Impact of Biogenic Ag-Containing Nanoparticles on Germination Rate, Growth, Physiological, Biochemical Parameters, and Antioxidants System of Tomato (Solanum tuberosum L.) In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biosynthesis of Ag-Containing NPs
2.2. Characterization of Ag-Containing NPs
2.3. Media Preparation and AgNP Treatment
2.4. Seed Germination Assay
2.4.1. Seed Germination Rate (%)
2.4.2. Germination Index (GI)
2.5. Photosynthetic Pigments Estimation
2.6. Antioxidant Enzymes Activity Estimation
2.6.1. Total Protein Content Estimation
2.6.2. Superoxide Dismutase Estimation
2.6.3. Catalase Estimation
2.6.4. Ascorbate Peroxidase Estimation
2.7. Determination of Phenolic Compounds
2.7.1. Determination of Total Phenolic Content (TPC)
2.7.2. Determination of Total Flavonoid Content (TFC)
2.7.3. Determination of Total Tannin Content (TTC)
2.8. Estimation of Total Sugar
2.9. Statistical Analysis
3. Results and Discussion
3.1. Green Synthesis and Characterization of Ag-Containing Nanoparticles
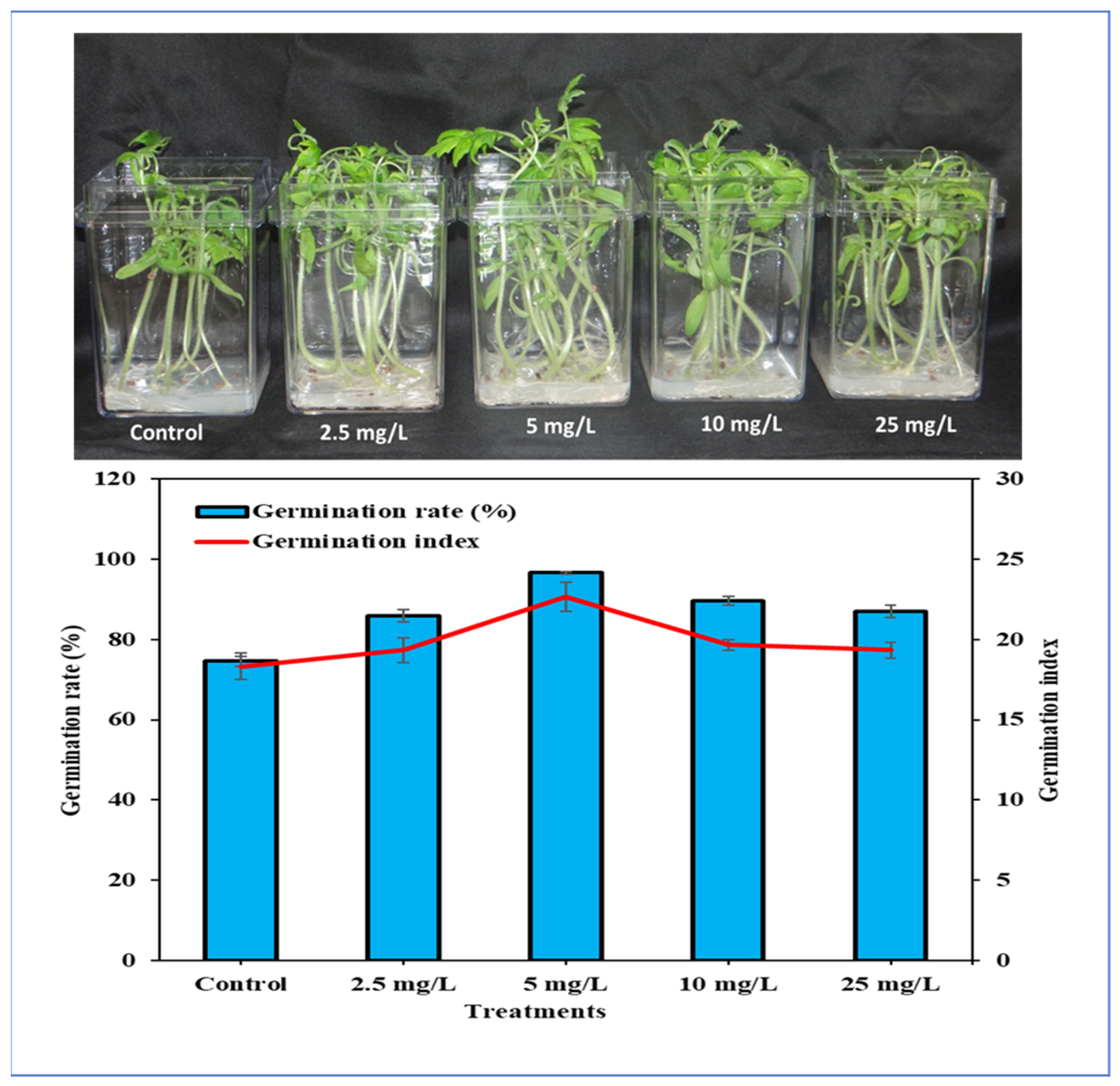
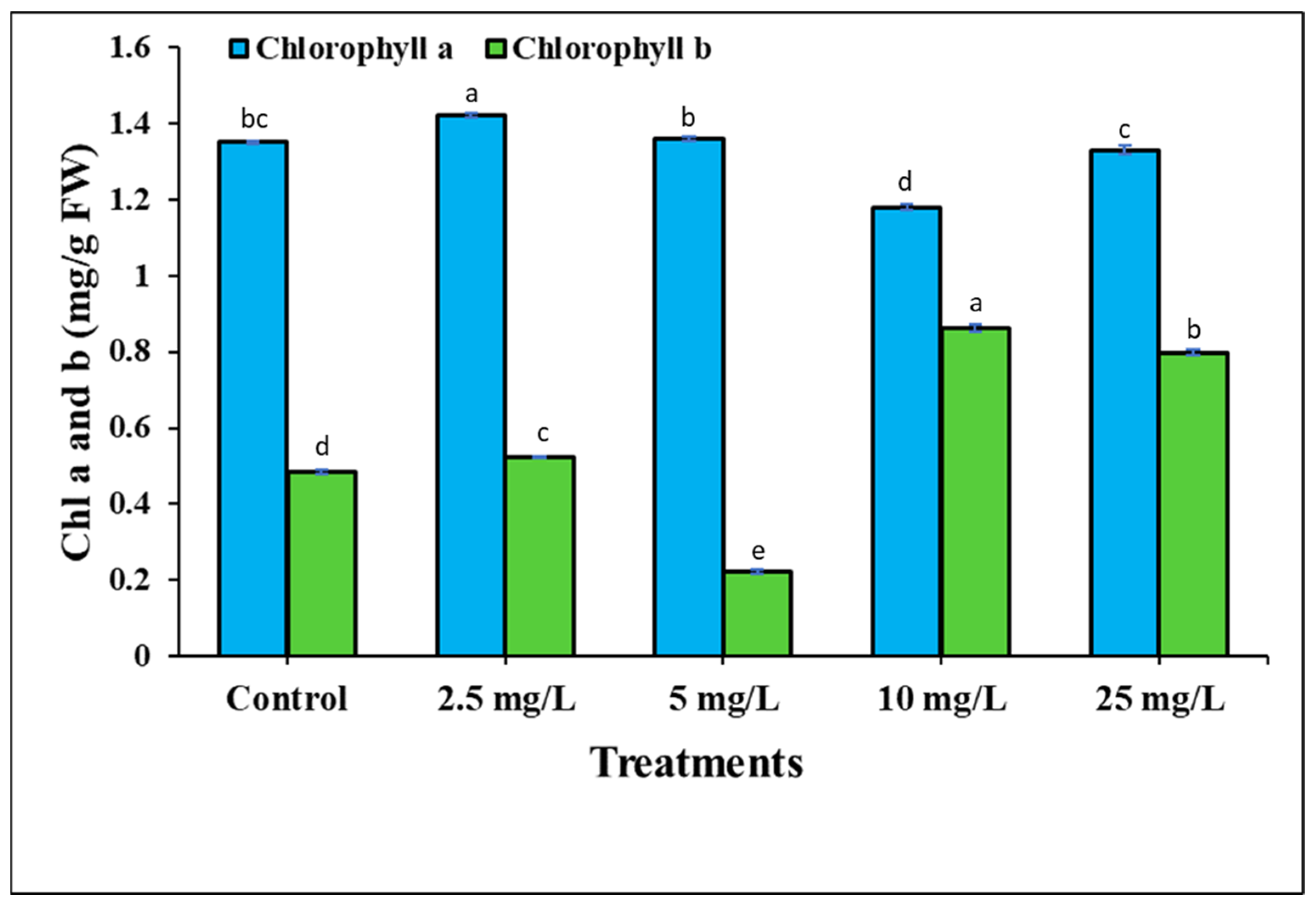
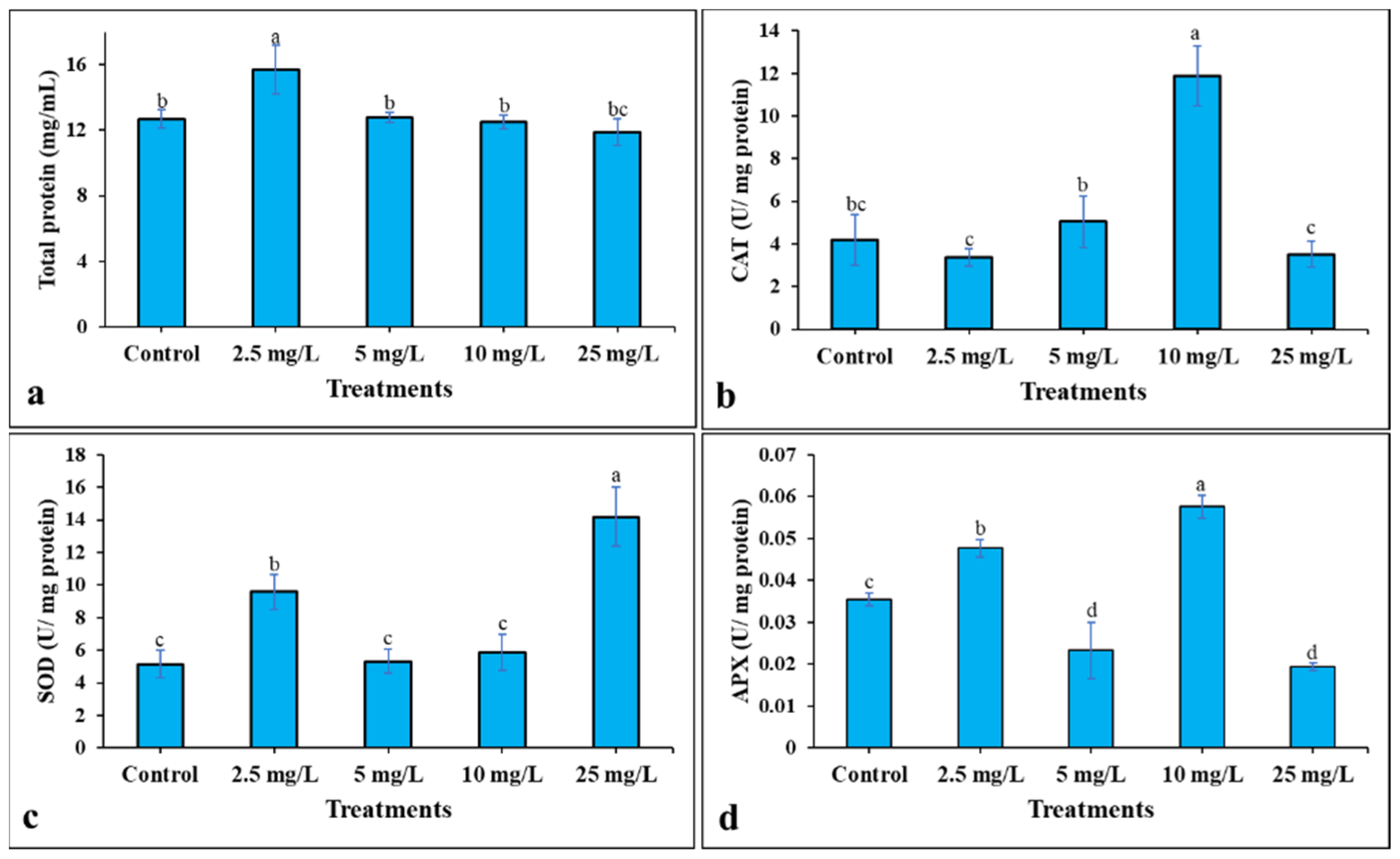
3.2. The Effect of Ag-Containing NPs on Tomato Germination Rate, Growth and Development
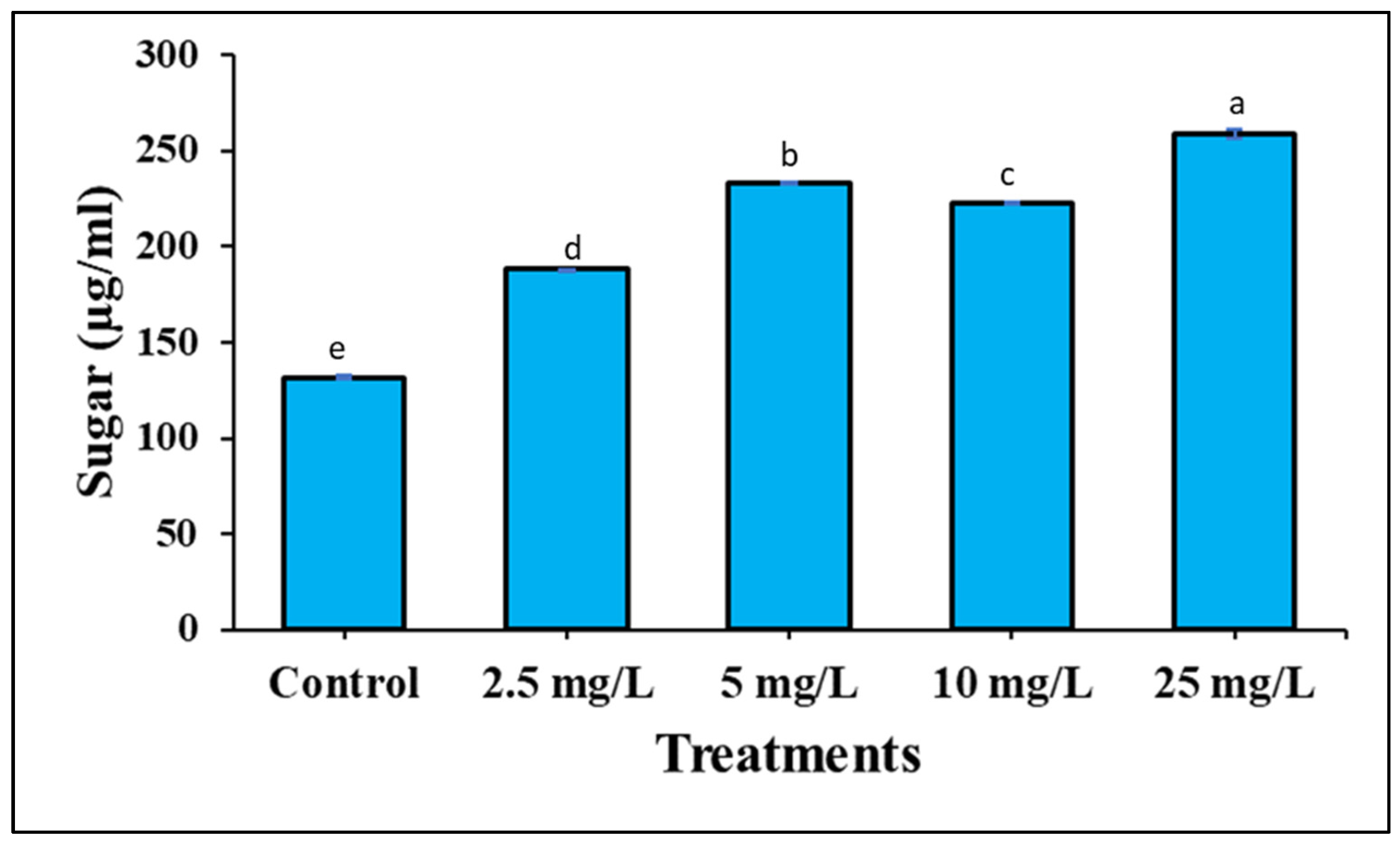
3.3. The Effect of Ag-Containing NPs on Phenolic Compounds and Total Soluble Sugar
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanveer, K.; Gilani, S.; Hussain, Z.; Ishaq, R.; Adeel, M.; Ilyas, N. Effect of salt stress on tomato plant and the role of calcium. J. Plant Nutr. 2020, 43, 28–35. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations—Statistic Division. Available online: https://www.fao.org/faostat/en/#data (accessed on 15 April 2019).
- Muzolf-Panek, M.; Kleiber, T.; Kaczmarek, A. Effect of increasing manganese concentration in nutrient solution on the antioxidant activity, Vitamin C, lycopene and polyphenol contents of tomato fruit. Food Addit. Contam. Part A 2017, 34, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Ditta, A. How Helpful is nanotechnology in Agricultural? Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 033002. [Google Scholar] [CrossRef]
- Tarafder, C.; Daizy, M.; Alam, M.M.; Ali, M.R.; Islam, M.J.; Islam, R.; Ahommed, M.S.; Aly Saad Aly, M.; Khan, M.Z.H. Formulation of a hybrid nanofertilizer for slow and sustainable release of micronutrients. ACS Omega 2020, 5, 23960–23966. [Google Scholar] [CrossRef] [PubMed]
- Rop, K.; Karuku, G.N.; Mbui, D.; Njomo, N.; Michira, I. Evaluating the effects of formulated nano-NPK slow release fertilizer composite on the performance and yield of maize, kale and capsicum. Ann. Agric. Sci. 2019, 64, 9–19. [Google Scholar] [CrossRef]
- Anand, R.; Bhagat, M. Silver nanoparticles (AgNPs): As nanopesticides and nanofertilizers. MOJ Biol. Med. 2019, 4, 19–20. [Google Scholar] [CrossRef]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Hossain, Z.; Mustafa, G.; Komatsu, S. Plant Responses to Nanoparticle Stress. Int. J. Mol. Sci. 2015, 16, 26644–26653. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, D.K.; Tripathi, A.; Shweta; Singh, S.; Singh, Y.; Vishwakarma, K.; Yadav, G.; Sharma, S.; Singh, V.K.; Mishra, R.K.; et al. Uptake, Accumulation and Toxicity of Silver Nanoparticle in Autotrophic Plants, and Heterotrophic Microbes: A Concentric Review. Front. Microbiol. 2017, 8, 7. [Google Scholar] [CrossRef]
- Qian, H.; Peng, X.; Han, X.; Ren, J.; Sun, L.; Fu, Z. Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J. Environ. Sci. 2013, 25, 1947–1956. [Google Scholar] [CrossRef]
- Guru, T.; Veronica, N.; Thatikunta, R.; Reddy, S. Crop nutrition management with nano fertilizers. Int. J. Environ. Sci. Technol. 2015, 1, 4–6. [Google Scholar]
- Jhanzab, H.M.; Razzaq, A.; Jilani, G.; Rehman, A.; Hafeez, A.; Yasmeen, F. Silver nano-particles enhance the growth, yield and nutrient use efficiency of wheat. Int. J. Agron. Agric. Res. 2015, 7, 15–22. [Google Scholar]
- Fouda, M.M.; Abdelsalam, N.R.; El-Naggar, M.E.; Zaitoun, A.F.; Salim, B.M.; Bin-Jumah, M.; Allam, A.; Abo-Marzoka, S.A.; Kandil, E.E. Impact of high throughput green synthesized silver nanoparticles on agronomic traits of onion. Int. J. Biol. Macromol. 2020, 149, 1304–1317. [Google Scholar] [CrossRef]
- Mehmood, A. Brief overview of the application of silver nanoparticles to improve growth of crop plants. IET Nanobiotechnol. 2018, 12, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Saifullah Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hojjat, S.S.; Kamyab, M. The effect of silver nanoparticle on Fenugreek seed germination under salinity levels. Russ. Agric. Sci. 2017, 43, 61–65. [Google Scholar] [CrossRef]
- Benech Arnold, R.; Fenner, M.; Edwards, P. Changes in germinability, ABA content and ABA embryonic sensitivity in developing seeds of Sorghum bicolor (L.) Moench. induced by water stress during grain filling. New Phytol. 1991, 118, 339–347. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [Green Version]
- Jogeswar, G.; Pallela, R.; Jakka, N.M.; Reddy, P.S.; Rao, J.V.; Sreenivasulu, N.; Kishor, P.B.K. Antioxidative response in different sorghum species under short-term salinity stress. Acta Physiol. Plant. 2006, 28, 465–475. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase activity. In Methods for Oxygen Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Ordonez, A.A.L.; Gomez, J.D.; Vattuone, M.A.; Lsla, M.I. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Rodrigues, C.I.; Marta, L.; Maia, R.; Miranda, M.; Ribeirinho, M.; Máguas, C. Application of solid-phase extraction to brewed coffee caffeine and organic acid determination by UV/HPLC. J. Food Compos. Anal. 2007, 20, 440–448. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Khalil, M.M.H.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloevera plant extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef]
- Banerjee, P.; Satapathy, M.; Mukhopahayay, A.; Das, P. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: Synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour. Bioprocess. 2014, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Bu, Y.; Lee, S.W. The characteristic AgcoreAushell nanoparticles as SERS substrates in detecting dopamine molecules at various pH ranges. Int. J. Nanomed. 2015, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Sastry, M.; Mayya, K.; Bandyopadhyay, K. pH Dependent changes in the optical properties of carboxylic acid derivatized silver colloidal particles. Colloids Surfaces A Physicochem. Eng. Asp. 1997, 127, 221–228. [Google Scholar] [CrossRef]
- Zou, J.; Xu, Y.; Hou, B.; Wu, D.; Sun, Y. Controlled growth of silver nanoparticles in a hydrothermal process. China Particuology 2007, 5, 206–212. [Google Scholar] [CrossRef]
- Masum, M.; Islam, M.; Siddiqa, M.M.; Ali, K.A.; Zhang, Y.; Abdallah, Y.; Ibrahim, E.; Qiu, W.; Yan, C.; Li, B. Biogenic Synthesis of Silver Nanoparticles Using Phyllanthus emblica Fruit Extract and Its Inhibitory Action Against the Pathogen Acidovorax oryzae Strain RS-2 of Rice Bacterial Brown Stripe. Front. Microbiol. 2019, 10, 820. [Google Scholar] [CrossRef]
- Hemlata, P.R.M.; Singh, A.P.; Tejavath, K.K. Biosynthesis of Silver Nanoparticles Using Cucumis prophetarum Aqueous Leaf Extract and Their Antibacterial and Antiproliferative Activity Against Cancer Cell Lines. ACS Omega 2020, 5, 5520. [Google Scholar] [CrossRef] [Green Version]
- Almutairi, Z.M.; Alharbi, A. Effect of Silver Nanoparticles on Seed Germination of Crop Plants. Int. J. Nucl. Quantum Eng. 2015, 9, 594–598. [Google Scholar] [CrossRef]
- Kaveh, R.; Li, Y.-S.; Ranjbar, S.; Tehrani, R.; Brueck, C.L.; Van Aken, B. Changes in Arabidopsis thaliana Gene Expression in Response to Silver Nanoparticles and Silver Ions. Environ. Sci. Technol. 2013, 47, 10637–10644. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef]
- Sharma, P.; Bhatt, D.; Zaidi, M.G.H.; Saradhi, P.P.; Khanna, P.K.; Arora, S. Silver Nanoparticle-Mediated Enhancement in Growth and Antioxidant Status of Brassica juncea. Appl. Biochem. Biotechnol. 2012, 167, 2225–2233. [Google Scholar] [CrossRef]
- Saeideh, N.; Rashid, J. Effect of silver nanoparticles and Pb(NO3)2 on the yield and chemical composition of mung bean (Vigna radiata). J. Stress Physiol. Biochem. 2014, 10, 316–325. [Google Scholar]
- Gruyer, N.; Dorais, M.; Bastien, C.; Dassylva, N.; Triffault-Bouchet, G. Interaction between silver nanoparticles and plant growth. In International Symposium on New Technologies for Environment Control, Energy-Saving and Crop Production in Greenhouse and Plant 1037; Son, J.E., Lee, I.b., Oh, M.M., Eds.; International Society for Horticultural Science: Leuven, Belgium, 2014; pp. 795–800. [Google Scholar] [CrossRef]
- Syu, Y.-Y.; Hung, J.-H.; Chen, J.-C.; Chuang, H.-W. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol. Biochem. 2014, 83, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Hong, F.; Lu, S.; Liu, C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [Google Scholar] [CrossRef]
- Hezaveh, T.A.; Rahmani, F.; Alipour, H.; Pourakbar, L. Effects of foliar application of ZnO nanoparticles on secondary metabolite and micro-elements of camelina (Camelina sativa L.) under salinity stress. J. Stress Physiol. Biochem. 2020, 16, 54–69. [Google Scholar]
- Kruszka, D.; Sawikowska, A.; Selvakesavan, R.K.; Krajewski, P.; Kachlicki, P.; Franklin, G. Silver nanoparticles affect phenolic and phytoalexin composition of Arabidopsis thaliana. Sci. Total Environ. 2020, 716, 135361. [Google Scholar] [CrossRef]
- Chung, I.-M.; Rajakumar, G.; Thiruvengadam, M. Effect of silver nanoparticles on phenolic compounds production and biological activities in hairy root cultures of Cucumis anguria. Acta Biol. Hung. 2018, 69, 97–109. [Google Scholar] [CrossRef] [Green Version]

| Stem Length (cm) | Stem FW (mg) | Root Length (cm) | Root FW (mg) | |
|---|---|---|---|---|
| Control | 8.77 ± 0.62 b | 337 ± 2.95 b | 8.33 ± 1.27 ac | 98.7 ± 04.7 c |
| 2.5 mg/L | 11.5 ± 1.11 ab | 504 ± 1.68 ab | 9.20 ± 0.61 a | 72.1 ± 1.34 d |
| 5 mg/L | 12.4 ± 0.56 a | 637 ± 1.12 a | 8.33 ± 0.59 ac | 127 ± 2.82 b |
| 10 mg/L | 11.0 ± 0.57 ab | 394 ± 2.45 ab | 9.03 ± 1.43 a | 184 ± 1.67 a |
| 25 mg/L | 9.60 ± 1.4 ab | 336 ± 2.17 b | 5.30 ± 0.71 c | 68.1 ± 1.36 d |
| Treatments | Phenol (mg GAE/g DW) | Flavonoid (mg QE/g DW) | Tannin (mg TAE/g DW) |
|---|---|---|---|
| Control | 194.6 ± 0.41 e | 118.0 ± 0.28 d | 56.51 ± 0.061 e |
| 2.5 mg/L | 266.0 ± 0.41 b | 127.8 ± 0.24 a | 76.94 ± 0.11 a |
| 5 mg/L | 281.7 ± 0.41 a | 121.5 ± 0.26 c | 76.64 ± 0.06 b |
| 10 mg/L | 231.5 ± 0.23 d | 125.1 ± 0.19 b | 64.97 ± 0.06 d |
| 25 mg/L | 263.9 ± 0.41 c | 121.0 ± 0.15 c | 72.62 ± 0.06 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salih, A.M.; Qahtan, A.A.; Al-Qurainy, F.; Al-Munqedhi, B.M. Impact of Biogenic Ag-Containing Nanoparticles on Germination Rate, Growth, Physiological, Biochemical Parameters, and Antioxidants System of Tomato (Solanum tuberosum L.) In Vitro. Processes 2022, 10, 825. https://doi.org/10.3390/pr10050825
Salih AM, Qahtan AA, Al-Qurainy F, Al-Munqedhi BM. Impact of Biogenic Ag-Containing Nanoparticles on Germination Rate, Growth, Physiological, Biochemical Parameters, and Antioxidants System of Tomato (Solanum tuberosum L.) In Vitro. Processes. 2022; 10(5):825. https://doi.org/10.3390/pr10050825
Chicago/Turabian StyleSalih, Abdalrhaman M., Ahmed A. Qahtan, Fahad Al-Qurainy, and Bander M. Al-Munqedhi. 2022. "Impact of Biogenic Ag-Containing Nanoparticles on Germination Rate, Growth, Physiological, Biochemical Parameters, and Antioxidants System of Tomato (Solanum tuberosum L.) In Vitro" Processes 10, no. 5: 825. https://doi.org/10.3390/pr10050825
APA StyleSalih, A. M., Qahtan, A. A., Al-Qurainy, F., & Al-Munqedhi, B. M. (2022). Impact of Biogenic Ag-Containing Nanoparticles on Germination Rate, Growth, Physiological, Biochemical Parameters, and Antioxidants System of Tomato (Solanum tuberosum L.) In Vitro. Processes, 10(5), 825. https://doi.org/10.3390/pr10050825








