Oily Wastewater Treatment: Methods, Challenges, and Trends
Abstract
:1. Introduction
2. Oily Industrial Wastewater
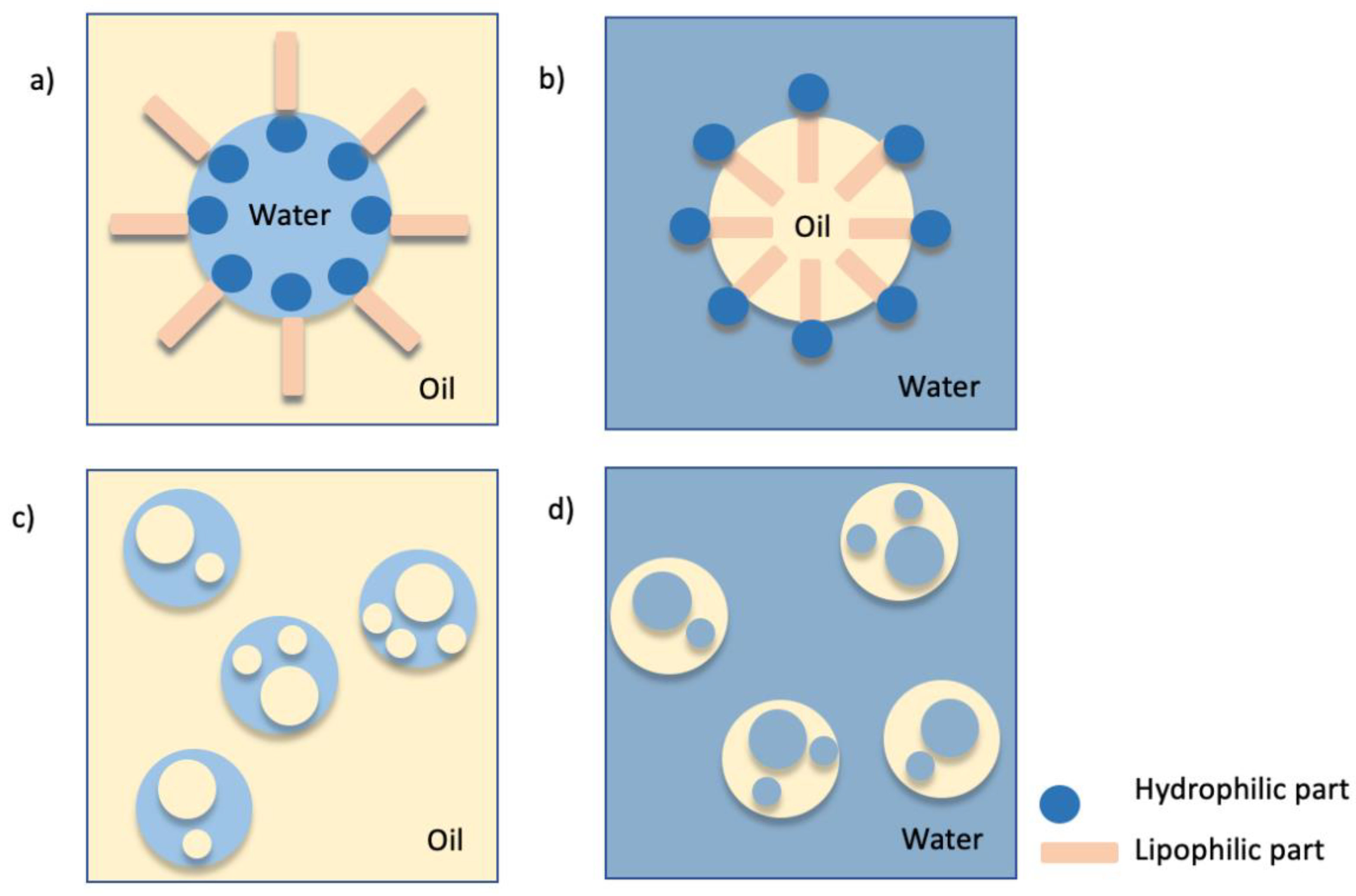
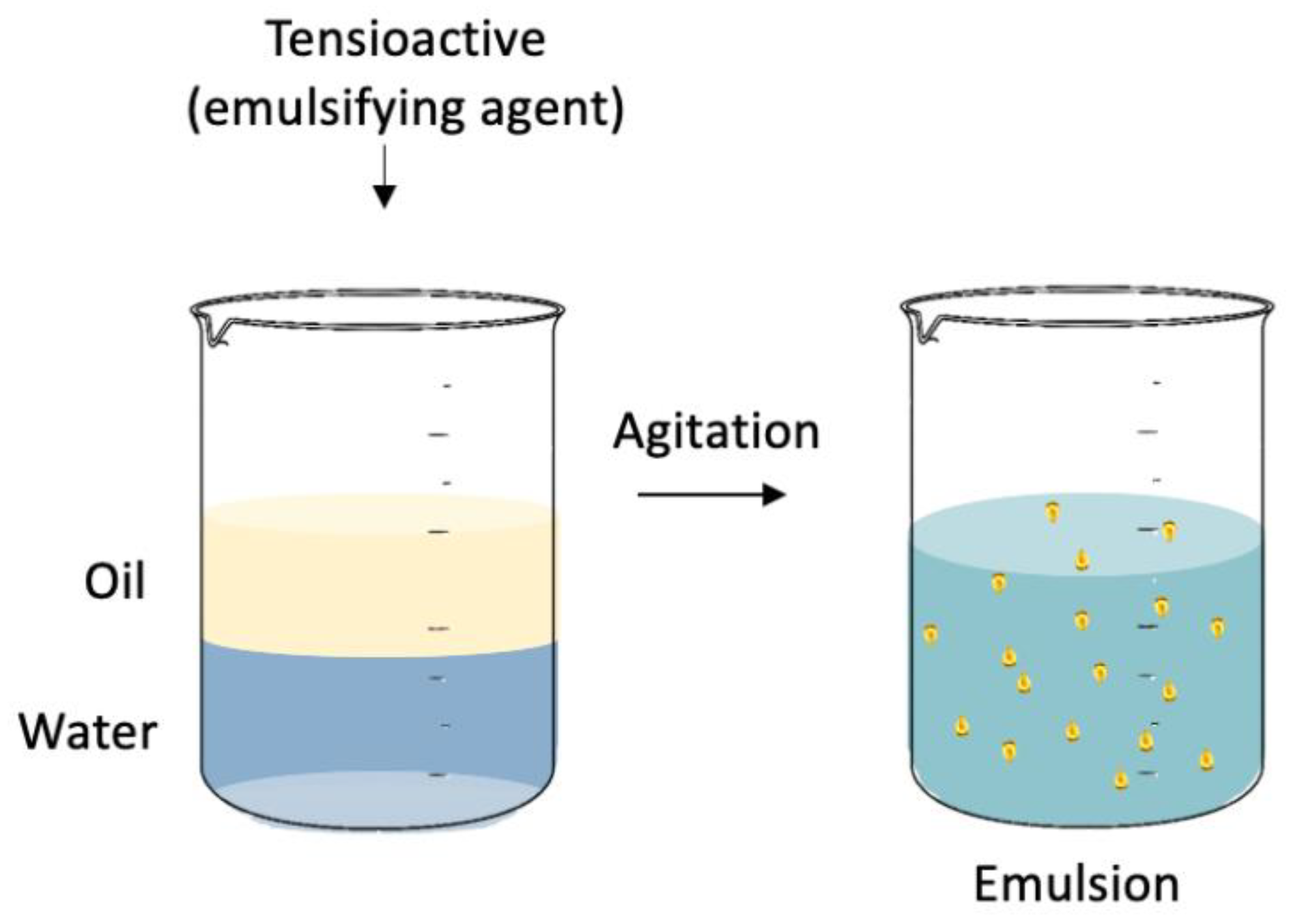
3. Emulsions
Demulsification Methods
4. Conventional Treatment Methods for Oily Wastewater
4.1. Evaporation Separation
4.2. Gravity Separation (Decantation or Settling Down)
4.3. Flotation
4.4. Coagulation (or Flocculation)
4.5. Filtration
4.6. Sorption Cleaning Methods
5. Modern Techniques for Cleaning Oily Wastewater
5.1. Biological Treatment
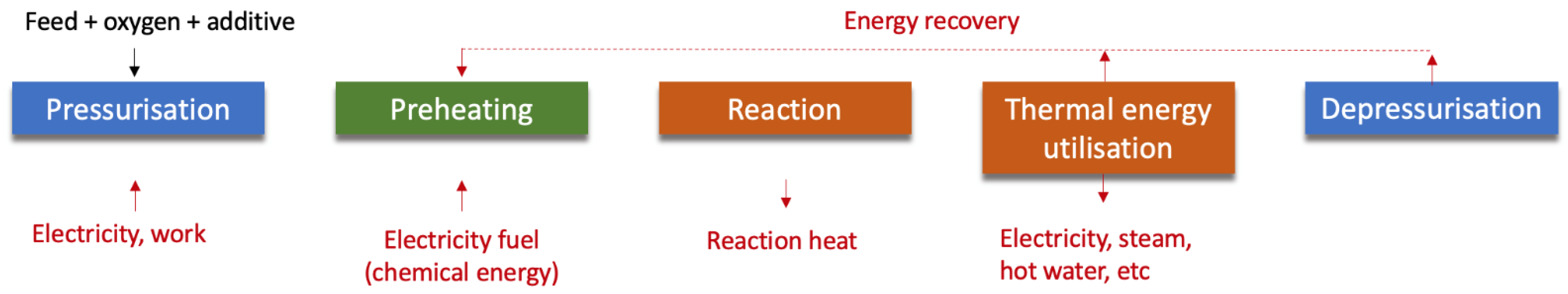
5.2. Advanced Treatment Processes
5.3. Membrane Separation Technology (Polymeric and Ceramic Membranes)
Biotechnological Filter/Strainer
6. Critical Analysis and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rocha e Silva, F.C.P.; Rocha e Silva, N.M.P.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Dissolved air flotation combined to biosurfactants: A clean and efficient alternative to treat industrial oily water. Rev. Environ. Sci. Biotechnol. 2018, 17, 591–602. [Google Scholar] [CrossRef]
- Galdino, C.J.S.; Maia, A.D.M.; Meira, H.M.; Souza, T.C.; Amorim, J.D.P.; Almeida, F.C.G.; Costa, A.F.S.; Sarubbo, L.A. Use of a bacterial cellulose filter for the removal of oil from wastewater. Process Biochem. 2020, 91, 288–296. [Google Scholar] [CrossRef]
- Abuhasel, K.; Kchaou, M.; Alquraish, M.; Munusamy, Y.; Jeng, Y.T. Oily Wastewater Treatment: Overview of Conventional and Modern Methods, Challenges, and Future Opportunities. Water 2021, 13, 980. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Avranas, A. Treatment of oil-in-water emulsions by coagulation and dissolved air flotation. Colloids Surf. A Physicochem. Eng. Asp. 2000, 172, 153–161. [Google Scholar] [CrossRef]
- Demore, J.P. Aspectos Sedimentares do Estuário da Lagoa dos Patos e Sua Interação com a Poluição por Petróleo: Subsídios Para um Plano de Contingência. Monography; Universidade Federal do Rio Grande: Rio Grande, Brazil, 2001. [Google Scholar]
- Njoku, K.L. Responses of accessions of Zea mays to crude oil pollution using growth indices and enzyme activities as markers. Pollution 2017, 4, 183–193. [Google Scholar] [CrossRef]
- Rajasulochana, P.; Preethy, V. Comparison on efficiency of various techniques in treatment of waste and sewage water—A comprehensive review. Resour.-Effic. Technol. 2016, 2, 175–184. [Google Scholar] [CrossRef] [Green Version]
- El-Gawad, H.S.A. Oil and grease removal from industrial wastewater using new utility approach. Adv. Environ. Chem. 2014, 2014, 916878. [Google Scholar] [CrossRef] [Green Version]
- Adetunji, A.I.; Olaniran, A.O. Treatment of industrial oily wastewater by advanced technologies: A review. Appl. Water Sci. 2021, 11, 98. [Google Scholar] [CrossRef]
- Zadymova, N.M.; Skvortsova, Z.N.; Traskine, V.Y.; Kulikov-Kostyushko, F.A.; Kulichikhin, V.G.; Malkin, A.Y. Rheological properties of heavy oil emulsions with different morphologies. J. Pet. Sci. Eng. 2017, 149, 522–530. [Google Scholar] [CrossRef]
- Yu, L.; Han, M.; He, F. A review of treating oily wastewater. Arab. J. Chem. 2017, 10, 1913–1922. [Google Scholar] [CrossRef] [Green Version]
- Putatunda, S.; Bhattacharya, S.; Sen, D.; Bhattacharjee, C. A review on the application of different treatment processes for emulsified oily wastewater. Int. J. Environ. Sci. Technol. 2019, 16, 2525–2536. [Google Scholar] [CrossRef]
- Li, M.; Deng, L.; Tan, Y.; Qi, K.; Tian, X.; Yu, J.; Qin, C.; Cheng, S. Superhydrophobic/Superoleophilic Polyacrylonitrile/Ag Aerogels for the High Efficient Oil/Water Separation and Sensitive Detection of Low-Concentration Oily Sudan Dyes. Adv. Mater. Interfaces 2021, 8, 2002174. [Google Scholar] [CrossRef]
- Chen, G.H.; He, G.H. Separation of water and oil from water-in-oil emulsion by freeze/thaw method. Sep. Purif. Technol. 2003, 31, 83–89. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, J.; Wang, Z.; Zhang, Y. Correlations between emulsification behaviors of crude oil–water systems and crude oil compositions. J. Pet. Sci. Eng. 2016, 146, 1–9. [Google Scholar] [CrossRef]
- Luo, F.; He, L.; He, N. Simulation and experimental study of working characteristics of an improved bioreactor for degrading oily sludge. Process Saf. Environ. Prot. 2021, 147, 1201–1208. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viraraghavan, T. Oil removal from water using biomaterials. Bioresour. Technol. 2010, 101, 6594–6600. [Google Scholar] [CrossRef]
- Lim, J.S.; Wong, S.F.; Law, M.C.; Samyudia, Y.; Dol, S.S. A review on the effects of emulsions on flow behaviours and common factors affecting the stability of emulsions. J. Appl. Sci. 2015, 15, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Khan, B.A.; Akhtar, N.; Khan, H.M.S.; Waseem, K.; Mahmood, T.; Rasul, A.; Iqbal, M.; Khan, H. Basics of pharmaceutical emulsions: A review. Afr. J. Pharm. Pharmacol. 2011, 5, 2715–2725. [Google Scholar] [CrossRef]
- Wong, S.F.; Lim, J.S.; Dol, S.S. Crude oil emulsion: A review on formation, classification and stability of water-in-oil emulsions. J. Pet. Sci. Eng. 2015, 135, 498–504. [Google Scholar] [CrossRef]
- Fingas, M. Water-in-oil emulsion formation: A review of physics and mathematical modelling. Spill Sci. Technol. Bull. 1995, 2, 55–59. [Google Scholar] [CrossRef]
- Karhu, M.; Kuokkanen, V.; Kuokkanen, T.; Ramo, J. Bench scale electrocoagulation studies of bio oil-in-water and synthetic oil-in-water emulsions. Sep. Purif. Technol. 2012, 96, 296–305. [Google Scholar] [CrossRef]
- Shaw, D.J. Introduction to Colloid and Surface Chemistry; Butterworths: London, UK, 1975. [Google Scholar]
- Adrio, J.L.; Demain, A.L. Microbial enzymes: Tools for biotechnological processes. Biomolecules 2014, 1, 117–139. [Google Scholar] [CrossRef] [Green Version]
- Durval, I.J.B.; Resende, A.H.M.; Figueiredo, M.A.; Luna, J.M.; Rufino, R.D.; Sarubbo, L.A. Studies on Biosurfactants Produced using Bacillus cereus Isolated from Seawater with Biotechnological Potential for Marine Oil-Spill Bioremediation. J. Surfactants Deterg. 2018, 22, 349–363. [Google Scholar] [CrossRef]
- Rocha e Silva, N.M.P.; Almeida, F.C.G.; Silva, F.C.P.R.; Luna, J.M.; Sarubbo, L.A. Formulation of a Biodegradable Detergent for Cleaning Oily Residues Generated during Industrial Processes. J. Surfactants Deterg. 2020, 23, 1111–1123. [Google Scholar] [CrossRef]
- Kulik, K.; Trapido, M.; Veressinina, Y.; Munter, R. Treatment of surfactant stabillized oil-in-water emulsions by means of chemical oxidation and coagulation. Environ. Technol. 2007, 28, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Santander, M.; Rodrigues, R.T.; Rubio, J. Modified jet flotation in oil (petroleum) emulsion/water separations. Colloids Surf. A Physicochem. Eng. Asp. 2011, 375, 237–244. [Google Scholar] [CrossRef]
- Zolfaghari, R.; Fakhru’l-Razi, A.; Abdullah, L.C.; Elnashaie, S.S.E.H.; Pendashteh, A. Demulsification techniques of water-in-oil and oil-in-water emulsions in petroleum industry. Sep. Purif. Technol. 2016, 170, 377–407. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Li, X.; Jia, W.; Zhao, Y.; Ren, S. Recyclable magnetic graphene oxide for rapid and efficient demulsification of crude oil-in-water emulsion. Fuel 2017, 189, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Abed, S.M.; Abdurahman, N.H.; Yunus, R.M.; Abdulbari, H.; Akbari, S. Oil emulsions and the different recent demulsification techniques in the petroleum industry—A review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 702, 012060. [Google Scholar] [CrossRef]
- Liang, H.; Esmaeili, H. Application of nanomaterials for demulsification of oily wastewater: A review study. Environ. Technol. Innov. 2021, 22, 101498. [Google Scholar] [CrossRef]
- Daaou, M.; Bendedouch, D. Water pH and surfactant addition effects on the stability of an Algerian crude oil emulsion. J. Saudi Chem. Soc. 2012, 16, 333–337. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Si, P.; Wei, L.; Wang, Y.; Tu, Y.; Zuo, G.; Yu, B.; Zhang, X.; Ye, S. Demulsification of acidic oil-in-water emulsions driven by chitosan loaded Ti3C2Tx. Appl. Surf. Sci. 2019, 476, 878–885. [Google Scholar] [CrossRef]
- Evdokimov, I.N.; Losev, A.P. Microwave treatment of crude oil emulsions: Effects of water content. J. Pet. Sci. Eng. 2014, 115, 24–30. [Google Scholar] [CrossRef]
- Spinelli, L.S.; Aquino, A.S.; Pires, R.V.; Barboza, E.M.; Louvisse, A.M.T.; Lucas, E.F. Influence of polymer bases on the synergistic effects obtained from mixtures of additives in the petroleum industry: Performance and residue formation. J. Pet. Sci. Eng. 2007, 58, 111–118. [Google Scholar] [CrossRef]
- Pérez-Calderón, J.; Santos, M.V.; Zaritzky, N. Optimal clarification of emulsified oily wastewater using a surfactant/chitosan biopolymer. J. Environ. Chem. Eng. 2018, 6, 3808–3818. [Google Scholar] [CrossRef]
- Dao, V.H.; Cameron, N.R.; Saito, K.; Bendoraitiene, J.; Zemaitaitis, A.; Liu, G.; Ye, J.; Cheng, R.; Cheng, R.; Lan, N.T. Synthesis, properties and performance of organic polymers employed in flocculation applications. Polym. Chem. 2016, 7, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Cai, Q.; Zhu, Z.; Chen, B.; Zhang, B. Oil-in-water emulsion breaking marine bacteria for demulsifying oily wastewater. Water. Res. 2019, 149, 292–301. [Google Scholar] [CrossRef]
- Luna, J.M.; Rufino, R.D.; Jara, A.M.A.T.; Brasileiro, P.P.F.; Sarubbo, L.A. Environmental applications of the biosurfactant produced by Candida sphaerica cultivated in low-cost substrates. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 413–418. [Google Scholar] [CrossRef]
- Rocha e Silva, F.C.P.; Roque, B.A.C.; Rocha e Silva, N.M.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Banat, I.M.; Sarubbo, L.A. Yeasts and bacterial biosurfactants as demulsifiers for petroleum derivative in seawater emulsions. AMB Express 2017, 7, 202. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Zu, Y.; Zhu, J.; Jin, M.; Cui, T.; Long, X. Application of biosurfactant surfactin as a pH-switchable biodemulsifier for efficient oil recovery from waste crude oil. Chemosphere 2020, 240, 124946. [Google Scholar] [CrossRef]
- Gidudu, B.; Chirwa, E.M.N. Biosurfactants as demulsification enhancers in bio-electrokinetic remediation of petroleum contaminated soil. Process Saf. Environ. Prot. 2020, 143, 332–339. [Google Scholar] [CrossRef]
- Hou, N.; Wang, Q.; Sun, Y.; Li, X.; Song, Q.; Jiang, X.; Li, B.; Zhao, X.; Zang, H.; Li, D. A novel biodemulsifier of Bacillus mojavensis XH1–Oxalate decarboxylase with the potential for demulsification of oilfield emulsion. J. Hazard. Mater. 2021, 407, 124737. [Google Scholar] [CrossRef]
- Rocha e Silva, N.M.; Meira, H.M.; Almeida, F.C.G.; Silva, R.C.F.S.; Almeida, D.G.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Natural Surfactants and Their Applications for Heavy Oil Removal in Industry. Sep. Purif. Rev. 2018, 48, 267–281. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Luna, J.M.; Rufino, R.D.; Brasileiro, P. Production of a low-cost biosurfactant for application in the remediation of sea water contaminated with petroleum derivates. Chem. Eng. Trans. 2016, 49, 523–528. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Meira, H.M.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Biosurfactant production from Candida lipolytica in bioreactor and evaluation of its toxicity for application as a bioremediation agent. Process Biochem. 2017, 54, 20–27. [Google Scholar] [CrossRef]
- Silva, I.A.; Veras, B.O.; Ribeiro, B.G.; Aguiar, J.S.; Campos Guerra, J.M.; Luna, J.M.; Sarubbo, L.A. Production of cupcake-like dessert containing microbial biosurfactant as an emulsifier. PeerJ 2020, 8, e9064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Sun, N.; Li, D.; Long, S.; Tang, X.; Xiao, G.; Wang, L. Optimization and characterization of biosurfactant production from kitchen waste oil using Pseudomonas aeruginosa. Environ. Sci. Pollut. Res. 2018, 25, 14934–14943. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.C.F.S.; Almeida, D.G.; Brasileiro, P.P.F.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Production, formulation and cost estimation of a commercial biosurfactant. Biodegradation 2018, 30, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Felix, A.K.N.; Martins, J.J.L.; Almeida, J.G.L.; Giro, M.E.A.; Cavalcante, K.F.; Melo, V.M.M.; Pessoa, O.D.L.; Rocha, M.V.P.; Gonçalves, L.R.B.; Aguiar, R.S.S. Purification and characterization of a biosurfactant produced by Bacillus subtilis in cashew apple juice and its application in the remediation of oil-contaminated soil. Colloids Surf. B Biointerfaces 2019, 175, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Markande, A.R.; Patel, D.; Varjani, S. A review on biosurfactants: Properties, applications and current developments. Bioresour. Technol. 2021, 330, 124963. [Google Scholar] [CrossRef]
- Singh, P.; Patil, Y.; Rale, V. Biosurfactant production: Emerging trends and promising strategies. J. Appl. Microbiol. 2019, 126, 2–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bach, H.; Gutnick, D.L. Potential applications of bioemulsifiers in the oil industry. Stud. Surf. Sci. Catal. 2004, 151, 233–281. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Hoek, E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef] [Green Version]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Lehtonen, J.; Chen, X.; Beaumont, M.; Hassinen, J.; Orelma, H.; Dumée, L.F.; Tardy, B.L.; Rojas, O.J. Impact of incubation conditions and post-treatment on the properties of bacterial cellulose membranes for pressure-driven filtration. Carbohydr. Polym. 2021, 251, 117073–117082. [Google Scholar] [CrossRef]
- Hu, X.; Bekassy-Molnar, E.; Vatai, G. Study of ultrafiltration behaviour of emulsified metalworking fluids. Desalination 2002, 149, 191–197. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Zherikova, K.V.; Martynenko, E.A. Molecular versus ionic liquids: Development of a thermodynamic framework for predicting vaporization thermodynamics. J. Mol. Liq. 2022, 350, 118576. [Google Scholar] [CrossRef]
- Aminmahalati, A.; Fazlali, A.; Safikhani, H. Study on the performance and optimization of CO boiler in the oil refinery. Appl. Therm. Eng. 2022, 201, 117790. [Google Scholar] [CrossRef]
- Djimasbe, R.; Varfolomeev, M.A.; Al-Muntaser, A.A.; Yuan, C.; Feoktistov, D.A.; Suwaid, M.A.; Kirgizov, A.J.; Davletshin, R.R.; Zinnatullin, A.L.; Fatou, S.D. Oil dispersed nickel-based catalyst for catalytic upgrading of heavy oil using supercritical water. Fuel 2022, 313, 122702. [Google Scholar] [CrossRef]
- Douglas, L.D.; Rivera-Gonzalez, N.; Cool, N.; Bajpayee, A.; Udayakantha, M.; Liu, G.-W.; Anita; Banerjee, S. A Materials Science Perspective of Midstream Challenges in the Utilization of Heavy Crude Oil. ACS Omega 2022, 7, 1547–1574. [Google Scholar] [CrossRef]
- Azimi, S.C.; Shirini, F.; Pendashteh, A. Treatment of wood industry wastewater by combined coagulation–flocculation–decantation and fenton process. Water Environ. Res. 2020, 93, 433–444. [Google Scholar] [CrossRef]
- Bach, M.; Schoenbrunn, F. Liquid–Solid Separation: De-sandin, flocculation, sedimentation and liquor filtration. In Smelter Grade Alumina Bauxite; Springer: Cham, Switzerland, 2022; pp. 241–267. [Google Scholar] [CrossRef]
- Nigro, A.; Finardi, A.; Ferraro, M.M.; Manno, D.E.; Quattrini, A.; Furlan, R.; Romano, A. Selective loss of microvesicles is a major issue of the differential centrifugation isolation protocols. Sci. Rep. 2021, 11, 3589–3601. [Google Scholar] [CrossRef] [PubMed]
- Casadei, E.; Valli, E.; Panni, F.; Donarski, J.; Gubern, J.F.; Lucci, P.; Conte, L.; Lacoste, F.; Maquet, A.; Brereton, P. Emerging trends in olive oil fraud and possible countermeasures. Food Control. 2021, 124, 107902–107915. [Google Scholar] [CrossRef]
- Rocha e Silva, F.C.P.; Rocha e Silva, N.M.P.; Moura, A.E.; Almeida, R.G.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Sarubbo, L.A. Effect of biosurfactant addition in a pilot scale dissolved air flotation system. Sep. Sci. Technol. 2015, 50, 618–625. [Google Scholar] [CrossRef]
- Menezes, C.T.B.; Barros, E.C.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Replacing Synthetic with Microbial Surfactants as Collectors in the Treatment of Aqueous Effluent Produced by Acid Mine Drainage, Using the Dissolved Air Flotation Technique. Appl. Biochem. Biotechnol. 2011, 163, 540–546. [Google Scholar] [CrossRef]
- Wang, L.K.; Shammas, N.K.; Selke, W.A.; Aulenbach, D.B. Flotation Technology; Humana Press: New York, NY, USA, 2010; Volume 12, p. 680. [Google Scholar]
- Yao, K.; Chi, Y.; Wang, F.; Yan, Y.; Ni, M.; Cen, K. The effect of microbubbles on gas-liquid mass transfer coefficient and degradation rate of COD in wastewater treatment. Water Sci. Technol. 2016, 73, 1969–1977. [Google Scholar] [CrossRef]
- Azevedo, A.; Etchepare, R.; Rubio, J. Raw water clarification by flotation with microbubbles and nanobubbles generated with a multiphase pump. Water Sci. Technol. 2017, 75, 2342–2349. [Google Scholar] [CrossRef]
- Brasileiro, P.P.F.; Silva, R.C.F.S.; Santos, V.A.; Sarubbo, L.A.; Benachour, M. Efficiency of microbubble production using surfactants for the treatment of oily water by flotation. Chem. Eng. Res. Des. 2021, 168, 254–263. [Google Scholar] [CrossRef]
- Wen, X.; Du, C.; Zeng, G.; Huang, D.; Zhang, J.; Yin, L.; Tan, S.; Huang, L.; Chen, H.; Yu, G. A novel biosorbent prepared by immobilized Bacillus licheniformis for lead removal from wastewater. Chemosphere 2018, 200, 173–179. [Google Scholar] [CrossRef]
- Wei, H.; Gao, B.; Ren, J.; Li, A.; Yang, H. Coagulation/flocculation in dewatering of sludge: A review. Water Res. 2018, 143, 608–631. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.L.; Ismail, S.; Bhatia, S. Optimization of Coagulation−Flocculation Process for Palm Oil Mill Effluent Using Response Surface Methodology. Environ. Sci. Technol. 2005, 39, 2828–2834. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.E.; Belian, M.F.; Lima, L.S.G.L.; Galembeck, A.; Alves, A.A. BR 10 2018 009736 9 A2–Filtros à Base de Membrana de Celulose Bacteriana Modificada; INPI: Rio de Janeiro, Brazil, 2018.
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Microfiltration membrane processes: A review of research trends over the past decade. J. Water Process Eng. 2019, 32, 100941. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-based separation for oily wastewater: A practical perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, S.; Shuai, S.; Zhou, Q.; Cheng, Q.; Liu, M.; Gao, C. Color removal and COD reduction of biologically treated textile effluent through submerged filtration using hollow fiber nanofiltration membrane. Desalination 2013, 314, 89–95. [Google Scholar] [CrossRef]
- Mo, X.; Ni, Y.; Liu, F. Preparation of different scale fibrous membranes and their filtration properties. Therm. Sci. 2021, 25, 1453–1459. [Google Scholar] [CrossRef]
- Ritt, C.L.; Stassin, T.; Davenport, D.M.; Duchanois, R.M.; Nulens, I.; Yang, Z.; Ben-Zvi, A.; Segev-Mark, N.; Elimelech, M.; Tang, C.Y. The open membrane database: Synthesis, structure and performance relationships of reverse osmosis membranes. J. Membr. Sci. 2022, 641, 119927. [Google Scholar] [CrossRef]
- Oliveira, L.M.T.M.; Saleem, J.; Bazargan, A.; Duarte, J.L.D.S.; Mckay, G.; Meili, L. Sorption as a rapidly response for oil spill accidents: A material and mechanistic approach. J. Hazard. Mater. 2021, 407, 124842. [Google Scholar] [CrossRef]
- Ravi, Y.; Prasanthi, I.; Behera, S.; Datta, K.K.R. MIL-101(Fe) Networks Supported on Fluorinated Graphene Nanosheets as Coatings for Oil Sorption. ACS Appl. Nano Mater. 2022. [Google Scholar] [CrossRef]
- Torres, C.E.I.; Quezada, T.E.S.; Kharissova, O.V.; Kharisov, B.I.; Lafuente, M.I.G.d. Carbon-based aerogels and xerogels: Synthesis, properties, oil sorption capacities, and dft simulations. J. Environ. Chem. Eng. 2021, 9, 104886. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Adebayo, A.R.; Hossain, M.E. A sustainable approach to controlling oil spills. J. Environ. Manag. 2012, 113, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Gheriany, I.A.E.; Saqa, F.A.E.; Amer, A.A.E.R.; Hussein, M. Oil spill sorption capacity of raw and thermally modified orange peel waste. Alex. Eng. J. 2020, 59, 925–932. [Google Scholar] [CrossRef]
- Paulauskiene, T. Ecologically friendly ways to clean up oil spills in harbor water areas: Crude oil and diesel sorption behavior of natural sorbents. Environ. Sci. Pollut. Res. 2018, 25, 9981–9991. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.; Joshi, R.; Srivastava, V.K.; Ngo, H.H.; Guo, W. Treatment of wastewater from petroleum industry: Current practices and perspectives. Environ. Sci. Pollut. Res. 2019, 27, 27172–27180. [Google Scholar] [CrossRef]
- Li, J.; Sun, S.; Yan, P.; Fang, L.; Yu, Y.; Xiang, Y.; Wang, D.; Gong, Y.; Gong, Y.; Zhang, Z. Microbial communities in the functional areas of a biofilm reactor with anaerobic–aerobic process for oily wastewater treatment. Bioresour. Technol. 2017, 238, 7–15. [Google Scholar] [CrossRef]
- Doshi, B.; Sillanpää, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef]
- Habibollahi, H.; Salehzadeh, A. Isolation, optimization, and molecular characterization of a lipase producing bacterium from oil contaminated soils. Pollution 2018, 4, 119–128. [Google Scholar] [CrossRef]
- Karlapudi, A.P.; Venkateswarulu, T.C.; Tammineedi, J.; Kanumuri, L.; Ravuru, B.K.; Dirisala, V.; Kodali, V.P. Role of biosurfactants in bioremediation of oil pollution—A review. Petroleum 2018, 4, 241–249. [Google Scholar] [CrossRef]
- Nejad, Y.S.; Jaafarzadeh, N.; Ahmadi, M.; Abtahi, M.; Ghafari, S.; Jorfi, S. Remediation of oily sludge wastes using biosurfactant produced by bacterial isolate Pseudomonas balearica strain Z8. J. Environ. Health Sci. Eng. 2020, 18, 531–539. [Google Scholar] [CrossRef]
- Ibrahim, M.L.; Ijah, U.J.J.; Manga, S.B.; Bilbis, L.S.; Umar, S. Production and partial characterization of biosurfactant produced by crude oil degrading bacteria. Int. Biodeterior. Biodegrad. 2013, 81, 28–34. [Google Scholar] [CrossRef]
- Sanghamitra, P.; Mazumder, D.; Mukherjee, S. Treatment of wastewater containing oil and grease by biological method- a review. J. Environ. Sci. Health A 2021, 56, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Elmobarak, W.F.; Hameed, B.H.; Almomani, F.; Abdullah, A.Z. A Review on the Treatment of Petroleum Refinery Wastewater Using Advanced Oxidation Processes. Catalysts 2021, 11, 782. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater Treatment by Advanced Oxidation Process and Their Worldwide Research Trends. Int. J. Environ. Res. Public Health 2020, 17, 170. [Google Scholar] [CrossRef] [Green Version]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Salimi, M.; Esrafili, A.; Gholami, M.; Jonidi Jafari, A.; Rezaei Kalantary, R.; Farzadkia, M.; Kermani, M.; Sobhi, H.R. Contaminants of emerging concern: A review of new approach in AOP technologies. Environ. Monit. Assess. 2017, 189, 414. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Oturan, M.A. Electrochemical advanced oxidation processes for wastewater treatment: Advances in formation and detection of reactive species and mechanisms. Curr. Opin. Electrochem. 2021, 27, 100678. [Google Scholar] [CrossRef]
- Darvishmotevalli, M.; Zarei, A.; Moradnia, M.; Noorisepehr, M.; Mohammadi, H. Optimization of saline wastewater treatment using electrochemical oxidation process: Prediction by RSM method. MethodsX 2019, 6, 1101–1113. [Google Scholar] [CrossRef]
- Duan, P.-G.; Yang, S.-K.; Xu, Y.-P.; Wang, F.; Zhao, D.; Weng, Y.-J.; Shi, X.-L. Integration of hydrothermal liquefaction and supercritical water gasification for improvement of energy recovery from algal biomass. Energy 2018, 155, 734–745. [Google Scholar] [CrossRef]
- Krause, M.J.; Thoma, E.; Sahle-Damesessie, E.; Crone, B.; Whitehill, A.; Shields, E.; Gullett, B. Supercritical Water Oxidation as an Innovative Technology for PFAS Destruction. J. Environ. Eng. 2022, 148, 05021006. [Google Scholar] [CrossRef]
- Brunner, G. Near and supercritical water. Part II: Oxidative processes. J. Supercrit. Fluids 2009, 47, 382–390. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Liang, Z.; Wu, T. Energy conversion and utilization in supercritical water oxidation systems: A review. Biomass Bioenergy 2022, 156, 106322. [Google Scholar] [CrossRef]
- Tang, X.; Zheng, Y.; Liao, Z.; Wang, Y.; Yang, J.; Cai, J. A review of developments in process flow for supercritical water oxidation. Chem. Eng. Comm. 2020, 208, 1494–1510. [Google Scholar] [CrossRef]
- Guo, S.; Xu, D.; Li, Y.; Guo, Y.; Wang, S.; Macdonald, D.D. Corrosion characteristics and mechanisms of typical Ni-based corrosion-resistant alloys in sub- and supercritical water. J. Supercrit. Fluids 2021, 170, 105138. [Google Scholar] [CrossRef]
- Xu, D.; Huang, C.; Wang, S.; Lin, G.; Guo, Y. Salt deposition problems in supercritical water oxidation. Chem. Eng. J. 2015, 279, 1010–1022. [Google Scholar] [CrossRef]
- Muddemann, T.; Haupt, D.; Sievers, M.; Kunz, U. Electrochemical Reactors for Wastewater Treatment. Chem. Ing. Technol. 2019, 91, 769–785. [Google Scholar] [CrossRef] [Green Version]
- Pahlevani, L.; Mozdianfard, M.R.; Fallah, N. Electrochemical oxidation treatment of offshore produced water using modified Ti/Sb-SnO2 anode by graphene oxide. J. Water Process Eng. 2020, 35, 101204. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Ruby-Figueroa, R.; Castro-Muñoz, R. Nanofiltration and tight ultrafiltration membranes for the recovery of polyphenols from agro-food by-products. Int. J. Mol. Sci. 2018, 19, 351. [Google Scholar] [CrossRef] [Green Version]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef] [Green Version]
- Hassan, E.; Hassan, M.; Abou-Zeid, R.; Berglund, L.; Oksman, K. Use of bacterial cellulose and crosslinked cellulose nanofibers membranes for removal of oil from oil-in-water emulsions. Polymers 2017, 9, 388. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.F.S.; Almeida, F.C.G.; Vinhas, G.M.; Sarubbo, L.A. Production of bacterial cellulose by Gluconacetobacter hansenii using corn steep liquor as nutrient sources. Front. microbiol. 2017, 8, 2027. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.F.S.; Amorim, J.D.P.; Gomes, E.A.S.; Araujo, L.M.; Sarubbo, L. Residue from the production of sugar cane: An alternative nutrient used in biocellulose production by Gluconacetobacter Hansenii. Chem. Eng. Trans. 2019, 64, 7–12. [Google Scholar] [CrossRef]
- Albuquerque, R.M.B.; Meira, H.M.; Silva, I.D.; Silva, C.J.G.; Almeida, F.C.G.; Amorim, J.D.P.; Vinhas, G.M.; Costa, A.F.S.; Sarubbo, L.A. Production of a bacterial cellulose/poly(3-hydroxybutyrate) blend activated with clove essential oil for food packaging. Polym. Polym. Compos. 2020, 29, 259–270. [Google Scholar] [CrossRef]
- Amorim, J.D.P.; Souza, K.C.; Duarte, C.R.; Duarte, I.S.; Ribeiro, F.A.S.; Silva, G.S.; Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. a review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Medeiros, A.D.M.; Silva Junior, C.J.G.; Amorim, J.D.P.; Nascimento, H.A.; Converti, A.; Costa, A.F.S.; Sarubbo, L.A. Biocellulose for Treatment of Wastewaters Generated by Energy Consuming Industries: A review. Energies 2021, 14, 5066. [Google Scholar] [CrossRef]
- Wanichapichart, P.; Kaewnopparat, S.; Buaking, K.; Puthai, W. Characterization of cellulose membranes produced by Acetobacter xyllinum. Songklanakarin J. Sci. Technol. 2002, 24, 855–862. [Google Scholar]
- Brown, R.; Kuga, S. Silver labeling of the reducing ends of bacterial cellulose. Carbohydr. Res. 1988, 180, 345–350. [Google Scholar] [CrossRef]
- Esa, F.; Tasirin, S.M.; Rahman, N.A. Overview of Bacterial Cellulose Production and Application. Agric. Agric. Sci. Procedia 2014, 2, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods–A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from indústrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Stasiak-różańska, L.; Płoska, J. Study on the use of microbial cellulose as a biocarrier for 1,3-dihydroxy-2-propanone and its potential application in industry. Polymers 2018, 10, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donini, Í.A.N.; Salvi, D.T.B.; Fukumoto, F.K.; Lustri, W.R.; Barud, H.S.; Marchetto, R.; Messaddeq, Y.; Ribeiro, S.J.L. Biossíntese e recentes avanços na produção de celulose bacteriana. Eclet. Quim J. 2010, 35, 165–178. [Google Scholar] [CrossRef]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown Jr, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Neera; Ramana, K.V.; Batra, H.V. Occurrence of cellulose-producing Gluconacetobacter spp. in fruit samples and kombucha tea, and production of the biopolymer. Appl. Biochem. Biotechnol. 2015, 176, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Sai, H.; Fu, R.; Xing, L.; Xiang, J.; Li, Z.; Li, F.; Zhang, T. Surface modification of bacterial cellulose aerogels’ web-like skeleton for oil/water separation. ACS Appl. Mater. Interfaces 2015, 7, 7373–7738. [Google Scholar] [CrossRef]
- Wang, Q.; Asoh, T.A.; Uyama, H. Facile fabrication of flexible bacterial cellulose/silica composite aerogel for oil/water separation. Bull. Chem. Soc. Jpn. 2018, 91, 1138–1140. [Google Scholar] [CrossRef]
- Hou, Y.; Duan, C.; Zhu, G.; Luo, H.; Liang, S.; Jin, Y.; Zhao, N.; Xu, J. Functional bacterial cellulose membranes with 3D porous architectures: Conventional drying, tunable wettability and water/oil separation. J. Membr. Sci. 2019, 591, 117312. [Google Scholar] [CrossRef]



| Microorganism | Biochemical Structure | Emulsion Type | Efficiency (%) | Concentration (g/L) | Reference |
|---|---|---|---|---|---|
| Candida sphaerica | Glycolipid | Motor oil/Seawater | 40 | 0.25 | [40] |
| Pseudomonas aeruginosa | Glycolipid | Motor oil/Distilled water | 62 | 8.00 | [41] |
| Halomonas venusta | Glycoprotein | Span–Tween–Kerosene/Water | 92 | 7.30 | [39] |
| Bacillus subtilis | Lipopeptide | Waste crude oil/Distilled water | 95 | 0.20 | [42] |
| Pseudomonas aeruginosa | Glycolipid | Distilled water/n-Hexane | 85 | - | [43] |
| Bacillus mojavensis | Oxalate decarboxylase | Tween-60/Deionised water | 62 | 0.17 | [44] |
| Classification | Pore size (nm) | Retention/Removal | Reference |
|---|---|---|---|
| Microfiltration (MF) | 100–5000 | Suspended particles, macromolecules, fungi, and bacteria | [79] |
| Ultrafiltration (UF) | 2–100 | Proteins and viruses | [114] |
| Nanofiltration (NF) | 0.5–2 | Dissolved organic matter, heavy metals, and multivalent ions | [114] |
| Reverse Osmosis (RO) | 0.2–1 | Monovalent salts and ions (ultrapure water) | [115] |
| Properties | Bacterial Cellulose Matrix | Vegetable Cellulose Matrix | Polypropylene Matrix |
|---|---|---|---|
| Crystallinity degree (%) | 90 | 62 | ~55 |
| Fibre size (nm) | 75 | 315 | 450 |
| Fibre density (g cm−3) | 1.5 | 0.99 | 0.95 |
| Sensitivity of fibres to water | Low | High | Low |
| Traction force (N) | ~70 | 47 | ~60 |
| Specific deformation (%) | 16 | 9 | 50 |
| Young’s modulus (GPa) | 5.0 | 0.85 | 1.90 |
| Tension force (MPa) | 85 | 0.83 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medeiros, A.D.M.d.; Silva Junior, C.J.G.d.; Amorim, J.D.P.d.; Durval, I.J.B.; Costa, A.F.d.S.; Sarubbo, L.A. Oily Wastewater Treatment: Methods, Challenges, and Trends. Processes 2022, 10, 743. https://doi.org/10.3390/pr10040743
Medeiros ADMd, Silva Junior CJGd, Amorim JDPd, Durval IJB, Costa AFdS, Sarubbo LA. Oily Wastewater Treatment: Methods, Challenges, and Trends. Processes. 2022; 10(4):743. https://doi.org/10.3390/pr10040743
Chicago/Turabian StyleMedeiros, Alexandre D’Lamare Maia de, Cláudio José Galdino da Silva Junior, Julia Didier Pedrosa de Amorim, Italo José Batista Durval, Andréa Fernanda de Santana Costa, and Leonie Asfora Sarubbo. 2022. "Oily Wastewater Treatment: Methods, Challenges, and Trends" Processes 10, no. 4: 743. https://doi.org/10.3390/pr10040743
APA StyleMedeiros, A. D. M. d., Silva Junior, C. J. G. d., Amorim, J. D. P. d., Durval, I. J. B., Costa, A. F. d. S., & Sarubbo, L. A. (2022). Oily Wastewater Treatment: Methods, Challenges, and Trends. Processes, 10(4), 743. https://doi.org/10.3390/pr10040743








