Abstract
The need to embrace a circular economy model for sustainable growth and development is increasing due to the rise in human population and the dwindling natural resources available to meet the demands for energy and food. In this study, anaerobic digestion of rice husk (RH) was carried out under mesophilic conditions to produce biogas and digestates. Two particle sizes (300 and 600 μm) and three dilution ratios (1:4, 1:6, and 1:8) were employed to determine the optimum conditions for biogas production. The best anaerobic digesters (300 μm/1:6 and 600 μm/1:4) in each of the categories produced a cumulative biogas of 3205 + 290 mL and 2310 + 320 mL, respectively. The digestates were separated into solid and liquid fractions and characterized to evaluate their potential as biofertilizers and nutrient sources for microalgae cultivation. The nitrogen and phosphorus contents of the solid fractions (1.00 ± 0.01 and 0.97 ± 0.04) were significantly higher (p < 0.05) than the liquid fractions whereas the liquid fractions had a higher potassium content than the solid fractions. The absence of heavy metals in the digestates confirmed their safe application as biofertilizers. The pH values of 4.70 and 5.50 reported in this study for liquid digestates are appropriate for the cultivation of some strains of microalgae that thrive in an acidic medium. The ammonium nitrogen contents of the liquid digestates (0.03% + 0.00% and 0.04% ± 0.00%) were moderate and not as high as some values reported to inhibit the growth of some species of microalgae. However, the brownish color of the liquid digestates could impair microalgae growth; thus, there is a need for dilution to increase light penetration.
1. Introduction
Nigeria, like many other developing countries in sub-Saharan Africa, is an agrarian country and, as such, produces several hundred million tonnes of agricultural waste annually [1]. Large quantities of such wastes accumulate every year, resulting not only in the deterioration of the environment but also in the loss of potentially valuable materials, which can be processed to yield a number of value-added products [2]. The use of agricultural wastes as raw materials is particularly convenient because it results in a significant reduction in the amount of waste that ends up in landfills and reduces the cost of production for biofuels and chemicals [3]. According to [4], agricultural wastes can be categorized into three groups: those generated in the fields, those generated after harvesting, and those generated during the processing of crops. Embedded in these wastes are bioresources waiting to be explored to meet the ever-increasing need of modern society for energy and chemicals from sustainable sources to mitigate problems associated with the use of fossil fuels.
The aggressive drive of the Nigerian government to make the country self-sufficient in rice production and the ambitious goal of exporting rice as a source of revenue have led to a noticeable recent increase in rice cultivation [5]. This laudable effort of the government has generated massive waste from rice processing, especially rice husks. In order not to lose the gains of the rice revolution to the degradation of the environment through the indiscriminate handling of waste, which can also lead to health problems, there is need to adopt sustainable ways of converting these wastes to valuable products.
Valorization of RH via anaerobic digestion (AD) is a useful tool for addressing these problems. The AD process generates biogas (which is a mixture of methane, carbon dioxide, hydrogen sulfide, and some trace elements) and digestates (both liquid and solid (fiber)) from organic wastes in a sustainable manner that is both safe for the environment and humans. AD technology also promotes the circular economy concept, which is a better alternative to the conventional linear economy of a “take-make-dispose” industrial model that is both unsustainable and environmentally unfriendly [6,7,8]. Fagerström et al. [9] reported that the multifunctionality of AD, which includes treatment of wastes, reduction in greenhouse gas emissions, the generation of heat and electricity, and the production of biofertilizers (digestates), gives AD a clear-cut advantage over other renewable energy processes, potentially playing a vital role in the future of the circular economy, which is currently at its early stage.
Digestates, which are the by-products of AD systems, can be used as biofertilizers and may serve as a source of additional income for local farmers. Horta and Carneiro [8] successfully used solid digestate from biogas plant fed with pig slurry and cereal straw as a nitrogen source for the cultivation of lettuce (Lactuna sativa L.) and kale (Brassica oleracea var. Winterborn). In another study [10], a three-year application of digestate from agricultural plants significantly increased the physico-chemical properties of the soil and the yield of switchgrass in comparison with soil without fertilizer. Koszel et al. [11] observed improvements in the soil quality and crop yields when liquid digestate from a biogas plant was used in the cultivation of alfalfa and winter wheat. From the aforementioned, the use of digestates as biofertilizers encourages sustainable agricultural practices, which can significantly reduce the carbon footprint of the production and use of mineral fertilizers [12]. Digestates are projected to be an alternative to mineral fertilizers, which are expensive and problematic for the environment. The use of mineral fertilizers has been reported to negatively impact the environment through greenhouse gas emissions, contamination of soil and water aquifers, eutrophication, etc. [13,14].
Biogas digestates are usually separated into liquid and solid fractions (fiber) through various methods such as filtration, centrifugation, sedimentation, and pressing [15]. Digestates are produced in large quantities because of the increasing acceptance of AD, and this is expected to rise in the near future. Therefore, research efforts are ongoing to find other applications for the digestates [16]. Some of the alternative applications of solid digestates include being used as biochar or bioadsorbents to remove heavy metals from aqueous solution, building materials, animal bedding, aquaculture to fertilize fish ponds, in biofuels, and biopesticide production [17,18].
Liquid digestates also have some applications in addition to being used as biofertilizers, including microalgae cultivation, struvite recovery, pretreatment of feedstocks for improved biogas yields, a source of nutrient for soilless farming, ammonia stripping, etc. [19,20]. Most of these alternative applications are still in their infancy and, in most cases, have yet to be effectively commercialized [21,22,23]. However, the most common application of digestates is still as a biofertilizer or soil conditioner. Based on the aforementioned, there is a need for more research evaluating the fertilizing potential and alternative applications of digestates in terms of their physico-chemical properties. This is imperative because different feedstocks are used in biogas plants and the composition and quality of these digestates largely depend on the type of feedstock used in the AD process [23,24]. Nonetheless, other factors affect the properties of digestates, namely retention time, pH, temperature, pretreatment conditions, etc. [25].
Microalgae cultivation has recently gained prominence because of the opportunities it offers in terms of biofuels and bioproducts production, wastewater treatment, and mitigation of CO2 emissions. However, the cost of nutrients for cultivating microalgae is a major setback in the commercialization of the process for derivation of greater benefits [26]. One of the solutions being explored to overcome this challenge is the use of digestates from the AD process as a sustainable nutrient source. Several studies have been conducted using digestates from biogas plants run on different classes of substrates for microalgae cultivation [27,28]. However, to the best of our knowledge, no study has been conducted on the possibility of using liquid digestates from AD of RH for the cultivation of microalgae. It is therefore important to have in-depth knowledge of the physico-chemical properties of digestates to evaluate their suitableness as a nutrient source for microalgae cultivation and proffer likely pretreatments that can improve their practical applications in this respect.
Hence, the objective of this study was to characterize the separated liquid and solid fractions of digestates from AD of RH to evaluate their possible applications as biofertilizers and/or as a nutrient source (liquid fractions) for microalgae cultivation for sustainable management of digestates.
2. Materials and Methods
2.1. Sample Collection and Preparation
Rice husk was collected from Auchi, Etsako West Local Government Area, Edo State, Nigeria. The sample was sun-dried, ground, and passed through 300 and 600 μm mesh sieves. The samples were kept in airtight containers prior to physico-chemical analyses and anaerobic digestion process. The seed inoculum was obtained from cow dung and kept in an anaerobic environment for about four weeks.
2.2. Batch Anaerobic Digestion
Batch AD of rice husk (RH) was performed at substrate-to-water ratios of 1:4, 1:6, and 1:8, separately, to determine the optimum dilution ratios of the two particle sizes for a retention time of twenty-one days. The substrates were charged into 1.5 L glass reactors and tightly closed with rubber stoppers. The digesters were codenamed RH314, RH316, RH318, RH614, RH616, and RH618 based on the particle size and substrate-to-water ratio. The experiments were conducted at an average ambient temperature range of 29 ± 2 °C for a retention time of 21 days. Biogas production was recorded by using the water displacement method. All experiments were performed in duplicate. The digestates from the most promising digesters based on biogas production were separated into solid and liquid fractions by filtration. The digestates were characterized to determine their potential as a biofertilizer and/or nutrient source for microalgae cultivation.
2.3. Analytical Methods
The following physico-chemical analyses were performed on the raw RH and cow dung: inoculum moisture content, ash content, carbon, nitrogen, total solid (TS), and volatile solid (VS) according to standard methods. The lignin, cellulose, and hemicellulose contents of raw RH were analyzed following the method by [29]. The mineral compositions of the digestates were determined using an atomic absorption spectrophotometer (AAS) (Buck Scientific, Norwalk, CT, USA), while phosphorus content was determined by colorimetric method [30]. Chemical oxygen demand was conducted on the liquid digestates using the titrimetric method [31]. The pH values were determined by a digital pH meter while electrical conductivity (EC) was determined by a handheld digital electrical conductivity meter (HANNA Instruments, Woonsocket, RI, USA).
2.4. Statistical Analysis
Analysis of variance (ANOVA) using SPSS software v. 20 (IBM SPSS Statistics for Windows, Armonk, NY, USA) was performed to compare confidence intervals and significance between treatments. The factors were considered significant when the probability (p value) was less than 0.05.
3. Results and Discussion
3.1. Characterization of Feedstocks and Inoculum
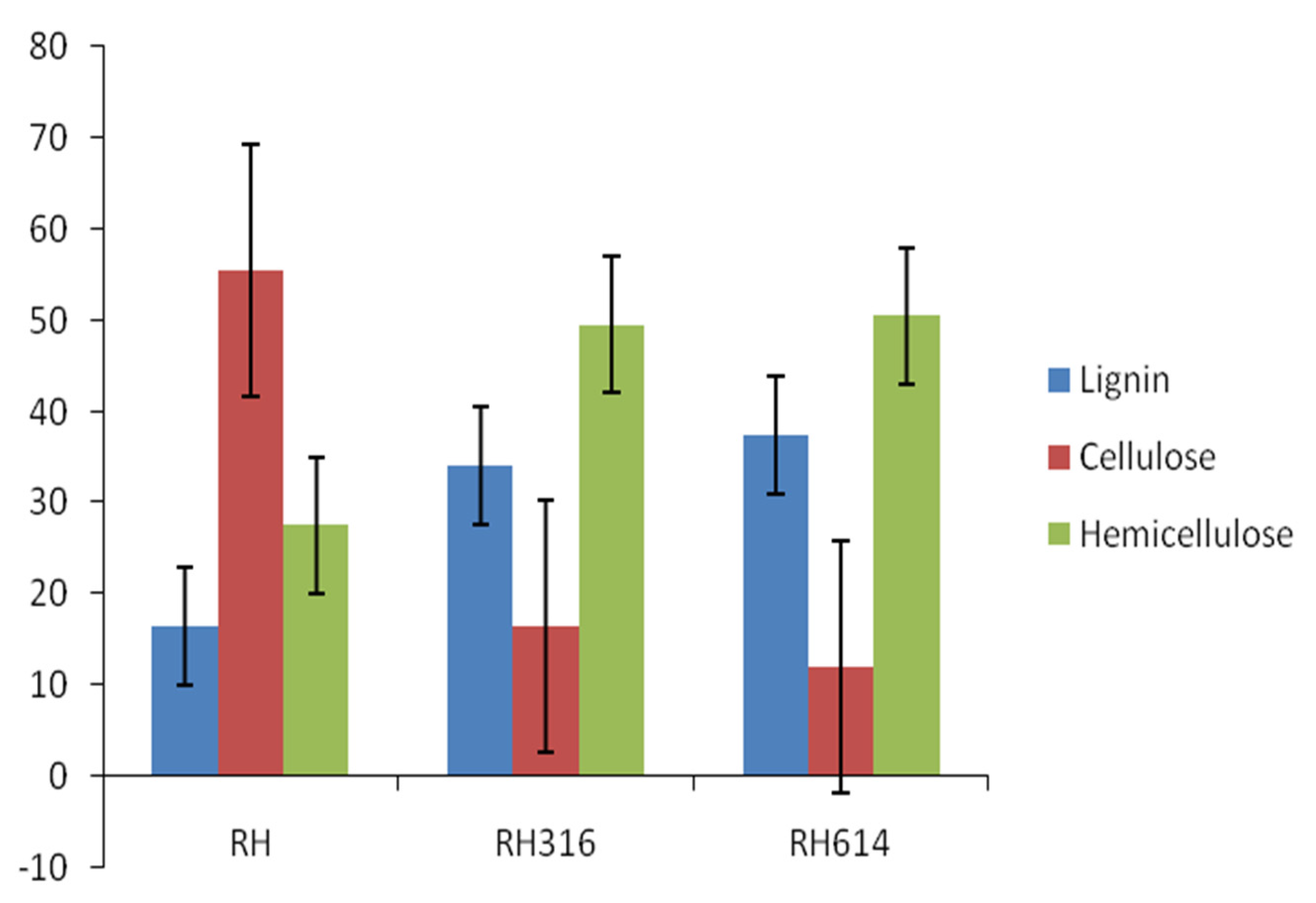
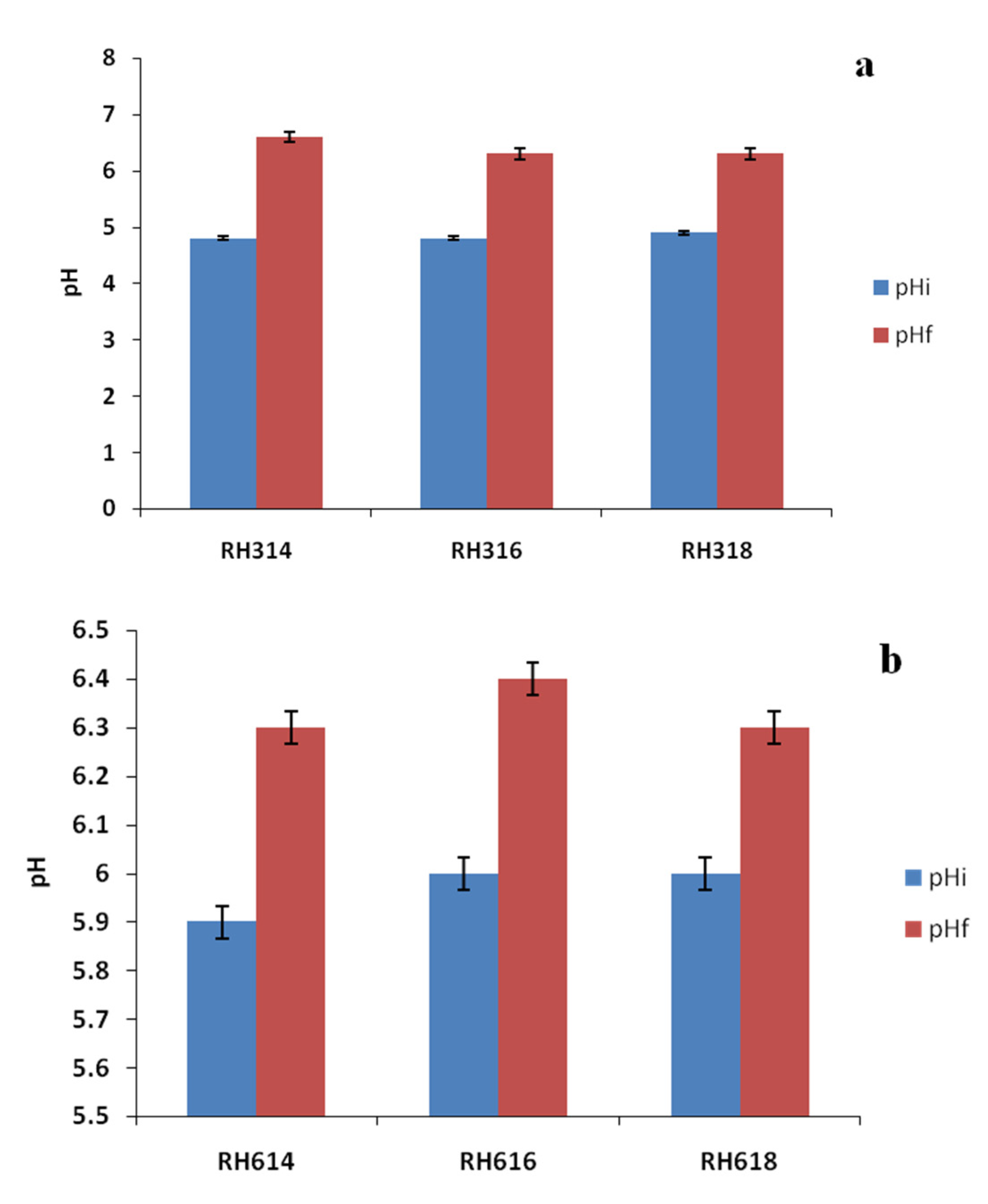
Table 1 shows the physicochemical properties of the raw RH and inoculum used in the experiment. The TS, VS, and VS/TS values were relatively high, which are advantageous to AD. The TS was higher than the 79.64% reported for raw bagasse by [32], but lower than the 94.56% reported for pawpaw stalk by [33], whereas the VS was similar to the 70.9% reported for vegetable waste by [34]. The VS/TS ratio was comparable to the 0.85 reported for food wastes by [35]. The C/N ratio is an important parameter of biogas production, and the value reported in this study for RH was within the range (20:1–30:1) recommended for the AD process [36]. Cellulose content (Figure 1) was the highest in the sample, with 55.53%, followed by hemicellulose with 27.54%, while lignin had the lowest value of 16.47%. The cellulose content of RH reported in this study was higher than the 39.60% reported for rice straw by [37]. The initial and final pH values (Figure 2) range from 4.8 to 6.6, with an increase in pH at the end of AD, which was indicative of the substrates resistance to decrease in pH.

Table 1.
Physicochemical properties of raw rice husk.
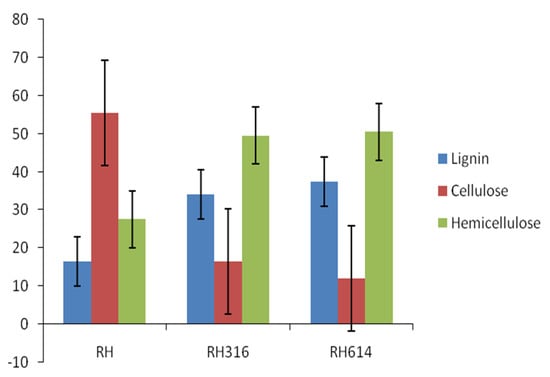
Figure 1.
Comparison of LCH contents of raw rice husk and solid digestates.
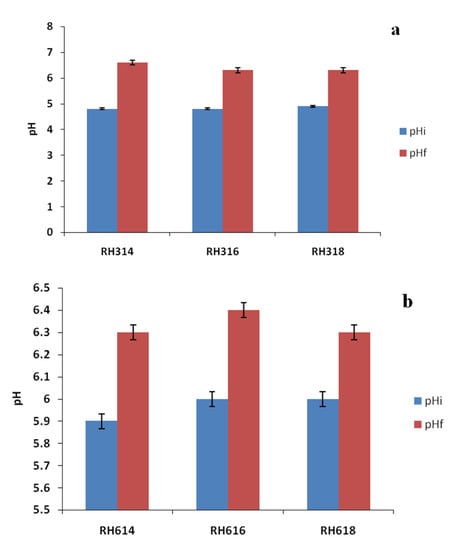
Figure 2.
Initial and final pH values of RH at particle sizes of 300 (a) and 600 μm (b).
3.2. Daily and Cumulative Biogas Production
All the digesters had a fast start-up, with biogas production commencing within the first 24 h, except for digesters RH318 and RH618 which produced no gas and incidentally both had the same dilution ratio. Samples in the 300 μm particle size category had an optimum substrate-to-water ratio of 1:6 while 1:4 was the optimum for samples in the 600 μm category. This confirmed that dilution ratios beyond 1:6 (for the 300 μm category) and 1:4 (for the 600 μm category) may not be desirable for the AD of RH under these experimental conditions. The substrate-to-water ratio plays a significant role in the efficiency of the AD process [38].
Generally, there was a sharp increase in biogas production in the first two days of the AD process, followed by a gradual decline until there was no production. The availability of substrates at the early stages of AD could be a factor, and as AD progressed, there was a depletion in the amount of substrates on which the microorganisms could feed [39].
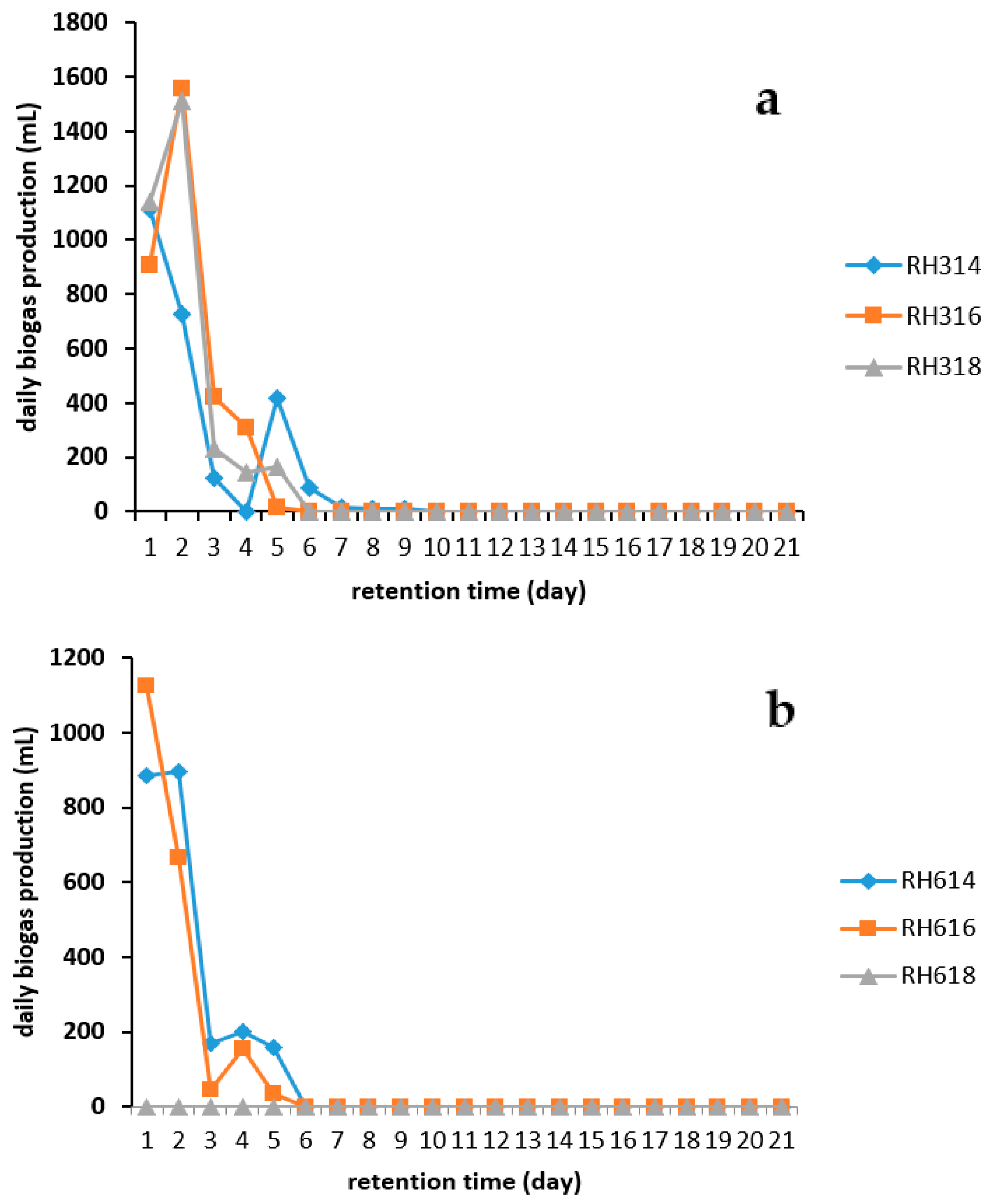
Figure 3 shows that digesters RH314 and RH616 attained their peak values on day 1, whereas RH316, RH318, and RH614 reached their daily peak production on day 2. Digester RH316 had the highest peak value of 1555 mL, followed by RH318 with 1510 mL, and RH614 had the lowest peak value of 895 mL.
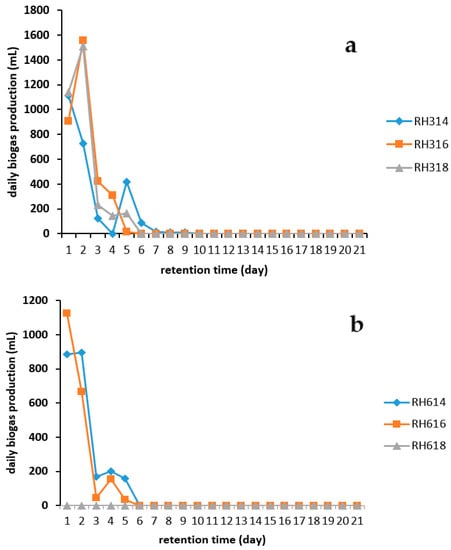
Figure 3.
Daily biogas production from RH for two particle sizes: (a) 300 and (b) 600 μm.
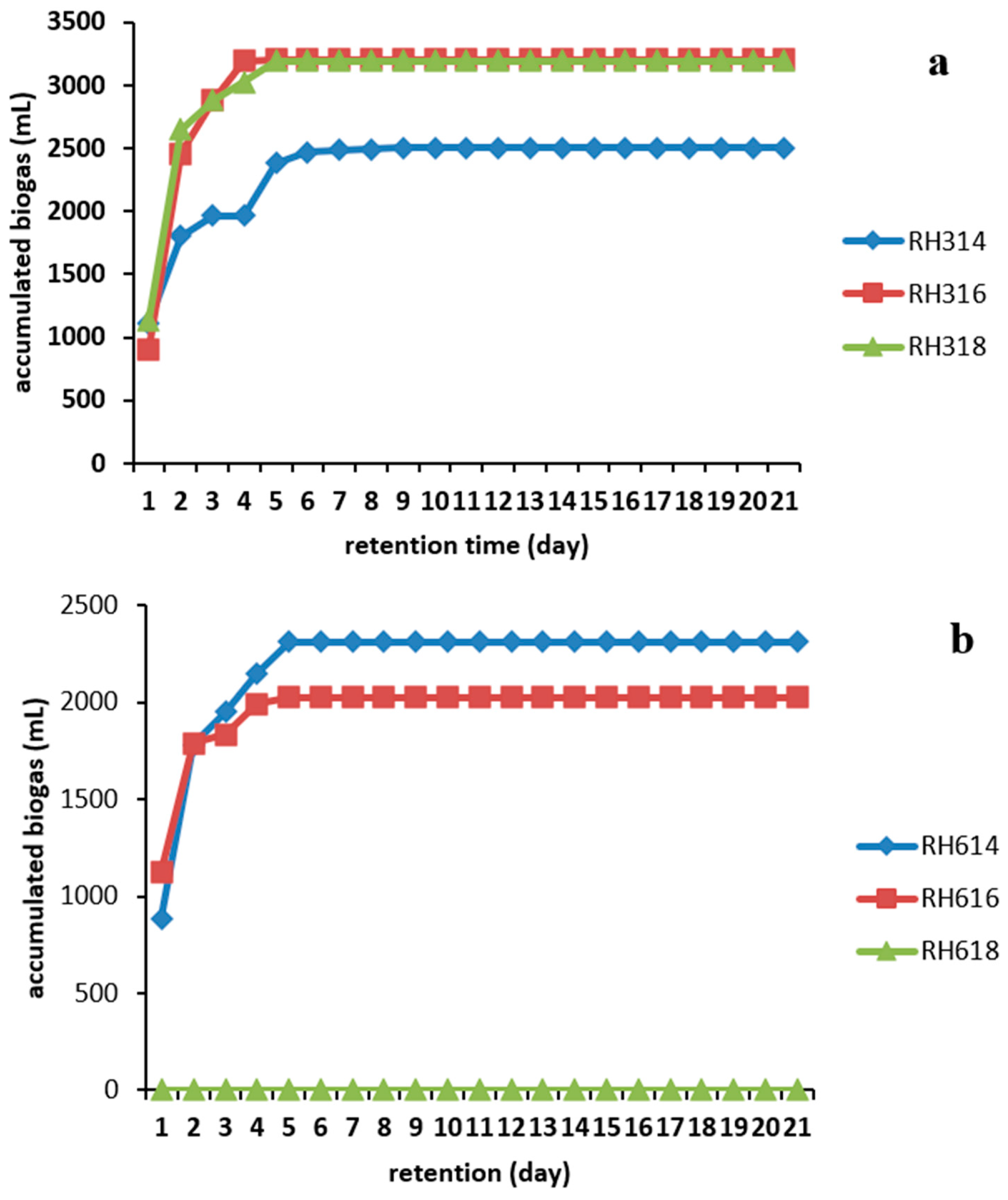
The cumulative biogas production shown in Figure 4 revealed that all the samples in the 300 μm particle size category produced more biogas in comparison than those in the 600 μm particle size sample. Digester RH316 produced the overall highest volume of 3205 ± 290 mL, followed by RH318 with 3190 ± 300 mL. The least production in this category was achieved by RH314 with 2505 ± 250 mL, whereas RH614 and RH616 produced 2310 ± 320 and 2025 ± 290 mL, respectively. This could be a result of increased available specific surface area, which made the substrates more easily accessible to microorganisms. However, this is not always the case in every AD process.
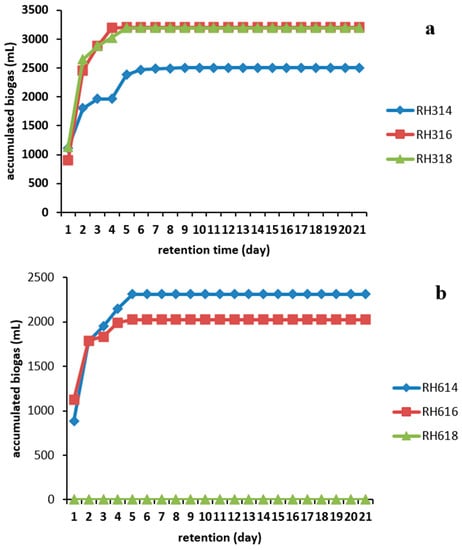
Figure 4.
Accumulated biogas production from RH for two particle sizes: (a) 300 and (b) 600 μm.
3.3. Technical Digestion Time T90
Technical digestion time T90 is the time taken to achieve the 90% production of biogas during AD process. AD was carried out in the present study for 21 days, and T90 was calculated. Digesters RH316, RH318, and RH616 had a T90 of 3 days while those of digesters RH314 and RH614 were 4 and 5 days respectively. The conditions at which a shorter T90 was achieved should be adopted when AD of RH is carried out in a continuous system in order to reduce the down time of a biogas plant and significantly increase profit.
3.4. Flame Test
All the healthy digesters tested positive for the flame test in the first two days of AD process. This provided an indication of the high methane content in the samples because methane is the only component with energy value in biogas [40].
3.5. Effect of AD on Lignin, Cellulose, and Hemicellulose
A comparison of the lignin, cellulose, and hemicellulose (LCH) contents of raw rice husk and digestates is presented in Figure 1. The degradation rates of cellulose were 70.45% and 78.23% for RH3316 and RH614, respectively, which established that most of the biogas was produced from the degradation of cellulose by the anaerobic microorganisms. The digestates had increased contents of both lignin and hemicellulose. An increase in these components can be attributed to the degradation of cellulose [41]. Pognani et al. [42] observed the same trend of significant increase in these macromolecules in the course of AD of some organic wastes. The presence of these macromolecules in solid digestates improves their quality as soil conditioners [25].
3.6. Characterization of Solid and Liquid Digestates
The physico-chemical properties of the separated digestates are presented in Table 2. The pH values of the digestates were in the acidic range (4.70–6.40), which encourages their usage in soils that are excessively alkaline in nature. The nitrogen contents were in the range of 0.05–1.00% for both solid and liquid digestates. The solid digestates had a higher nitrogen content than the liquid fractions. The nitrogen contents of the solid fractions were higher than the 0.45% reported for solid digestate from an agricultural biogas plant by [7]. The same trend was observed in the phosphorus contents of the digestates; the solid digestates had higher (p < 0.05) values than the liquid digestates. This observation is in agreement with the findings of [43] on phosphorus distribution in untreated and mechanically separated digestates. Logan and Visvanathan [44] found 55% to 65% of the total phosphorus in the solid fraction of the digestates after separation, while the remaining 35% to 45% was found in the liquid digestates. The phosphorus values of the solid digestates were higher than 0.11% (1.10 g/kg) reported for Virginia fanpetals digestate (DVF) by [24] and the 0.26% reported for Napier grass digestate by [45]. The value of RH614 (solid) is similar to 0.84% (8.4 g/kg) reported for whey-permeate codigested with manure reported by [46].

Table 2.
Physicochemical properties of liquid and solid digestates.
The potassium values of the samples ranged from 0.08% to 0.81%, with the liquid digestate fractions having higher (p < 0.05) values than the solid fractions, in agreement with the report by [36]. The N:P ratio of the samples ranged from 0.83% to 1.67%, which is below the 5–30 range reported by [47] for some strains of microalgae; yet, the N:P ratio of 1.67 ± 0.00 for RH316 (liquid) is similar to the 1.71 reported by [48] for the cultivation of Haematococcus species of microalgae. The values for NH4-N concentration for the liquid digestates were 0.03% and 0.04% (at 300 and 400 mg/L, respectively). These values were higher than the 0.006% (0.06 g/kg) reported by [49] for liquid digestate from swine manure, but lower than the 0.32% (3.2 g/L) of filtered liquid digestate from the organic fraction of municipal solid waste (OFMSW) used in the cultivation of five species of microalgae [50]. The liquid digestate in the aforementioned study was diluted due to having a high concentration of NH4-N to enhance microalgae growth. It was reported that a high concentration of NH4-N could inhibit the growth of microalgae [27].
The calcium contents of the samples ranged from 0.06 to 0.23%, which is lower than the 2.96% reported for the digestate from the codigestion of cattle slurry, maize silage, and haylage by [51]. The sodium concentration ranged from 0.19% to 4.33%, with RH316 (liquid) having the highest value and RH614 (solid) having the least value. All the values were, however, higher than the 0.12% (1200 mg/L) reported for the liquid digestate obtained from the AD of food wastes from households and industry by [52]. The magnesium concentration was higher in the liquid fraction than in the solid digestates. The values (0.75% and 0.78%, respectively) for RH316 and RH614 (liquid) were higher than the 0.62% recorded for the digestate from a biogas plant run on pig slurry and maize silage [53]. The values for manganese and iron ranged from 0.001% to 0.009% and 0.01% to 0.56%, respectively. The solid fractions had higher values for these micronutrients than the liquid fractions. The values for RH316 and RH614 were higher than the 0.003% reported for digestate from Carica papaya fruit peels by [54]. Their iron contents were equally higher than the 0.20% reported by the same author for digestate from Tithonia diversifolia shoot.
A negative correlation was reported between the lignin content of digestates and nitrogen release: the higher the lignin content, the lower the nitrogen release [55]. According to the aforementioned, RH316 is a better nitrogen-releasing digestate than RH614. Nitrogen and phosphorus contents in the solid digestates were significantly (p < 0.05) (Table S4 Supplementary Material) higher than in liquid digestates; on the other hand, the potassium, calcium, magnesium, and sodium contents of the liquid digestates were significantly (p < 0.05) higher than those of the solid digestates. Liquid biofertilizers are preferred by farmers to solid ones due to ease of handling and the possibility of mixing them with herbicides [56].
The COD concentrations of the liquid digestates are shown in Table 2, with RH316 having a higher value of 784 mg/L and that of RH614 being 522 mg/L. The value for RH614 was lower than the 672.65% reported for rubber processing effluent digestate used as a soil conditioner by [57]. The COD concentration of RH316 (liquid) was similar to the 720 mg/L reported for 50% diluted distilled liquid digestates obtained from an agricultural biogas plant, operated with maize silage and distillery stillage, which was found not to inhibit the growth of two algal strains, Chlorella vulgaris and Arthrospira platensis, cultivated in it [58].
The EC of the liquid digestates were 20,552 and 24,702 µS/cm (20.55 and 24.70 mS/cm, respectively) for RH316 and RH614 respectively. The values are much higher than 3000 µS/cm (3 dS/m) recommended as the threshold value for the prevention of soil salinity [8]. Additionally, the values were outside the acceptable range (750–3000 µS/cm) for liquid digestates as irrigation water [47]. Jamison et al. [59] suggested that digestates high in electrical conductivity should be used in combination with other fertilizers to improve their suitability for plant cultivation. The pH values of the liquid fractions were 4.70 and 5.50, which are outside the reported optimal range of 6.8–7.4 for microalgae cultivation [35]. However, the Parachlorella species of microalgae was successfully cultivated in an acidic liquid digestate [27]. This shows that some strains of microalgae can thrive at the pH values recorded in this study. Digestates for microalgae cultivation can therefore be tailor-made based on appropriate pretreatments to meet the nutrient requirements of a specific strain of microalgae to ensure optimum yield. As pointed out by [48], to obtain an appropriate cultivation medium, it is important to consider the species of microalgae to be cultivated and the properties of the digestates. Figure 5 shows the pelletized digestates (biofertilizers) and liquid digestates produced in this study. The liquid digestates were brownish in color and, as such, will need to be diluted to increase light penetration which is one of the conditions for effective microalgae cultivation [48,58]. Zuliani et al. [26] reported significant growth rates in three strains of microalgae (C. vulgaris, C. sorokiniana, and Scenedesmus) when the digestate was diluted 30 times. Nevertheless, digestates should be diluted with caution to avoid the loss of nutrients which could be detrimental to the growth of microalgae [26,27].

Figure 5.
Pelletized solid and liquid digestates from rice husk.
3.7. Heavy Metal Loads in Digestates
The standards for the limits of heavy metals in digestates adopted by some European countries are shown in Table 3. A comparison of the results obtained in this study of heavy metal contents with these standards revealed that both solid and liquid digestates are safe for application as biofertilizers or soil conditioners.

Table 3.
Biofertilizer standards from some European countries.
4. Conclusions
In this study, we characterized solid and liquid digestates from the AD of RH under mesophilic conditions. The most promising digesters from the two categories were RH314 and RH61. Digester RH314 produced 3205 ± 290 mL of biogas which was significantly higher (p < 0.05) than the 2310 ± 320 mL produced by RH618. The characterization of solid and liquid digestates from the AD process revealed that the solid fractions have better fertilizing potential than the liquid fractions in terms of nitrogen and phosphorus contents. The nitrogen contents of RH314 (solid) and RH618 (solid) were 1.00% ± 0.01% and 0.97% ± 0.04%, respectively, which were significantly higher (p < 0.05) than the 0.05% ± 0.00% recorded for the liquid digestates. The phosphorus values for RH314 (solid) and RH618 (solid) were 0.68% ± 0.00% and 0.80% + 0.00%, respectively which were equally higher than the values obtained for the liquid digestates. The heavy metal contents of the digestates revealed that they are environmentally benign and do not pose any threat to the environment. The findings further revealed the microalgae cultivation capability of the liquid digestates. The results showed that the liquid digestates can be used for the cultivation of some strains of microalgae but they might not be suitable for others. Therefore, some pretreatments may be necessary to improve their qualities as profitable media for microalgae cultivation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr10051007/s1.
Author Contributions
Conceptualization, A.D.O.; methodology, A.D.O.; formal analysis, A.D.O.; investigation, A.D.O.; data curation, A.D.O.; writing—original draft preparation, A.D.O.; writing—reviewing and editing, B.L.; funding acquisition, A.D.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Tertiary Education Trust Fund (TETfund) grant number TETfund/IBR/2021 and the APC was funded by ARRS P2-152.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Damisa, D.; Ameh, J.B.; Umoh, V.J. Effect of chemical pre-treatment of some lignocellulosic wastes on the recovery of cellulose from Aspergillus niger AH3 mutant. Afr. J. Biotechnol. 2008, 7, 2444–2450. [Google Scholar]
- Someet, N.; Virenda, S.; Bisaria, V.S. Optimization of xylanase production by Melanocarpus albomyces IIS 68 in solid state fermentation using response surface methodology. J. Biosci. Bioeng. 2001, 91, 425–427. [Google Scholar] [CrossRef]
- Hahn-Hagerdal, B.; Galbe, M.; Gorwa-Grauslund, M.; Liden, G.; Zacch, G. Bioethanol-the fuel of tomorrow from the residues of today. Trends Biotechnol. 2006, 24, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Santolini, E.; Bovo, M.; Barbaresi, A.; Torreggiani, D.; Tassinari, P. Turning agricultural wastes into biomaterials: Assessing the sustainability of scenarios of circular valorization of corn cob in a life-cycle perspective. Appl. Sci. 2021, 11, 6281. [Google Scholar] [CrossRef]
- Abdulwaheed, A.; Opadotun, O.O.; Amusat, M.A. Rice self-sufficiency: A review of government policies on rice production. IJSER 2017, 8, 1289–1302. [Google Scholar] [CrossRef]
- Ericsson, N.; Nordberg, Å.; Berglund, M. Biogas plant management decision support—A temperature and time-dependent dynamic methane emission model for digestate storages. Bioresour. Technol. Rep. 2020, 11, 100454. [Google Scholar] [CrossRef]
- Jurgutis, L.; Šlepetienė, A.; Šlepetys, J.; Cesevičienė, J. Towards a full circular economy in biogas plants: Sustainable management of digestate for growing biomass feedstocks and use as biofertilizer. Energies 2021, 14, 4272. [Google Scholar] [CrossRef]
- Horta, C.; Carneiro, J.P. Assessment of Fertilising Properties of a Solid Digestate in Comparison with Undigested Cattle Slurry Applied to an Acidic Soil. Open J. Soil Sci. 2020, 10, 307. [Google Scholar] [CrossRef]
- Fagerström, A.; Al Seadi, T.; Rasi, S.; Briseid, T. The role of anaerobic digestion and biogas in the circular economy. IEA Bioenergy 2018, 37, 1–10. [Google Scholar]
- Głowacka, A.; Szostak, B.; Klebaniuk, R. Effect of biogas digestate and mineral fertilisation on the soil properties and yield and nutritional value of switchgrass forage. Agronomy 2020, 10, 490. [Google Scholar] [CrossRef]
- Koszel, M.; Przywara, A.; Santoro, F.; Anifantis, A.S. Evaluation of use of biogas plant digestate as fertilizer in alfalfa and winter wheat. In Proceedings of the Conference: 17th International Scientific Conference Engineering for Rural Development, Jelgava, Latvija, 23–25 May 2018; Volume 23, pp. 1413–1418. [Google Scholar]
- Brentrup, F.; Hoxha, A.; Christensen, B. Carbon footprint analysis of mineral fertilizer production in Europe and other world regions. In Proceedings of the 10th International Conference on Life Cycle Assessment of Food (LCA Food 2016), University College Dublin (UCD), Dublin, Ireland, 19–21 October 2016. [Google Scholar]
- Srivastav, A.L. Chemical fertilizers and pesticides: Role in groundwater contamination. In Agrochemicals Detection, Treatment and Remediation; Heinemann: Butterworth, Malaysia, 2020; pp. 143–159. [Google Scholar]
- Onwe, H.O.; Adesiji, A.R.; Agbese, E. Effect of chemical fertilizers on groundwater quality in an unconfined aquifer. In Proceedings of the 2nd International Civil Engineering Conference (ICEC 2020), Department of Civil Engineering Federal University of Technology, Held at Federal University of Technology Minna (FUT Minna), Minna, Nigeria, 9–11 June 2022. [Google Scholar]
- Kodymová, J.; Švehláková, H.; Kyncl, M.; Bártková, M. The distribution of macro and micronutrients in the maize within separated digestate fertilizing: Field trial. GeoSci. Eng. 2016, 62, 10–18. [Google Scholar] [CrossRef]
- Czekała, W. Solid fraction of digestate from biogas plant as a material for pellets production. Energies 2021, 14, 5034. [Google Scholar] [CrossRef]
- Aso, S.N. Digestate: The coproduct of biofuel production in a circular economy and new results for cassava peeling residue digestate. In Renewable Energy-Technologies and Applications; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Ogwang, I.; Kasedde, H.; Nabuuma, B.; Kirabira, J.B.; Lwanyaga, J.D. Characterization of Biogas Digestate for Solid Biofuel Production in Uganda. Sci. Afr. 2021, 12, e00735. [Google Scholar] [CrossRef]
- Siddhu, M.A.H.; Li, J.; Zhang, R.; Liu, J.; Ji, J.; He, Y.; Chen, C.; Liu, G. Potential of black liquor of potassium hydroxide to pretreat corn stover for biomethane production. BioResources 2016, 11, 4550–4563. [Google Scholar] [CrossRef]
- Lind, O.P.; Hultberg, M.; Bergstrand, K.J.; Larsson-Jönsson, H.; Caspersen, S.; Asp, H. Biogas digestate in vegetable hydroponic production: pH dynamics and pH management by controlled nitrification. Waste Biomass Valorization 2021, 12, 123–133. [Google Scholar] [CrossRef]
- Hu, Y.; Pang, Y.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Chufo, W.A.; Jaffar, M.; Li, X. Promoting anaerobic biogasification of corn stover through biological pretreatment by liquid fraction of digestate (LFD). Bioresour. Technol. 2015, 175, 167–173. [Google Scholar] [CrossRef]
- Akhiar, A.; Battimelli, A.; Torrijos, M.; Carrere, H. Comprehensive characterization of the liquid fraction of digestates from full-scale anaerobic co-digestion. Waste Manag. 2017, 59, 118–128. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Liu, Y.; Jiang, N.; Zhao, Q.; Deng, L. Managing liquid digestate to support the sustainable biogas industry in China: Maximizing biogas-linked agro-ecosystem balance. GCB Bioenergy 2021, 13, 880–892. [Google Scholar] [CrossRef]
- Sienkiewicz, S.; Wierzbowska, J.; Kovacik, P.; Krzebietke, S.; Zarczynski, P. Digestate as a substitute of fertilizers in the cultivation of Virginia fanpetals. Fresenius Environ. Bull. 2018, 27, 3970–3976. [Google Scholar]
- Makádi, M.; Tomócsik, A.; Orosz, V. Digestate: A new nutrient source-review. Energy 2012, 4, 295–311. [Google Scholar] [CrossRef]
- Zuliani, L.; Frison, N.; Jelic, A.; Fatone, F.; Bolzonella, D.; Ballottari, M. Microalgae cultivation on anaerobic digestate of municipal wastewater, sewage sludge and agro-waste. Int. J. Mol. Sci. 2016, 17, 1692. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Kim, J.; Rhee, C.; Shin, J.; Shin, S.G.; Lee, C. Effects of different pH control strategies on microalgae cultivation and nutrient removal from anaerobic digestion effluent. Microorganisms 2022, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Pulgarin, A.; Kapeller, A.G.; Tarik, M.; Egloff, S.; Mariotto, M.; Ludwig, C.; Refardt, D. Cultivation of microalgae at high-density with pretreated liquid digestate as a nitrogen source: Fate of nitrogen and improvements on growth limitations. J. Clean. Prod. 2021, 324, 129238. [Google Scholar] [CrossRef]
- ASTM D1 107-ASTM; Standard Methods of Test for Alcohol-Benzene Solubility of Wood. ASTM (American Society for Testing and Materials): West Conshohocken, PA, USA, 1972.
- Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists, 20th ed.; ASTM: Washington, DC, USA, 2016. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Clesceri, L.S., Eds.; American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF): Washington, DC, USA, 2012. [Google Scholar]
- Budiyono, B.; Riyanta, A.B.; Sumardiono, S.; Jos, B.; Syaichurrozi, I. Optimization of parameters for biogas production from bagasse using Taguchi Method. Pol. J. Environ. Stud. 2021, 30, 4453–4461. [Google Scholar] [CrossRef]
- Jensen, C.D.; Olugbemide, A.D.; Akpa, F.A.O.; Oladipo, A. Pretreatment Chemometrics in Holistic Biogas Life Cycle Assessment: Framing Case Study with Carica papaya. Waste Biomass Valorization 2020, 11, 7029–7042. [Google Scholar] [CrossRef]
- Nabaterega, R.; Banadda, N.; Muyonga, J.H.; Kiggundu, N.; Kabenge, I.; Tumutegyereize, P. Determining the most appropriate and optimum ratios of Organic waste for Biogas generation from small-scale food processing units. Int. J. Appl. Sci. Eng. 2015, 4, 758–766. [Google Scholar] [CrossRef]
- Batool, N.; Qazi, J.I.; Aziz, N.; Hussain, A.; Shah, S.Z.H. Bio-methane production potential assays of organic waste by anaerobic digestion and co-digestion. Pak. J. Zool. 2020, 52, 971–976. [Google Scholar] [CrossRef]
- Chong, C.C.; Cheng, Y.W.; Ishak, S.; Lam, M.K.; Lim, J.W.; Tan, I.S.; Lee, K.T. Anaerobic digestate as a low-cost nutrient source for sustainable microalgae cultivation: A way forward through waste valorization approach. Sci. Total Environ. 2022, 803, 150070. [Google Scholar] [CrossRef]
- Yavini, T.D.; Taura, U.H.; Mohammed, N.; Namo, J.M. Comparative study of mesophilic biogas production potentials of selected agro-wastes. Int. J. Eng. Sci. 2014, 3, 1–6. [Google Scholar]
- Imasuen, A.O.; Olugbemide, A.D.; Ogungbemide, D.I.; Osula, J. Biogas production from fresh maize leaves (Zea mays): Effect of dilution ratios. Cont. J. Appl. Sci. 2011, 6, 21–24. [Google Scholar]
- Darimani, H.S.; Pant, D.C. Biogas Production from Co-Digestion of Grass with Food Waste. J. Agric. Chem. Environ. 2019, 9, 27–36. [Google Scholar] [CrossRef][Green Version]
- Atelge, M.R.; Senol, H.; Djaafri, M.; Hansu, T.A.; Krisa, D.; Atabani, A.; Kıvrak, H.D. A Critical Overview of the state-of-the-art methods for biogas purification and utilization processes. Sustainability 2021, 13, 11515. [Google Scholar] [CrossRef]
- Mulat, D.G.; Dibdiakova, J.; Horn, S.J. Microbial biogas production from hydrolysis lignin: Insight into lignin structural changes. Biotechnol. Biofuels 2018, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Pognani, M.; D’Imporzano, G.; Scaglia, B.; Adani, F. Substituting energy crops with organic fraction of municipal solid waste for biogas production at farm level: A full-scale plant study. Process Biochem. 2009, 44, 817–821. [Google Scholar] [CrossRef]
- Bachmann, S.; Uptmoor, R.; Eichler-Löbermann, B. Phosphorus distribution and availability in untreated and mechanically separated biogas digestates. Sci. Agric. 2016, 73, 9–17. [Google Scholar] [CrossRef]
- Logan, M.; Visvanathan, C. Management strategies for anaerobic digestate of organic fraction of municipal solid waste: Current status and future prospects. Waste Manag. Res. 2019, 37, 27–39. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C. Anaerobic Digestion Biorefinery to Produce Bioenergy and Biobased Products Using High Yielding Tropical Feedstock. Ph.D. Thesis, University of Hawai’i at Manoa, Honolulu, HI, USA, 2017. [Google Scholar]
- Sogn, T.A.; Dragicevic, I.; Linjordet, R.; Krogstad, T.; Eijsink, V.G.; Eich-Greatorex, S. Recycling of biogas digestates in plant production: NPK fertilizer value and risk of leaching. Int. J. Recycl. Org. Waste Agric. 2018, 7, 49–58. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.M. Effect of the N/P ratio on biomass productivity and nutrient removal from municipal wastewater. Bioprocess Biosyst. Eng. 2015, 38, 761–766. [Google Scholar] [CrossRef]
- Bauer, L.; Ranglová, K.; Masojídek, J.; Drosg, B.; Meixner, K. Digestate as sustainable nutrient source for microalgae—Challenges and prospects. Appl. Sci. 2021, 11, 1056. [Google Scholar] [CrossRef]
- Odales, B.L.; López, D.E.; López, G.L.; Jiménez, H.J.; Barrera, C.E.L. Biofertilizer potential of digestates from small-scale biogas plants in the Cuban context. Revista de Ciencias Agrícolas 2020, 37, 14–26. [Google Scholar] [CrossRef]
- Barreiro-Vescovo, S.; Barbera, E.; Bertucco, A.; Sforza, E. Integration of microalgae cultivation in a biogas production process from organic municipal solid waste: From laboratory to pilot scale. ChemEngineering 2020, 4, 25. [Google Scholar] [CrossRef]
- Barłóg, P.; Hlisnikovský, L.; Kunzová, E. Effect of digestate on soil organic carbon and plant-available nutrient content compared to cattle slurry and mineral fertilization. Agronomy 2020, 10, 379. [Google Scholar] [CrossRef]
- Valeur, I. Speciation of Heavy Metals and Nutrient Elements in Digestate. Master’s Thesis, Norwegian University of Life Sciences, As, Norway, 2011. [Google Scholar]
- Losak, T.; Hlusek, J.; Zatloukalova, A.; Musilova, L.; Vitezova, M.; Skarpa, P.; Zlamalova, T.; Fryc, J.; Vitez, T.; Marecek, J.; et al. Digestate from biogas plants is an attractive alternative to mineral fertilisation of kohlrabi. J. Sustain. Dev. Energy Water Environ. Syst. 2014, 2, 309–318. [Google Scholar] [CrossRef]
- Dahunsi, S.O. Optimization of Biogas and Digestate Biofertilizer Produced from Five Locally Available Biomass. Ph.D. Thesis, Landmark University, Omu-Aran, Nigeria, 2017. [Google Scholar]
- Prasad, M.; Lee, A.; Gaffney, M.T. A Detailed Chemical and Nutrient Characterization of Compost and Digestate Including Comparative Releases of Nitrogen and Phosphorus. rx3, Rethink, Recycle, Remake. 2012, pp. 1–43. Available online: http://www.cre.ie/web/wp-content/uploads/2010/12/Compost-Digestate-Characterisation.pdf (accessed on 26 April 2022).
- Chandini, K.R.; Kumar, R.; Prakash, O. The impact of chemical fertilizers on our environment and ecosystem. Res. Trends Environ. Sci. 2019, 69–86. [Google Scholar]
- Maliki, M.; Ifijen, I.H.; Khan, M.E. Effect of Digestate from Rubber Processing Effluent on Soil Properties. Uganda J. Agric. Sci. 2019, 19, 27–33. [Google Scholar] [CrossRef]
- Kisielewska, M.; Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Quattrocelli, P.; Bordiean, A. Effects of liquid digestate treatment on sustainable microalgae biomass production. BioEnergy Res. 2021, 15, 357–370. [Google Scholar] [CrossRef]
- Jamison, J.; Khanal, S.K.; Nguyen, N.H.; Deenik, J.L. Assessing the effects of digestates and combinations of digestates and fertilizer on yield and nutrient use of Brassica juncea (Kai Choy). Agronomy 2021, 11, 509. [Google Scholar] [CrossRef]
- Foster, P.; Prasad, M. Development of Quality Standards for Compost and Digestate in Ireland; Environmental Protection Agency: Wexford, Ireland, 2021.
- Guilayn, F.; Jimenez, J.; Rouez, M.; Crest, M.; Patureau, D. Biogas digestate typologies. In Proceedings of the Sixteenth International Waste Management and Landfill Symposium, Sardinia, Italy, 2–6 October 2017. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).