Unveiling the Evolution of Madeira Wine Key Metabolites: A Three-Year Follow-Up Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
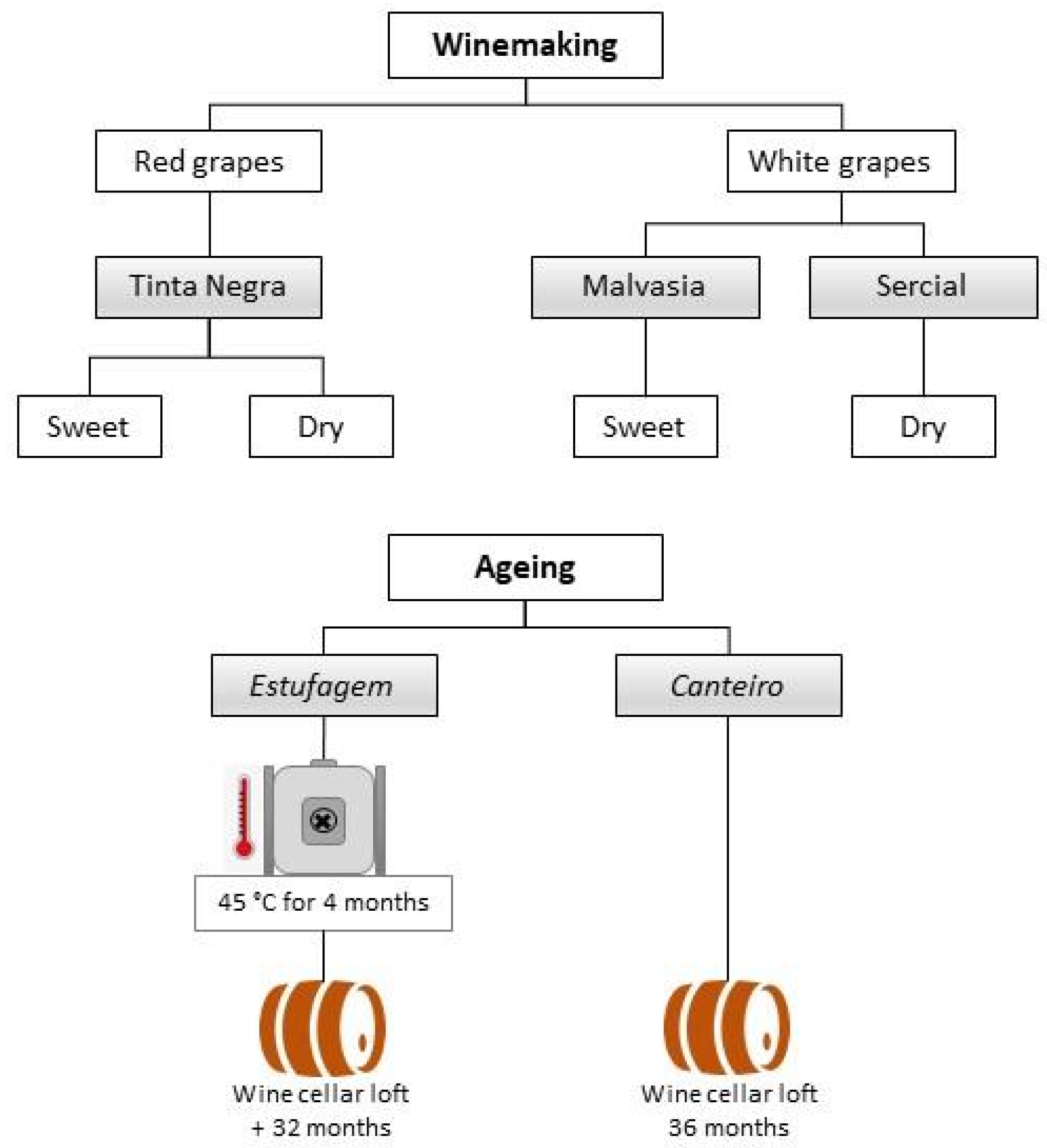
2.2. Samples
2.3. HMF and Furfural Quantification
2.4. Sotolon and Ethyl Carbamate Quantification
2.5. CIELAB Analyses
2.6. Data Analysis
3. Results and Discussion
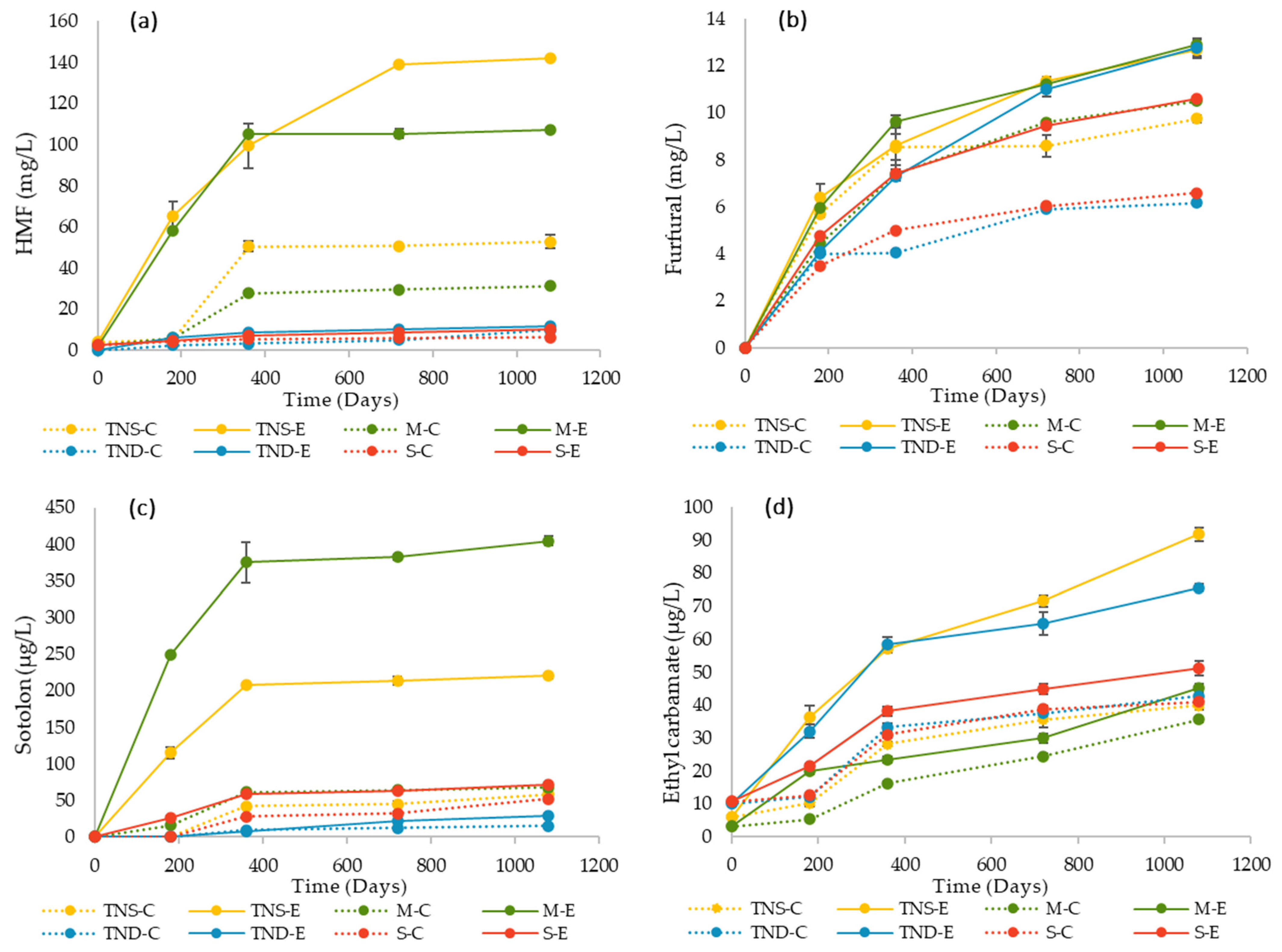
3.1. HMF and Furfural Quantification
3.2. Sotolon Quantification
3.3. Ethyl Carbamate Quantification
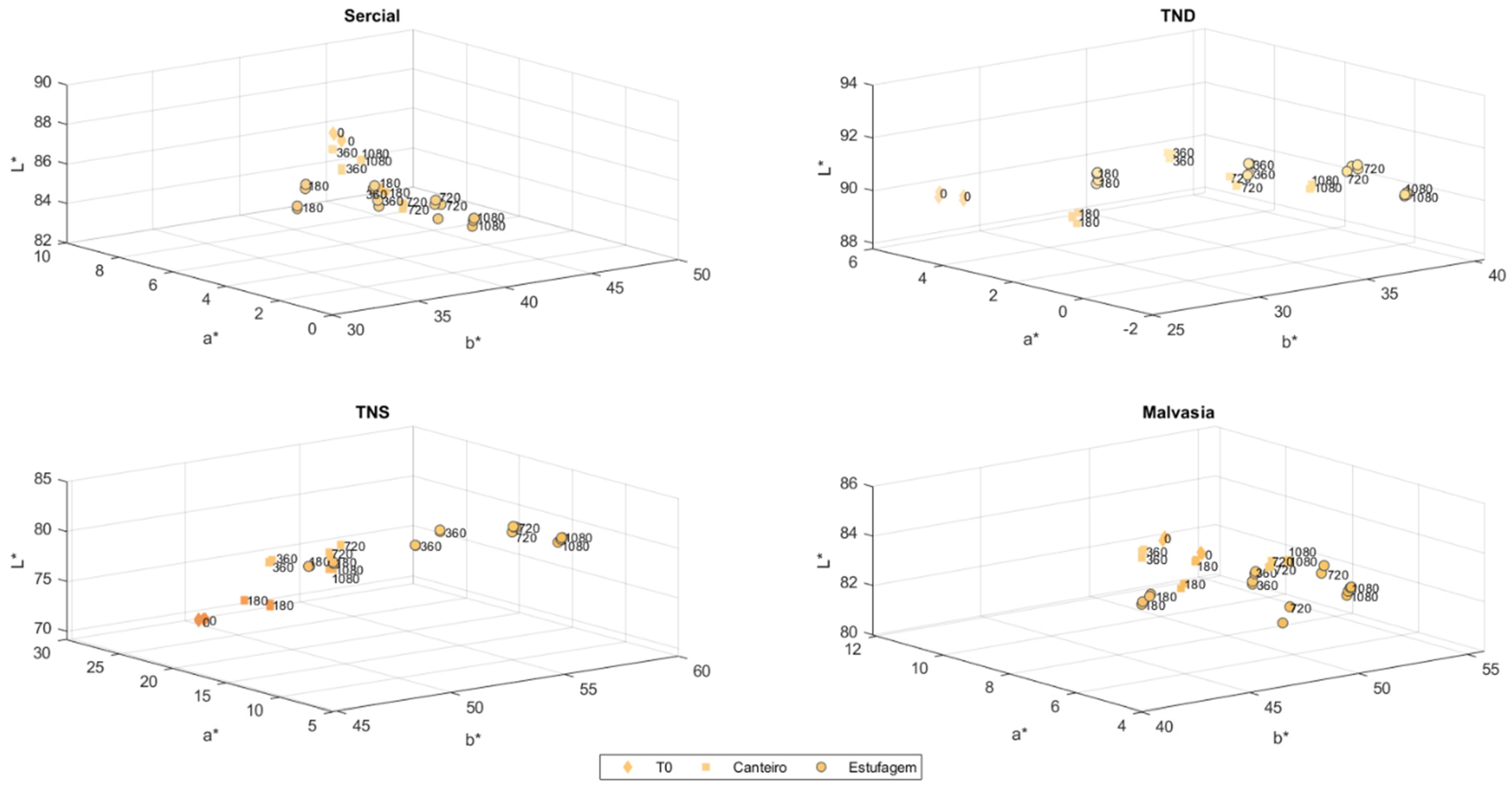
3.4. CIELAB Analyses
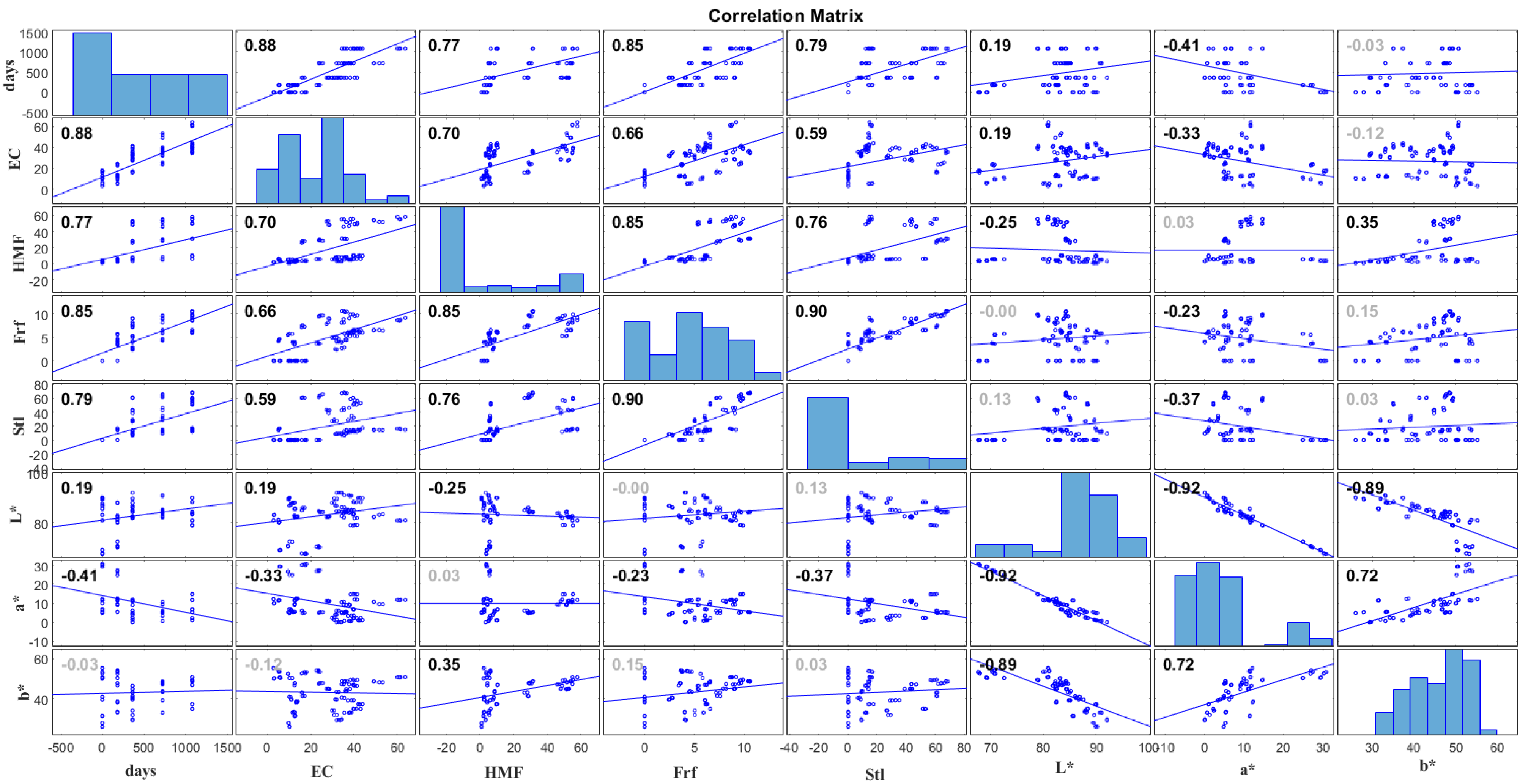
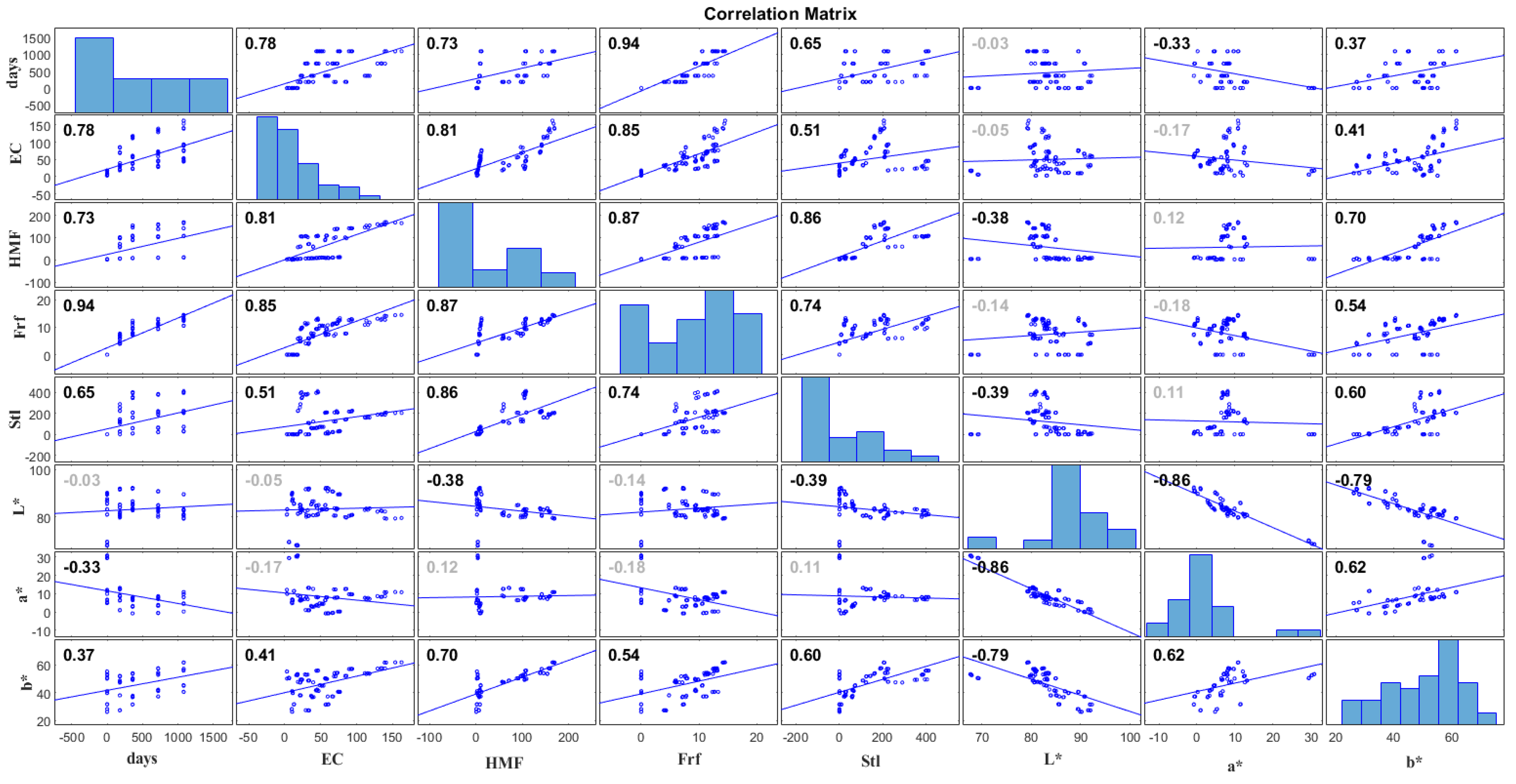
3.5. Correlation Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, V.; Pereira, A.C.; Marques, J.C. Emerging Trends in Fortified Wines: A Scientific Perspective. In Alcoholic Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; Volume 7, Chapter 13; pp. 419–470. [Google Scholar]
- Oliveira e Silva, H.; Guedes de Pinho, P.; Machado, B.P.; Hogg, T.; Marques, J.C.; Câmara, J.S.; Albuquerque, F.; Silva Ferreira, A.C. Impact of Forced-Aging Process on Madeira Wine Flavor. J. Agric. Food Chem. 2008, 56, 11989–11996. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.; Cacho, J.; Marques, J.C. Volatile Profile of Madeira Wines Submitted to Traditional Accelerated Ageing. Food Chem. 2014, 162, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Albuquerque, F.; Rocha, S.M.; Câmara, J.S. Distinctive Characteristics of Madeira Wine Regarding Its Traditional Winemaking and Modern Analytical Methodologies. In Advances in Food and Nutrition Research; Jackson, R.S., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 63, Chapter 7; pp. 207–249. [Google Scholar]
- Perestrelo, R.; Silva, C.; Gonçalves, C.; Castillo, M.; Câmara, J.S. An Approach of the Madeira Wine Chemistry. Beverages 2020, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Alves, R.F.; Nascimento, A.M.D.; Nogueira, J.M.F. Characterization of the Aroma Profile of Madeira Wine by Sorptive Extraction Techniques. Anal. Chim. Acta 2005, 546, 11–21. [Google Scholar] [CrossRef]
- Campo, E.; Ferreira, V.; Escudero, A.; Marqués, J.C.; Cacho, J. Quantitative Gas Chromatography–Olfactometry and Chemical Quantitative Study of the Aroma of Four Madeira Wines. Anal. Chim. Acta 2006, 563, 180–187. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Câmara, J.S. Madeira Wine Volatile Profile. A Platform to Establish Madeira Wine Aroma Descriptors. Molecules 2019, 24, 3028. [Google Scholar] [CrossRef] [Green Version]
- Perestrelo, R.; Silva, C.L.; Silva, P.; Câmara, J.S. Impact of Storage Time and Temperature on Volatomic Signature of Tinta Negra Wines by LLME/GC-ITMS. Food Res. Int. 2018, 109, 99–111. [Google Scholar] [CrossRef]
- Câmara, J.S.; Alves, M.A.; Marques, J.C. Changes in Volatile Composition of Madeira Wines During their Oxidative Ageing. Anal. Chim. Acta 2006, 563, 188–197. [Google Scholar] [CrossRef] [Green Version]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Carbohydrates. In Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2004; pp. 248–339. [Google Scholar]
- Pereira, V.; Albuquerque, F.M.; Ferreira, A.C.; Cacho, J.; Marques, J.C. Evolution of 5-Hydroxymethylfurfural (HMF) and Furfural (F) in Fortified Wines Submitted to Overheating Conditions. Food Res. Int. 2011, 44, 71–76. [Google Scholar] [CrossRef]
- Pereira, V.; Santos, M.; Cacho, J.; Marques, J.C. Assessment of the Development of Browning, Antioxidant Activity and Volatile Organic Compounds in Thermally Processed Sugar Model Wines. LWT 2017, 75, 719–726. [Google Scholar] [CrossRef]
- Pons, A.; Lavigne, V.; Landais, Y.; Darriet, P.; Dubourdieu, D. Distribution and Organoleptic Impact of Sotolon Enantiomers in Dry White Wines. J. Agric. Food Chem. 2008, 56, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Guichard, E.; Etiévant, P.; Henry, R.; Mosandl, A. Enantiomeric Ratios of Pantolactone, Solerone, 4-Carboethoxy-4-Hydroxy-Butyrolactone and of Sotolon, A Flavour Impact Compound of Flor-Sherry and Botrytized Wines. Z. Lebensm. Unters. Forsch. 1992, 195, 540–544. [Google Scholar] [CrossRef]
- Martin, B.; Etievant, P.X.; Le Quere, J.L.; Schlich, P. More Clues About Sensory Impact of Sotolon in Some Flor Sherry Wines. J. Agric. Food Chem. 1992, 40, 475–478. [Google Scholar] [CrossRef]
- Silva Ferreira, A.C.; Barbe, J.-C.; Bertrand, A. 3-Hydroxy-4,5-dimethyl-2(5H)-furanone: A Key Odorant of the Typical Aroma of Oxidative Aged Port Wine. J. Agric. Food Chem. 2003, 51, 4356–4363. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.S.; Marques, J.C.; Alves, M.A.; Silva Ferreira, A.C. 3-Hydroxy-4,5-dimethyl-2(5H)-furanone Levels in Fortified Madeira Wines: Relationship to Sugar Content. J. Agric. Food Chem. 2004, 52, 6765–6769. [Google Scholar] [CrossRef]
- Gaspar, J.M.; Freitas, A.I.; Zhao, Q.; Leça, J.M.; Pereira, V.; Marques, J.C. Is Sotolon Relevant to the Aroma of Madeira Wine Blends? Biomolecules 2019, 9, 720. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Coello, M.S.; Sanz, J.; Cabezudo, M.D. Determination of Volatile Compounds in Hydroalcoholic Extracts of French and American Oak Wood. Am. J. Enol. Vitic. 1999, 50, 162. [Google Scholar]
- Perestrelo, R.; Rodriguez, E.; Câmara, J.S. Impact of Storage Time and Temperature on Furanic Derivatives Formation in Wines Using Microextraction by Packed Sorbent Tandem with Ultrahigh Pressure Liquid Chromatography. LWT 2017, 76, 40–47. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Bin-Jumah, M.; Othman, S.I.; Khafaga, A.F.; Shaheen, H.M.; Samak, D.; Shehata, A.M.; Allam, A.A.; Abd El-Hack, M.E. The Toxicological Aspects of the Heat-Borne Toxicant 5-Hydroxymethylfurfural in Animals: A Review. Molecules 2020, 25, 1941. [Google Scholar] [CrossRef] [Green Version]
- Abraham, K.; Gürtler, R.; Berg, K.; Heinemeyer, G.; Lampen, A.; Appel, K.E. Toxicology and Risk Assessment of 5-Hydroxymethylfurfural in Food. Mol. Nutr. Food Res. 2011, 55, 667–678. [Google Scholar] [CrossRef]
- Monakhova, Y.B.; Lachenmeier, D.W. The Margin of Exposure of 5-Hydroxymethylfurfural (HMF) in Alcoholic Beverages. Environ. Anal. Health. Toxicol. 2012, 27, e2012016. [Google Scholar] [CrossRef] [PubMed]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) Levels in Honey and other Food Products: Effects on Bees and Human Health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Kumar, V.; Kumar, S.; Majid, I.; Aggarwal, P.; Suri, S. 5-Hydroxymethylfurfural (HMF) Formation, Occurrence and Potential Health Concerns: Recent Developments. Toxin Rev. 2020, 40, 545–561. [Google Scholar] [CrossRef]
- Perestrelo, R.; Petronilho, S.; Câmara, J.S.; Rocha, S.M. Comprehensive Two-Dimensional Gas Chromatography with Time-Of-Flight Mass Spectrometry Combined with Solid Phase Microextraction as a Powerful Tool for Quantification of Ethyl Carbamate in Fortified Wines. The Case Study of Madeira Wine. J. Chromatogr. A 2010, 1217, 3441–3445. [Google Scholar] [CrossRef] [PubMed]
- Leça, J.M.; Pereira, V.; Pereira, A.C.; Marques, J.C. A Sensitive Method for the Rapid Determination of Underivatized Ethyl Carbamate in Fortified Wine by Liquid Chromatography-Electrospray Tandem Mass Spectrometry. Food Anal. Methods 2018, 11, 327–333. [Google Scholar] [CrossRef]
- Jiao, Z.; Dong, Y.; Chen, Q. Ethyl Carbamate in Fermented Beverages: Presence, Analytical Chemistry, Formation Mechanism, and Mitigation Proposals. Compr. Rev. Food Sci. Food Saf. 2014, 13, 611–626. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Alcohol Consumption and Ethyl Carbamate; International Agency for Research on Cancer: Lyon, France, 2010; p. 1440. [Google Scholar]
- Weber, J.V.; Sharypov, V.I. Ethyl Carbamate in Foods and Beverages: A Review. Environ. Chem. Lett. 2009, 7, 233–247. [Google Scholar] [CrossRef]
- Pereira, V.; Câmara, J.S.; Cacho, J.; Marques, J.C. HPLC-DAD Methodology for the Quantification of Organic Acids, Furans and Polyphenols by Direct Injection of Wine Samples. J. Sep. Sci. 2010, 33, 1204–1215. [Google Scholar] [CrossRef] [Green Version]
- Pereira, V.; Leça, J.M.; Gaspar, J.M.; Pereira, A.C.; Marques, J.C. Rapid Determination of Sotolon in Fortified Wines Using a Miniaturized Liquid-Liquid Extraction Followed by LC-MS/MS Analysis. J. Anal. Methods Chem. 2018, 2018, 4393040. [Google Scholar] [CrossRef]
- International Organization of Vine and Wine. Compendium of International Methods of Spirituous Beverages of Vitivinicultural Origin; International Organization of Vine and Wine: Paris, France, 2018; Volume 1. [Google Scholar]
- Martins, R.C.; Monforte, A.R.; Silva Ferreira, A. Port Wine Oxidation Management: A Multiparametric Kinetic Approach. J. Agric. Food Chem. 2013, 61, 5371–5379. [Google Scholar] [CrossRef]
- Pereira, V.; Pereira, A.C.; Pérez Trujillo, J.P.; Cacho, J.; Marques, J.C. Amino Acids and Biogenic Amines Evolution during the Estufagem of Fortified Wines. J. Chem. 2015, 2015, 494285. [Google Scholar] [CrossRef] [Green Version]
- Leça, J.M.; Pereira, V.; Miranda, A.; Vilchez, J.L.; Marques, J.C. New Insights into Ethyl Carbamate Occurrence in Fortified Wines. LWT 2021, 150, 111566. [Google Scholar] [CrossRef]
- Teissedre, P.-L.; Jourdes, M. Tannins and Anthocyanins of Wine: Phytochemistry and Organoleptic Properties. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2255–2274. [Google Scholar]
- Carvalho, M.J.; Pereira, V.; Pereira, A.C.; Pinto, J.L.; Marques, J.C. Evaluation of Wine Colour Under Accelerated and Oak-Cask Ageing Using CIELab and Chemometric Approaches. Food Bioprocess Technol. 2015, 8, 2309–2318. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, V.; Leça, J.M.; Freitas, A.I.; Pereira, A.C.; Pontes, M.; Albuquerque, F.; Marques, J.C. Unveiling the Evolution of Madeira Wine Key Metabolites: A Three-Year Follow-Up Study. Processes 2022, 10, 1019. https://doi.org/10.3390/pr10051019
Pereira V, Leça JM, Freitas AI, Pereira AC, Pontes M, Albuquerque F, Marques JC. Unveiling the Evolution of Madeira Wine Key Metabolites: A Three-Year Follow-Up Study. Processes. 2022; 10(5):1019. https://doi.org/10.3390/pr10051019
Chicago/Turabian StylePereira, Vanda, João M. Leça, Ana I. Freitas, Ana C. Pereira, Marisela Pontes, Francisco Albuquerque, and José C. Marques. 2022. "Unveiling the Evolution of Madeira Wine Key Metabolites: A Three-Year Follow-Up Study" Processes 10, no. 5: 1019. https://doi.org/10.3390/pr10051019
APA StylePereira, V., Leça, J. M., Freitas, A. I., Pereira, A. C., Pontes, M., Albuquerque, F., & Marques, J. C. (2022). Unveiling the Evolution of Madeira Wine Key Metabolites: A Three-Year Follow-Up Study. Processes, 10(5), 1019. https://doi.org/10.3390/pr10051019









