473–479. [Google Scholar] [CrossRef]
- Galvin, K.P.; Nicol, S.K.; Waters, A.G. Selective ion flotation of gold. Colloids Surf. 1992, 64, 21–33. [Google Scholar] [CrossRef]
- Galvin, K.P.; Engel, M.D.; Nicol, S.K. The potential for reagent recycle in the ion flotation of gold cyanide—A pilot scale field trial. Int. J. Miner. Proc. 1994, 42, 75–98. [Google Scholar] [CrossRef]
- Ting, Y.P.; Mittal, A.K. Effect of pH on the biosorption of gold by a fungal biosorbent. Resour. Environ. Biotechnol. 2002, 3, 229–239. [Google Scholar]
- Bayramoğlu, G.; Arica, M.Y. Removal of heavy mercury (II), cadmium (II) and zinc (II) metal ions by live and heat inactivated Lentinus edodes pellets. Chem. Eng. J. 2008, 143, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, C. Biosorbent for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Filipović-Kovačević, Ž.; Sipos, L.; Briški, F. Biosorption of chromium, copper, nickel and zinc onto fungal pellets of Aspergillus niger 405 from aqueous solutions. Food Technol. Biotechnol. 2000, 38, 211–216. [Google Scholar]
- García-Reyes, M. Efecto del pH en la Generación, Producción y Morfología de los Gránulos de Cladosporium cladosporioides Crecidos en SUSTRATOs de Opuntia sp. y Caldo Papa Dextrosa (PDB). Bachelor’s Thesis, Universidad Politécnica de Pachuca, Zempoala, Mexico, 2016. (In Spanish). [Google Scholar]
- Singh, K.; Nizam, S.; Sinha, M.; Verma, P.K. Comparative transcriptome analysis of the necrotrophic fungus Ascochyta rabiei during oxidate stress: Insight for fungal survival in the host plant. PLoS ONE 2012, 7, e33128. [Google Scholar]
- Bujdák, J. Adsorption kinetics models in clay systems. The critical analysis of pseudo-second order mechanism. Appl. Clay Sci. 2020, 191, 105630. [Google Scholar] [CrossRef]
- Kumar, K.V. Linear and non-linear regression analysis for the sorption kinetics of methylene blue onto activated carbon. J. Hazard. Mater. 2006, 137, 1538–1544. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Vigdorovich, V.I.; Vigdorowitsch, M.V.; Tsygankova, L.E.; Uryadnikova, M.N.; Shel, N.V. A lean method for determination of dynamic capacity of a sorbent at multiple sorption of cations. Int. J. Environ. Sci. Technol. 2019, 16, 6227–6236. [Google Scholar] [CrossRef]
- Foster, G.C.; Lane, D.; Scott, D.; Hebl, M.; Guerra, R.; Osherson, D.; Zimmer, H. Post-hoc tests. In An Introduction to Psychological Statistics; University of Missouri’s Affordable and Open Access Educational Resources Initiative: Houston, TX, USA, 2021. [Google Scholar]
- Zabala, Y.; Pomares-Alfonso, M.S.; Govin, A.; Villanueva-Tagle, M.E. Study of Ag (I) sorption on biomass of Aspergillus niger O-5 chemically treated with cetyltrimethylammonium bromide. Afinidad 2020, 77, 31–41. [Google Scholar]
- Strizkho, L.S.; Zakharova, V.I.; Bezrukova, Z.N. Extraction of silver from the diluted solutions by biosorbents. Nonferrous Metal. 2006, 2, 32–40. [Google Scholar]
- Tuzen, M.; Soylak, M. Column solid-phase extraction of nickel and silver in environmental samples prior to their flame atomic absorption spectrometric determinations. J. Hazard. Mater. 2009, 164, 1428–1432. [Google Scholar] [CrossRef]
- Rakhila, Y.; Elmchaouri, A.; Mestari, A.; Korili, S.; Abouri, M.; Gil, A. Adsorption recovery of Ag (I) and Au (III) from an electronics industry wastewater on a clay mineral composite. Int. J. Miner. Metall. Mater. 2019, 26, 673–680. [Google Scholar] [CrossRef]
- Li, L.; Liang, T.; Liu, W.; Liu, Y.; Ma, F.A. Comprehensive Review of the Mycelial Pellet: Research Status, Applications, and Future Prospects. Ind. Eng. Chem. Res. 2020, 59, 16911–16922. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Mahadevan, A.; Vijayaraghavan, K.; Pavagadhi, S.; Balasubramanian, R. Green recovery of gold through biosorption, biocrystallization, and pyro-crystallization. Ind. Eng. Chem. Res. 2010, 49, 7129–7135. [Google Scholar] [CrossRef]
- Colica, G.; Caparrotta, S.; Bertini, G.; De Philippis, R. Gold biosorption by exopolysaccharide producing cyanobacteria and purple nonsulphur bacteria. J. Appl. Microbiol. 2012, 113, 1380–1388. [Google Scholar] [CrossRef]
- Pethkar, A.V.; Paknikar, K.M. Recovery of gold from solutions using Cladosporium cladosporioides biomass beads. J. Biotechnol. 1998, 63, 121–136. [Google Scholar] [CrossRef]
- Figueroa, G.; Valenzuela, J.; Parga, J.R.; Vázquez, V.; Valenzuela, A. Recovery of gold and silver and removal of copper, zinc and lead ions in pregnant and barren cyanide solutions. Mater. Sci. Appl. 2015, 6, 171. [Google Scholar] [CrossRef] [Green Version]
- Low, B.T.; Ting, Y.P.; Deng, S. Surface modification of Penicillium chrysogenum mycelium for enhanced anionic dye removal. Chem. Eng. J. 2008, 141, 9–17. [Google Scholar] [CrossRef]
- Tsuruta, T. Biosorption and recycling of gold using various microorganisms. J. Gen. Appl. Microbiol. 2004, 50, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, N.; Chirwa, E.M.N. Biosorption and desorption potential of gold (III) by freshwater microalgae Scenedesmus obliquus AS-6-1. Chem. Eng. Trans. 2018, 65, 19–24. [Google Scholar]
- Singleton, I.; Simmons, P. Factors affecting silver biosorption by an industrial strain of Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 1996, 65, 21–28. [Google Scholar] [CrossRef]
- Pethkar, A.V.; Kulkarni, S.K.; Paknikar, K.M. Comparative studies on metal biosorption by two strains of Cladosporium cladosporioides. Bioresour. Technol. 2001, 80, 211–215. [Google Scholar] [CrossRef]
- Akthar, M.N.; Sastry, K.S.; Mohan, P.M. Biosorption of silver ions by processed Aspergillus niger biomass. Biotechnol. Lett. 1995, 17, 551–556. [Google Scholar] [CrossRef]
- Han, Y.L.; Wu, J.H.; Cheng, C.L.; Nagarajan, D.; Lee, C.R.; Li, Y.H.; Chang, J.S. Recovery of gold from industrial wastewater by extracellular proteins obtained from a thermophilic bacterium Tepidimonas fonticaldi AT-A2. Bioresour. Technol. 2017, 239, 160–170. [Google Scholar] [CrossRef]
- Vázquez-Rodríguez, G.A.; Mailly, J.; Stoquart, C.; Barbeau, B. Adsorption capacity of biological powdered activated carbon in a hybrid membrane process for drinking water treatment. In Proceedings of the II AMIDIQ International Conference, San José del Cabo, Mexico, 1–4 May 2012. [Google Scholar]
- Ayele, L.; Pérez, E.; Mayoral, Á.; Chebude, Y.; Díaz, I. Synthesis of zeolite A using raw kaolin from Ethiopia and its application in removal of Cr (III) from tannery wastewater. J. Chem. Technol. Biotechnol. 2018, 93, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Das, D.; Das, N.; Mathew, L. Kinetics, equilibrium and thermodynamic studies on biosorption of Ag (I) from aqueous solution by macrofungus Pleurotus platypus. J. Hazard. Mater. 2010, 184, 765–774. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, D.; Xie, H.; Won, S.W.; Cui, L.; Wu, G. Adsorption of Ag (I) from aqueous solution by waste yeast: Kinetic, equilibrium and mechanism studies. Bioproc. Biosyst. Eng. 2015, 38, 69–77. [Google Scholar] [CrossRef]
- do Nascimento, J.M.; Cruz, N.D.; de Oliveira, G.R.; Sa, W.S.; de Oliveira, J.D.; Ribeiro, P.R.S.; Leite, S.G. Evaluation of the kinetics of gold biosorption processes and consequent biogenic synthesis of AuNPs mediated by the fungus Trichoderma harzianum. Environ. Technol. Innov. 2021, 21, 101238. [Google Scholar] [CrossRef]





| Metal | Biosorbent | Amount of Metal Adsorbed (mg/g) | Ci * (mg/L) |
|---|---|---|---|
| Ag | Chemically-modified chitosan resin | 226.5 | 647.2 |
| Ag | Dried and ground Cladosporium cladosporioides pellets | 13.3–42.6 | 107.9 |
| Ag | Dried Bacillus cereus | 91.75 | 203.5 |
| Au | Chemically-modified chitosan resin | 709.1 | 1181 |
| Au | Dried and ground Cladosporium cladosporioides pellets | 81.1–101 | 197 |
| Au | Dried and ground Sargassum natans | 420 | 8.5–1000 |
| Biomass Quantity (g ww *) | Biomass Quantity (g dw *) | qe (mg/g dw) | k (g dw/mg·min) | r2 |
|---|---|---|---|---|
| 0.025 | 0.0019 | 42.373 | 0.0384 | 0.9929 |
| 0.050 | 0.0035 | 31.847 | 0.0151 | 0.9851 |
| 0.075 | 0.0050 | 23.095 | 0.0185 | 0.9933 |
| 0.100 | 0.0065 | 17.513 | 0.0275 | 0.9903 |
| 0.125 | 0.0076 | 14.970 | 0.0351 | 0.9857 |
| 0.150 | 0.0108 | 7.610 | 0.1366 | 0.9938 |
| Biomass Quantity (g ww *) | Biomass Quantity (g dw *) | qe (mg/g dw) | k (g dw/mg·min) | r2 |
|---|---|---|---|---|
| 0.025 | 0.0020 | 24.390 | 0.0129 | 0.9946 |
| 0.050 | 0.0035 | 21.277 | 0.0164 | 0.9937 |
| 0.075 | 0.0050 | 17.422 | 0.0163 | 0.9878 |
| 0.100 | 0.0063 | 14.409 | 0.0150 | 0.9851 |
| 0.125 | 0.0075 | 13.351 | 0.0209 | 0.9880 |
| 0.150 | 0.0101 | 8.375 | 0.0348 | 0.9845 |
| Biomass Quantity (g ww *) | Biomass Quantity (g dw *) | qe (mg/g dw) | k (g dw/mg·min) | r2 |
|---|---|---|---|---|
| 0.025 | 0.0023 | 47.170 | 0.0050 | 0.9837 |
| 0.050 | 0.0036 | 36.101 | 0.0036 | 0.9902 |
| 0.075 | 0.0051 | 26.178 | 0.0057 | 0.9943 |
| 0.100 | 0.0063 | 17.825 | 0.0061 | 0.9947 |
| 0.125 | 0.0077 | 17.123 | 0.0065 | 0.9917 |
| 0.150 | 0.0106 | 11.377 | 0.0122 | 0.9858 |
| Biomass Quantity (g ww *) | Biomass Quantity (g dw *) | qe (mg/g dw) | k (g dw/mg·min) | r2 |
|---|---|---|---|---|
| 0.025 | 0.0025 | 30.864 | 0.0148 | 0.9908 |
| 0.050 | 0.0036 | 29.762 | 0.0076 | 0.9902 |
| 0.075 | 0.0048 | 26.738 | 0.0072 | 0.9934 |
| 0.100 | 0.0062 | 20.243 | 0.0126 | 0.9914 |
| 0.125 | 0.0078 | 17.065 | 0.0167 | 0.9919 |
| 0.150 | 0.0100 | 12.136 | 0.0323 | 0.9980 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legorreta-Castañeda, A.J.; Lucho-Constantino, C.A.; Coronel-Olivares, C.; Beltrán-Hernández, R.I.; Vázquez-Rodríguez, G.A. Biosorption of Precious Metals Present at Dilute Concentrations on Fungal Pellets. Processes 2022, 10, 645. https://doi.org/10.3390/pr10040645
Legorreta-Castañeda AJ, Lucho-Constantino CA, Coronel-Olivares C, Beltrán-Hernández RI, Vázquez-Rodríguez GA. Biosorption of Precious Metals Present at Dilute Concentrations on Fungal Pellets. Processes. 2022; 10(4):645. https://doi.org/10.3390/pr10040645
Chicago/Turabian StyleLegorreta-Castañeda, Adriana Jazmín, Carlos Alexander Lucho-Constantino, Claudia Coronel-Olivares, Rosa Icela Beltrán-Hernández, and Gabriela A. Vázquez-Rodríguez. 2022. "Biosorption of Precious Metals Present at Dilute Concentrations on Fungal Pellets" Processes 10, no. 4: 645. https://doi.org/10.3390/pr10040645
APA StyleLegorreta-Castañeda, A. J., Lucho-Constantino, C. A., Coronel-Olivares, C., Beltrán-Hernández, R. I., & Vázquez-Rodríguez, G. A. (2022). Biosorption of Precious Metals Present at Dilute Concentrations on Fungal Pellets. Processes, 10(4), 645. https://doi.org/10.3390/pr10040645








